Abstract
Nitrogen (N) is essential for sustaining life on Earth and plays a vital role in plant growth and thus agricultural production. The excessive use of N fertilizers not only harms the economy, but also the environment. In the context of the environmental impacts caused by agriculture, global maize improvement programs aim to develop cultivars with high N-use efficiency (NUE) to reduce the use of N fertilizers. Since N is highly mobile in plants, NUE is related to numerous little-known morphophysiological and molecular mechanisms. In this review paper we present an overview of the morpho-physiological adaptations of shoot and root, molecular mechanisms involved in plant response to low nitrogen environment, and the genetic effects involved in the control of key traits for NUE. Some studies show that the efficiency of cultivars growing under low N is related to deep root architecture, more lateral roots (LR), and sparser branching of LR, resulting in lower metabolic costs. The NUE cultivars also exhibit more efficient photosynthesis, which affects plant growth under suboptimal nitrogen conditions. In this sense, obtaining superior genotypes for NUE can be achieved with the exploitation of heterosis, as non-additive effects are more important in the expression of traits associated with NUE.
1. Introduction
With the growing demand for increased food production and the demand for more economically sustainable agriculture, there is a pressing need to use efficient genotypes in the use of nitrogen (N) in crop fields [1]. From the economic point of view, only 33% of the N applied to the soil is used by wheat, rice, and corn plants [2], resulting in a significant increase in expenditure [3,4]. The excessive use of nitrogen fertilizers causes not only economic damage, but also environmental damage, with soil acidification and water and air pollution [5,6,7]. Damage caused by excessive N use in Europe has been estimated at between $91 USD and $466 USD billion annually. The annual cost of nitrogen fertilizer could be reduced by about $2.3 billion USD if nitrogen uptake efficiency was improved by only 1% [8].
Worldwide, corn (Zea mays L.) alone consumes almost a fifth of the nitrogen produced in the world. According to the United States Department of Agriculture (USDA) [9], in 2020, corn production in the United States (US), China, and Brazil—the three largest grain producers in the world, respectively—corresponded to 64.63% of global production. Additionally, according to data from the Assessment of Fertilizer Use by Crop at the Global Level [10], of all the N used in agriculture (102.50 million metric tons—Mt), the three countries consumed about 39.8% in the cultivation of wheat, rice, soybean, and corn. In terms of N consumption in agriculture, China ranks first, consuming about 24.5% of global N, followed by the United States, with a consumption of 11.5%, and Brazil with a consumption of 3.8%. In China, the highest consumption of N occurs in corn cultivation, with 4.65 Mt of N applied in crop fields, followed by rice (3.90 Mt), wheat (3.40 Mt), and soybean (201 thousand metric tons). In the United States, corn also accounts for the highest consumption of N in agriculture, with a use of approximately 5.58 Mt, followed by wheat (1.56 Mt), rice (201 Mt), and soybean (178 thousand metric tons). In Brazil, similarly, the highest consumption of N in agriculture occurs in corn (1.06 Mt), followed by soybean (266 thousand metric tons), rice (182 thousand metric tons) and wheat (179 thousand metric tons).
Since 1960, the global application of nitrogen fertilizers has increased by 9 times and, in the next forty years, it is expected that there will be an increase of 40 to 60% [11]. Therefore, reducing the consumption of nitrogen fertilizers and the environmental impacts resulting from them, through the cultivation of more efficient N-use genotypes (NUE) is an effective strategy to make agriculture more sustainable from an economic and environmental point of view [12,13]. Nitrogen-use efficiency is a complex characteristic consisting of two main components: N-uptake efficiency (NUpE) and N-utilization efficiency (NUtE), which involve biochemistry, phenology, root architecture, and responses to the environment [14,15,16].
Although nitrogen-use efficiency has been extensively studied in maize, little is known about the molecular mechanisms underlying the plant response to this stress [17]. Such information is important not only for improving plant yields under insufficient nitrogen supply, but also for the development of potential molecular tools for the selection of nitrogen-efficient genotypes. It is believed that there is a diversity of genes involved in molecular mechanisms related to N metabolism [18,19], resulting from the various metabolic processes that this nutrient participates during plant growth and development, namely: uptake, assimilation, recycling, and remobilization [18].
Given the complexity of the processes involved in the efficiency and use of N and the great diversity of metabolic pathways involved, it is necessary to fill the gap in knowledge regarding the morphological, physiological and molecular mechanisms involved in the response to corn tolerance to low N input, so that plant breeding has supported to obtain more efficient genotypes in the use of the nutrient. This review aims to explore corn responses to N-limiting conditions and seeks to give an overview about the morphophysiological and molecular mechanisms underlying this abiotic stress.
2. The Physiological and Morphological Shoot Responses of Maize under Low Nitrogen Conditions
As an essential component of key macromolecules, nitrogen is of great importance to plants [17] and, in maize, it is an extremely important nutrient. N represents up to 5% of the total dry matter [4], and is a constituent of leaf pigments, such as chlorophyll, amino acids, nucleic acids, proteins, and plant hormones [20,21], and plays an important role in photosynthesis. The nitrogen used in all photosynthetic apparatus can be divided into two categories: (i) N associated with enzymes related to CO2 assimilation; and (ii) N present in thylakoids and associated with photochemical efficiency.
Regarding the association with enzymes, N is present in the structure of ribulose-1,5-bisphosphate carboxylase (Rubisco), phosphoenolpyruvate carboxylase (PEPC) and pyruvate orthophosphate dikinase (PPDK), which are directly involved in carbon reduction reactions, and are the most abundant enzymes in the assimilation of CO2 [22].
Regarding the N associated with thylakoids, the nutrient can be distributed between two types of proteins, which are related to bioenergetics-including Cyt b6f and CF1/CF0, involved in electron transport and phosphorylation [23,24]—and those involved in the photosystem I (PSI) and photosystem II (PSII) [light-harvesting complex II (LHCII) and I (LHCI)] [25]. In plants with C4 metabolisms, about 45% of nitrogen is allocated to soluble proteins (20% of which is Rubisco) and 28% to thylakoids. Of this total present in thylakoids, about 75% of N is associated with light-harvesting proteins and the remainder is allocated to bioenergetics [22].
Therefore, the reduced supply of nitrogen negatively affects many aspects of plant growth and development in diverse morphophysiological stages (Figure 1), such as germination, seedling emergence, tillering, flowering, pollination, and ultimately in yield and grain quality [26,27,28,29]. The impact of low N stress depends upon the magnitude and duration of exposure, on the genotype response, soil moisture and status and other environmental aspects. In maize, these negative effects impact plant growth and development in many aspects [30]. At early seedling stage it causes poor seedling establishment [31]. At the beginning of the vegetative development, it affects root and shoot growth, impacting on the acquisition of mineral and water supply, resulting in reduction of leaf area. The prolonged exposure causes reduction in photosynthesis, which directly affects the grain yield by reducing the weight and length of ears [31], the weight of the grains, and the prolificity [32,33].
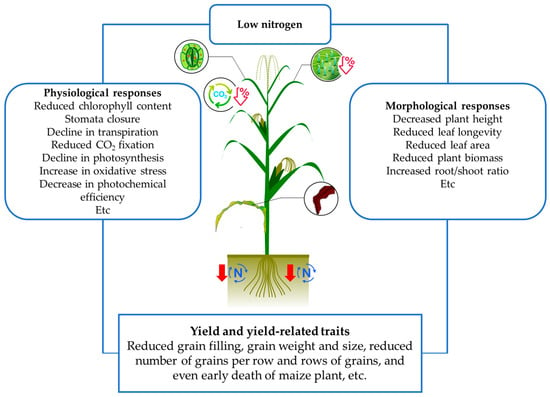
Figure 1.
Schematic diagram showing impacts of plants to low nitrogen stress.
However, plants have numerous morphophysiological mechanisms to respond to the different stresses to which they are subjected that were obtained through natural or artificial selection [34], and grant them tolerance to unfavorable environments, as is the case of low nutritional availability. In terms of low nitrogen availability, as a positive response, corn—which is a C4 plant—can increase the amount of CO2 available to Rubisco, investing much less N for the synthesis of this protein, which makes the crop more efficient at assimilating carbon per unit of N when compared to rice, for example, a C3 plant [35]. In addition, the low amount of Rubisco in corn allows for greater investment in thylakoid components [36], allowing the crop to perform well in scenarios where N demand is not met. Another important mechanism of low N tolerance is the reduction in the relative content of chlorophyll in leaves for the protection of the photosynthetic apparatus, which, according to Khamis et al. [37] and Lu et al. [38], means an important strategy for the protection of PSII, since the reduction in the content of this pigment reduces the amount of excitation energy in the system that, in a stressful scenario, could generate oxidative damage due to the presence of reactive oxygen species (ROS)—resulting in low values of stay-green.
Regarding the stay-green, the damage caused by ROS in the plant cells directly affects this trait—which was the most important phenotype for breeding selection, particularly for maize—resulting in early leaf senescence [39]. Stay-green is a term used to describe genotypes with a delay in leaf senescence when compared other genotypes. Stay-green has a strong correlation with productivity because higher values for this characteristic translates into greater photosynthetic activity after the flowering period and, therefore, greater production of photoassimilates, positively impacting productivity [40].
In a scenario of adequate N supply, plants use different mechanisms to distribute N among the various metabolic processes in which the nutrient is involved. Among these processes, the remobilization and recycling of N stands out, which, during the reproductive growth, supplies 35 to 55% of the N destined to grains [41,42]. However, in an infra-optimal condition of N supply, this mechanism is disrupted and the N that should be destined to filling the grain will increasingly depend on remobilized N, since the availability of soil N to be absorbed is low. Therefore, yield is reduced to the extent that, at the expense of N remobilization from leaves—as a component of photosynthetic pigments, nucleic acids, etc.—several cell functions are affected, causing damage to the leaves, through reductions in chlorophyll content, Fv/Fm, and photosynthesis activity (measured as CO2 interchange) [43] and to the roots—which has reduced its supply of photoassimilates. All these effects ultimately significantly affect productivity. Therefore, more N-use efficient genotypes with higher values of stay-green have been the focus of improvement programs for the low N condition, since these plants have, as one of the tolerance mechanisms, a more effective absorption of N in an environment of limiting N, reducing the need to activate remobilization mechanisms that would damage photosynthetic processes [44].
Another existing hypothesis about the tolerance mechanisms of maize plants under low N conditions—proposed by Plénet and Lemaire [45] and by Ciampitti and Vyn [46]—is that some genotypes would be able to accumulate luxury N, that is, N which is absorbed during vegetative growth and is stored into storage pools, instead of being used in the increment of biomass. According to the authors, this luxury N would then be used later in a scenario of nutritional deprivation during grain filling, a critical phase for the occurrence of stress in corn. Although later studies have found a strong association between high levels of productivity and the accumulation of luxury N, the mechanisms underlying this response of luxury N uptake and greater shoot longevity, i.e., greater stay-green, have remained unstudied. In order to clarify this response, subsequent studies with 15N isotope tracking sought to quantify the response of maize to the stress of low N availability during grain filling. Paponov and Engels [47], Oliveira Silva et al. [48] and, more recently Nasielski et al. [44], in greenhouse and field experiments proved that during post-silking N stress, maize metabolism adapts to ensure that the necessary N is allocated to the grain at the expense of the N accumulated during the vegetative stage. Therefore, more efficient genotypes with a positive tolerance response to low amounts of N in the soil are more effective in accumulating luxury N in the vegetative stages. In view of the urgent issues related to climate change and the environmental damage caused by agriculture, breeding programs aimed at selecting superior materials for this condition can be highly successful when aimed at evaluating materials that are more efficient for these conditions, so that to reduce the consumption of N.
3. The Root Adaptation of Maize under Low N Conditions
Roots play a crucial role from the initial development of seedlings to harvest, whether under stress conditions or optimal growth conditions, in addition, root plasticity plays a vital role in the adaptation of plants to stressful environments, whether in the acquisition or transport of nutrients [49]. For this, root systems have developed anatomical and physiological strategies to exploit resources in complex environments [50,51]. Corn has an embryonic root system—which includes the primary root/radicle and seminal roots—and a post-embryonic one, which comprises shoot-borne roots, including crown roots (formed below ground) and brace roots (formed at aboveground nodes) [52]. Primary, seminal, crown, and brace roots are also called axial roots, while roots arising from axial roots are called lateral roots (LRs) (Figure 2).
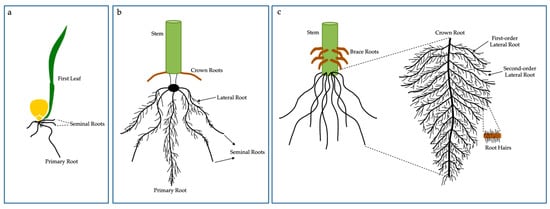
Figure 2.
Maize embryonic (a), early post-embryonic (b) and adult post-embryonic (c) root systems are represented. (a) The primary root and seminal roots initiate from the embryo. (b) Lateral roots initiate from primary root and seminal root, while crown roots initiate from the stem. (c) The root system of adult maize is comprised of numerous crown roots and their lateral roots, which goes through several branching orders. The different root organs are terminated by root hairs. The scheme also demonstrates structural brace roots that start above-ground.
Some corn root system responses have been documented. Lynch et al. [53] proposed a root ideotype called “steep, cheap, and deep” to optimize water and N capture in maize. This ideotype consists of a set of architectural, anatomical, and physiological attributes that promote a quick exploration of more distant soil profiles and better acquisition of nitrate—the predominant form of N acquired by plants, highly mobile and soluble. In this more efficient root phenotype proposed by Lynch et al. [53], regarding architectural attributes, more accentuated angles (in relation to the ground) of root growth favor the exploration of more distant profiles in the soil and, therefore, better acquisition of nitrogen—which, due to its greater mobility when compared to other nutrients— tends to be concentrated on deeper soil layers. Therefore, a greater depth of the root system, that is, a smaller root angle, has a direct effect on the accumulation of plant biomass. Another feature associated with the root architecture proposed by Lynch [53] is the presence de few nodal roots and sparser LR branching which contributes to the reduction of competition between root axes for resources such as carbohydrates (since a greater number of ramifications would require redistribution of carbohydrate between these structures—which represents greater energy expenditure) and available nitrate. This root phenotype has been corroborated by other studies, such as the one developed by Wang et al. [54] who sought to evaluate the influence of nitrate supply availability on root morphology and N uptake efficiency in 5 corn lines. According to the authors, under an N deficient situation, a larger root system with a great root length allowed the plants to explore the deep strata of soil, contributed to the efficient N accumulation. Trachsel et al. [55], evaluating 108 inbred lines of maize in grown in high and low nitrogen under field conditions in the USA and South Africa found that the angles of crown roots were significantly associated with rooting depth—calculated as the depth containing 95% of the root mass, which means that the biggest portion of the crown roots were in the deep soil strata, contributing to a better N acquisition. The study confirms that more accentuated root angles allowed more adapted genotypes, under conditions of N limitation, to potentially explore soil volumes like those genotypes under optimal conditions of N availability, thus avoiding the reduction in grain yield.
Given the large number of characteristics and root phenotypes that affect the search for water and mineral resources in the soil, and the diversity of genetic and molecular mechanisms, it is quite unlikely that genotypes with maximum productive capacity will be reached in breeding programs based on up only on components such as productivity and other secondary characteristics. Despite being rare, breeding programs that employ these characteristics in the search for more adapted root phenotypes are successful [56,57,58].
In terms of the important role of root system adaptation for the maintenance of plants in an environment of low nutritional availability, we cannot fail to highlight the crucial performance of the microbiota present in the rhizosphere for the availability of N to plants. Because the productivity gains in maize cultivation of the last years were achieved through genotype selection in the presence of optimal N conditions, cultivars developed over the years are less and less associated with beneficial microbial communities. In this sense, modern cultivars no longer provide the microbiota with supplies for its maintenance— such as photosynthates and nutrients since, due to the high amounts of N applied, they no longer need these communities [59]. Despite being abundant and composing about 80% of the atmosphere, nitrogen in its natural form is not available to plants since N can only be absorbed in the forms of ammonium (NH4+) or nitrate (NO3−) by the roots (Figure 3).
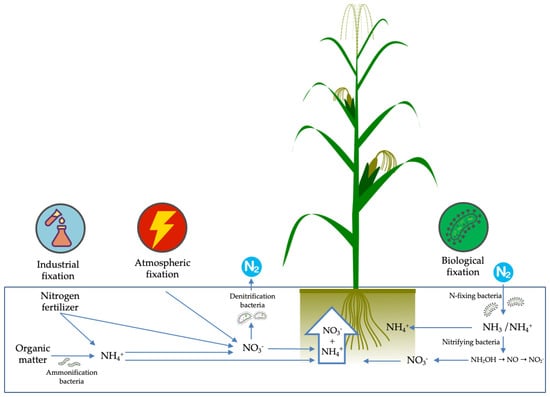
Figure 3.
Summary of the industrial, atmospheric and biological N fixation.
Therefore, for plant nutrition, it is necessary that the N2 gas be transformed into assimilable forms that can be carried out through biological, industrial or atmospheric fixation. Of these processes, biological fixation is the one that has the best potential to add nitrogen to the soil with the best cost/benefit ratio [60].
In terms of biological fixation, it is well known that plants can influence their microbiome, and this regulation takes place through the ability of plants to send signals into their environment—which can have a positive or negative effect on other plants and members of their microbiome [61,62,63,64,65,66]. The exudates produced by the roots, composed of allelochemicals, have been associated with signaling in the plant-microbiome interaction and the positive response of plants to various environmental stresses [61]. Regarding the low availability of N in the soil, an example is the recent study carried out by Van Deynze et al. [67], who demonstrated that the carbohydrate-rich mucilage produced by the aerial roots of an indigenous maize landrace supports a complex diazotrophic microbiota, enriched for homologs of genes encoding nitrogenase subunits that harbor active nitrogenase activity, and that nitrogen is transferred efficiently from the nitrogen-fixing bacteria to the host plant tissues. These results demonstrate that in addition to the selection of more adapted genotypes in terms of root architecture, plant breeding becomes more effective when genotypes with greater associations with their microbiome are selected.
4. The Molecular Mechanisms Involved in Nitrogen-Use Efficiency in Maize
Below, we highlight other important mechanisms for the tolerance response and efficiency of plants in using available nitrogen in environments with nutrient limitation—such as those related to the acquisition, transport, and remobilization of N.
Nitrogen is a nutrient that has great mobility in plants, so its metabolism involves several processes, including absorption, reduction, assimilation, translocation and remobilization. The genetic differences between nitrogen uptake or productivity per unit of nitrogen applied to the soil have been studied for several grasses, especially those of commercial importance, such as wheat, rice, oats and, mainly, corn. The latter, in general, is one of the most produced cereals worldwide and one that most requires nitrogen fertilizers to increase productivity [68]. However, little is known about the regulation of nitrate assimilation at the molecular and genetic levels in this crop [69]. In this perspective, each process involved in the use of nitrogen has been widely explored, in order to elucidate the routes by which efficient plants in the use of N can avoid the effects of the lack of this nutrient in the soil. Below, each of these steps involved in the use of nitrogen is highlighted, including the molecular mechanisms described in the literature and how they influence plants in the efficient use of N.
4.1. Nitrogen Uptake and Transport
Nitrogen is a limiting macronutrient for plant growth and development [70,71]. Soil nitrogen availability is normally low and can be influenced by factors such as precipitation, temperature, pH and soil type. The form of nitrogen preferred by plants is intrinsically related to their adaptation and soil conditions [72]. Plants can use both nitrate (NO3−) and ammonium (NH4+) as sources of N [8,73,74]. NO3− is the main and most readily available source of nitrogen for most higher plants [75,76], however, NO3− concentrations in soils can be very varied, depending on environmental variations and, therefore, plants have developed several specific adaptations for the uptake of available NO3− [72,77].
Nitrate is actively transported into cells, mainly by the NO3− transcarriers of the NRT family, which depend on the energy supply and electrochemical gradient and are divided into two systems existing in the cell membranes of the roots [77,78,79,80]. One of them is the high affinity transport system (HATS), which is activated when there is a high concentration of nitrate available to the plant. The other, the low affinity transport system (LATS), is activated under conditions of low nitrate concentration [71,72,78,81].
NRT transporters belong to three main families: the first, NRT1—or NPF—contains many genes, which can be divided into 8 to 10 subfamilies in Arabidopsis, which are predominantly low-affinity transporters [71,78,80,82]. The other families—NRT2/NRT3 (NAR2)—play an important role in the high-affinity transport of NO3− [72,80]. As in Arabidopsis, NRTs have been identified in rice, sorghum and maize and show differences in gene numbers and family structure. Studies show that in the corn genome there are four copies of genes of the ZmNRT2 family, namely: ZmNRT2.1, ZmNRT2.2, ZmNRT2.3 and ZmNRT2.5, of which only two were studied: ZmNRT2.1 and ZmNRT2.2, that have about 98% homology in their amino acid sequence [81]. Both are inducible to NO3− in seedling roots, having been found transcripts of the former in the region of the root cortex while, for the latter, in the cortex, stele, and lateral primordia of the roots [83].
For the NRT3 family, two copies of the NRT3.1 gene were described in the maize genome: ZmNRT3.1A and ZmNRT3.1B, both never characterized in maize, but which are strong candidates in the NO3− transport complex [72,84,85] More recently, Wang et al. [86], after analyzing the proteome of two contrasting hybrids for the efficiency in the use of nitrogen, verified the function of the ZmTGA gene and the study found that the gene has an important role in maintaining the tolerance of plants in low conditions. N, giving greater length and area of the root system, greater shoot/root ratio, in addition to lower leaf senescence compared when comparing the mutant with the wild type. TGA transcription factors are a very important group of the bZIP (basic leucine zipper) family [87] and can bind to the -1 (as-1) activation sequence with TGACG as the core and activate or inhibit the translation of downstream target genes, having, therefore, an important role in the defense and response of plants to various biotic and abiotic stresses, such as low nitrogen availability. Furthermore, studies show that plants with overexpression of the TGA1 or TGA4 genes show a significant increase in the nitrate transporter genes NRT2.1 and NRT2.2 [88,89], whose functions we discussed earlier.
Ammonium is also a direct source of nitrogen taken up by plant roots, but, in general, the ammonium content in unfertilized soils is up to 1000 times lower than that of nitrate. [90]. However, efficient ammonium uptake is critical for optimal plant growth and development, as it confers several beneficial effects, such as root density and corn seedling length [91,92] or enhanced H+ proton extrusion, which subsequently acidifies the rhizosphere and leads to increased bioavailability of poorly soluble nutrients such as P or Fe [93]. Therefore, this NH4+ absorption process is expected to be highly regulated under adverse conditions of N availability in the soil.
Whenever the ammonium concentration in the soil solution is low, the contribution of high-affinity transport systems (HATSs) becomes more relevant to the overall ammonium uptake by roots [93]. In general, high-affinity ammonium transport is mediated by AMT1-type ammonium transporters: ZmAMT1; 1a and ZmAMT1; 3, which belong to the ammonium/methylammonium permease/rhesus transporters (AMT/MEP/Rh) [93,94].
The two ZmAMTs confer high-affinity ammonium transport activities and are localized in the plasma membrane of maize root epidermal cells. Furthermore, their gene expressions are induced by ammonium, and one study revealed high correlations with high-affinity ammonium and increased root influx rates [93]. Although ZmAMT1; 1a e ZmAMT1; 3 are likely to be the main components for ammonium uptake in the root, not much is known about their physiological contribution to N uptake and use efficiency [94].
4.2. Nitrogen Reduction and Assimilation
Once incorporated, nitrate is reduced to nitrite in plant cells in a reaction that takes place in the cytosol and is catalyzed by a nitrate reductase (NR) [95,96]. The nitrite is then translocated to plastids and chloroplasts, where it is reduced to ammonium by the enzyme nitrite reductase (NiR). Nitrate-derived ammonium, or that produced by photorespiration or amino acid recycling, is more assimilated in plastids by the GS/GOGAT cycle [97,98].
Ammonium is attached to glutamate to form glutamine by glutamine synthase (GS; family of Gln genes), of which there is a plastid isoform (GS2) and a cytosolic isoform (GS1). In maize, a single gene encodes GS2 (Gln2), while at least 5 genes encode GS1 (Gln1-1 to Gln1-5), which are differentially expressed during development [77,99,100,101].
Glutamate can serve as an amino acid donor to other amino acids and nitrogen-requiring compounds, or act as an amine acceptor in the GS-GOGAT cycle to regenerate glutamine. Plants have two types of GOGAT enzymes—NADH-GOGAT and Fd-GOGAT—which use NADH and ferredoxin as electron donors, respectively [102]. Different parallels of GOGAT show constitutive or tissue-specific activity in plants, including maize [99]. Ferridoxine-GOGAT is localized in leaf chloroplasts, while NADH-GOGAT is expressed in non-photosynthetic tissues, including root plastids [97]. After nitrogen assimilation, glutamine, glutamate and other amino acids, including asparagine and aspartate, are transported by vascular tissues to growing organs [97]. Nitrate and ammonium can also be stored in vacuoles before or after long-distance transport.
4.3. Translocation and Remobilization of Nitrogen
During senescence, leaf proteins and photosynthetic proteins are extensively degraded, becoming a huge source of nitrogen, which plants can exploit to supplement the nutrition of growing organs [97].
During the grain filling period, the absorption and assimilation of nitrogen are often insufficient for the high demand required at this stage, making re-mobilization in the different organs of the plant necessary to direct nitrogen to the seeds [98]. The contribution of this process to supplying N in cereals such as rice, wheat and corn varies according to the cultivar, at rates of 50 to 90% [97]. N remobilization also depends on the environment and is favored under conditions of nitrate limitation [103].
In this sense, glutamine (Gln) is the main amino acid translocated in cereals as a source of N. Therefore, in senescence, glutamine concentrations increase in the phloem sap, being remobilized to the reproductive organs. In this process, GS and GOGAT enzymes are important for the remobilization and reuse of N in senescent and developing organs, respectively [80,104]. Some studies have shown that GS1-1 is responsible for this process and that NADH-GOGAT1 plays a key role in the reuse of Gln transported in the developing organs of rice [104,105]. In maize, wheat, and barley, grain N content is correlated with senescence of flag leaves and appears to play a significant role in N availability for grain filling [72,100,105].
5. Maize Improvement for Low N Conditions
The development of genotypes tolerant to low N conditions along with the adoption of improved agronomic practices—as reduction of nitrogen fertilizers applied in crop fields—are required to sustain corn productivity under the negative effects of climate changes. Recent advances in plant breeding for improving corn tolerance to low N conditions, thus harvesting better yields under infraoptimal conditions of N in the soil, are discussed below.
The Conventional Breeding Approach and the Genetic Basis of Nitrogen-Use Efficiency under Conditions of Limited N in Soil
The development of superior varieties for N use is the most viable and efficient strategy to mitigate the negative effects of climate change—which increasingly requires the development of a sustainable agricultural model. Therefore, one of the initial steps in breeding for N-use efficiency involves testing candidate environments and genotypes, then selecting superior varieties. The selection process can be effective for traits that are highly heritable and positively correlated with high productivity under conditions of N limitation [106,107]. However, for the most part, the traits that contribute to grain yield and productivity are of a polygenic nature and have relatively low heritability, making direct selection difficult [108,109]. In these cases, the use of secondary traits with positive correlation with productivity can serve to assess gains in the selection process. As we mentioned in Topic 2.2, different are the responses of plants under infra-optimal conditions of N availability and, given the advances achieved in plant phenotyping in recent years, evaluating a greater number of secondary characteristics has been less challenging.
Therefore, several breeding programs have sought to evaluate secondary characters that are shown to be associated with higher productivity in maize under low N conditions [110], such as photosynthetic rate, relative content of pigments (such as chlorophyll, flavonoids and anthocyanins), chlorophyll fluorescence, stay-green duration and other traits associated with plant growth. Regarding foliar pigments, several studies with abiotic stresses have pointed to the effectiveness and speed of the evaluation of these parameters, which can be carried out with the aid of portable meters that use non-destructive methods for evaluation [58,111]. Portable chlorophyll meters have been applied in the diagnosis of N status in maize and the significant relationship between chlorophyll meter readings and N status as well as productivity of maize plants has been well documented [112,113,114,115,116,117]. Parameters associated with leaf gas exchange and chlorophyll fluorescence have also proven to be powerful tools to monitor the photochemical efficiency of leaves because they are reliable, non-destructive and can be obtained in vivo to assess important physiological phenomena that have a high correlation with maize productivity under optimal conditions and deprivation of N in the soil, according to results demonstrated in some studies [37,118,119].
Once the stages of selection of superior genotypes for limiting N conditions have been advanced, defining the strategy that will guide the breeding program is crucial for obtaining adapted varieties. In this sense, studies aimed at understanding the genetic control of target traits (and correlated with productivity, as we discussed) are important to understand how to increase the frequency of favorable alleles associated with these traits, as well as assist in the selection of the best genotypes parental. In this sense, several key traits have been studied to understand genetic control, tolerance and nitrogen use efficiency in maize (Table 1), in order to guide crop improvement programs regarding the most appropriate improvement methods for gains in productivity [120].

Table 1.
Genetic parameters for traits associated with maize under limiting nitrogen conditions.
Studies conducted in recent years on common corn and popcorn (Zea mays everta), using classical and molecular genetic approaches, have shown that the inheritance of traits linked to tolerance to low N and efficiency in the use of nitrogen can be additive or non-additive, linked to dominance and/or epistasis effects. Through classical approaches, such as diallel analysis or generation mean analysis, authors show that grain yield and yield-related traits—such as length and diameter of the ear, number of grains per row, number of rows of grain and one hundred grains weight, protein content, etc.—are controlled mostly by non-additive effects, either under optimal or nitrogen-limiting conditions [2,29,122,123,124,125,126]. In a recent study, for example, Amegbor et al. [127] valuated 100 corn hybrids under optimum and limiting nitrogen conditions and, through combinatorial analysis, obtained estimates of the general (GCA) and specific (SCA) combining abilities for traits associated with phenology (days for male and female flowering and interval between flowering) and plant architecture as well as productivity and secondary characters. According to the authors, GCA e SCA varied for grain yield demonstrating the importance of additive and non-additive genetic effects for the hybrids evaluated under contrasting N conditions. Even though significant variations were detected for GCA and SCA, GCA which is the additive gene action component mainly controlled the heritage of grain yield under both conditions [127].
In terms of efficiency indices in the use of nitrogen and its secondary components—NUpE and NUtE-DoVale et al. [123] and Mastrodomenico et al. [121] report a greater contribution of additive effects on these characteristics with the others reporting a greater contribution of additive non-additive effects in common corn [125,126] and in popcorn [29,128], for example. In a work developed by Souza et al. (2008), thirty-one corn genotypes (28 crosses between commercial hybrids and three controls) were evaluated in soils with high and low N application rates. The authors found that in corn grown in soil with high N content, the GCA and SCA were significant for grain yield, NUE and NUpE. In corn from soils with low N content, only GCA, in NUpE, was significant. As for NUte, GCA and SCA were not significant in any of the environments. Thus, the authors concluded that for the conditions studied, the additive and non-additive genetic effects are responsible for the genetic control of NUE and grain yield in maize cultivated in soils with high availability of N. Riache et al. [129], evaluating diallel hybrids of Alegrian varieties of corn under two contrasting N conditions—optimal and infraoptimal—proposed that for the evaluated characteristics (plant height, interval between flowering, productivity, etc.) the best method to assess significant gains would be by exploring the interpopulation recurrent selection method (or reciprocal recurrent selection), since additive and non-additive effects contributed to the expression of genotypic variation in the studied material.
For popcorn, a crop of significant commercial importance [58], although studies aimed at elucidating the inheritance and genetic control of important traits are less expressive [120,130,131,132,133,134,135,136], especially when it comes to abiotic stresses as it is the case of low N availability. In this perspective, Santos et al. [29] evaluating 90 hybrids from a complete diallel, under contrasting N availability conditions, for two indices of N use efficiency, grain yield and popping expansion, concluded that both additive and non-additive gene effects were important for selection for NUE. Moreover, the authors also concluded that there was allelic complementarity between the lines and a reciprocal effect for NUE, indicating the importance of the choice of the parents used as male or female. Considering the mentioned studies, the exploitation of heterosis is still the most viable alternative from an economic and sustainable point of view to obtain more tolerant and efficient genotypes in the use of N and, consequently, more productive.
However, not departing from the quantitative nature of the inheritance of essential traits related to low N tolerance, such as those mentioned above, genomic prediction (GP) has been applied to explore the additive effects in order to improve the response of maize to environments with low N availability (Table 1). Ertiro et al. [110], using GP to explore these effects on traits such as grain yield, flowering, plant height, ear height, and number of ears per plant, found prediction accuracies ranging between 0.24 and 0.67.
6. Conclusions and the Way Forward
Over the past few years, researchers have shed light on the complex genetic architectures and regulatory mechanisms involved in the nitrogen use efficiency in corn. There are several adaptive responses of maize in conditions of low nitrogen availability, which involve complex pathways of absorption, transport, remobilization, and recycling of the nutrient. However, several key traits can be used in order to select genotypes more efficient, but the use of methodologies that also explore the root phenotype can help to achieve even more satisfactory results in corn productivity for environments with limited nitrogen.
The traits related to tolerance or efficiency in the use of N are governed by multiple genes with mostly non-additive effect, which allows breeding programs to develop more efficient genotypes by exploiting heterosis in the release of hybrids. superiors. However, the possibility of advances in intrapopulational recurrent selection programs should not be ruled out, so that the frequency of additive effect genes can be increased, which also have some contribution to the expression of traits associated with tolerance and efficiency in the use of N.
With advances in the research topics presented in this review, the complex network of responses associated with corn tolerance and/or efficiency to low N soil conditions may be the subject of future studies. Undoubtedly, this acquired knowledge will help to improve the development of new corn cultivars that can adapt and resist the challenges presented by the need to reduce nitrogen fertilization in crop fields.
Author Contributions
T.d.O.S. conceived the topic and wrote the section on the physiological and morphological shoot responses of maize under low nitrogen conditions and about root adaptation. M.M.M. and A.T.d.A.J. wrote on the sections molecular mechanisms involved in nitrogen-use efficiency in maize and the genetic basis of nitrogen-use efficiency under conditions of limited N in soil, respectively. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing Nitrogen for Sustainable Development. Nature 2015, 528, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Almeida, V.C.; Viana, J.M.S.; Risso, L.A.; Ribeiro, C.; DeLima, R.O. Generation Mean Analysis for Nitrogen and Phosphorus Uptake, Utilization, and Translocation Indexes at Vegetative Stage in Tropical Popcorn. Euphytica 2018, 214, 103. [Google Scholar] [CrossRef]
- Tilman, D.; Reich, P.B.; Knops, J.; Wedin, D.; Mielke, T.; Lehman, C. Diversity and Productivity in a Long-Term Grassland Experiment. Science 2001, 294, 843–845. [Google Scholar] [CrossRef] [PubMed]
- Mascia, M.; Sega, D.; Zamboni, A.; Varanini, Z. Nitrogen Starvation Differentially Influences Transcriptional and Uptake Rate Profiles in Roots of Two Maize Inbred Lines with Different NUE. Int. J. Mol. Sci. 2019, 20, 4856. [Google Scholar] [CrossRef]
- Khan, S.; Amaral Júnior, A.T.d.; Ferreira, F.R.A.; Kamphorst, S.H.; Gonçalves, G.M.B.; Simone Mendonça Freitas, M.; Silveira, V.; Apolinário de Souza Filho, G.; Francisco Teixeira do Amaral, J.; Enrique Bresssan Smith, R.; et al. Limited Nitrogen and Plant Growth Stages Discriminate Well Nitrogen Use, Uptake and Utilization Efficiency in Popcorn. Plants 2020, 9, 893. [Google Scholar] [CrossRef]
- Ren, B.; Guo, Y.; Liu, P.; Zhao, B.; Zhang, J. Effects of Urea-Ammonium Nitrate Solution on Yield, N2O Emission, and Nitrogen Efficiency of Summer Maize Under Integration of Water and Fertilizer. Front. Plant Sci. 2021, 12, 700331. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, B.-L.; Yu, X.; Gao, J.; Sun, J.; Su, Z.; Yu, S. Physiological Basis of Heterosis for Nitrogen Use Efficiency of Maize. Sci. Rep. 2019, 9, 18708. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.; Lea, P.J. Our Nitrogen ‘Footprint’: The Need for Increased Crop Nitrogen Use Efficiency. Ann. Appl. Biol. 2013, 163, 165–169. [Google Scholar] [CrossRef]
- USDA Agricultural Baseline Database. Available online: https://www.ers.usda.gov/data-products/agricultural-baseline-database (accessed on 4 January 2023).
- Heffer, P.; Gruère, A.; Roberts, T. Assessment of Fertilizer Use by Crop at the Global Level; International Fertilizer Industry Association: Paris, France, 2017. [Google Scholar]
- Sheoran, S.; Kumar, S.; Kumar, P.; Meena, R.S.; Rakshit, S. Nitrogen Fixation in Maize: Breeding Opportunities. Theor. Appl. Genet. 2021, 134, 1263–1280. [Google Scholar] [CrossRef]
- Han, M.; Wong, J.; Su, T.; Beatty, P.H.; Good, A.G. Identification of Nitrogen Use Efficiency Genes in Barley: Searching for QTLs Controlling Complex Physiological Traits. Front. Plant Sci. 2016, 7, 1587. [Google Scholar] [CrossRef]
- Pampana, S.; Mariotti, M. Durum Wheat Yield and N Uptake as Affected by N Source, Timing, and Rate in Two Mediterranean Environments. Agronomy 2021, 11, 1299. [Google Scholar] [CrossRef]
- Hawkesford, M.J. Genetic Variation in Traits for Nitrogen Use Efficiency in Wheat. J. Exp. Bot. 2017, 68, 2627–2632. [Google Scholar] [CrossRef] [PubMed]
- Hawkesford, M.J.; Griffiths, S. Exploiting Genetic Variation in Nitrogen Use Efficiency for Cereal Crop Improvement. Curr. Opin. Plant Biol. 2019, 49, 35–42. [Google Scholar] [CrossRef]
- Congreves, K.A.; Otchere, O.; Ferland, D.; Farzadfar, S.; Williams, S.; Arcand, M.M. Nitrogen Use Efficiency Definitions of Today and Tomorrow. Front. Plant Sci. 2021, 12, 637108. [Google Scholar] [CrossRef]
- Quan, X.; Zeng, J.; Chen, G.; Zhang, G. Transcriptomic Analysis Reveals Adaptive Strategies to Chronic Low Nitrogen in Tibetan Wild Barley. BMC Plant. Biol. 2019, 19, 68. [Google Scholar] [CrossRef]
- He, X.; Ma, H.; Zhao, X.; Nie, S.; Li, Y.; Zhang, Z.; Shen, Y.; Chen, Q.; Lu, Y.; Lan, H.; et al. Comparative RNA-Seq Analysis Reveals That Regulatory Network of Maize Root Development Controls the Expression of Genes in Response to N Stress. PLoS ONE 2016, 11, e0151697. [Google Scholar] [CrossRef]
- Kumari, S.; Sharma, N.; Raghuram, N. Meta-Analysis of Yield-Related and N-Responsive Genes Reveals Chromosomal Hotspots, Key Processes and Candidate Genes for Nitrogen-Use Efficiency in Rice. Front. Plant Sci. 2021, 12, 627955. [Google Scholar] [CrossRef] [PubMed]
- Gan, H.; Jiao, Y.; Jia, J.; Wang, X.; Li, H.; Shi, W.; Peng, C.; Polle, A.; Luo, Z.-B. Phosphorus and Nitrogen Physiology of Two Contrasting Poplar Genotypes When Exposed to Phosphorus and/or Nitrogen Starvation. Tree Physiol. 2016, 36, 22–38. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2016. [Google Scholar]
- Mu, X.; Chen, Y. The Physiological Response of Photosynthesis to Nitrogen Deficiency. Plant Physiol. Biochem. 2021, 158, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Urban, A.; Rogowski, P.; Wasilewska-Dębowska, W.; Romanowska, E. Understanding Maize Response to Nitrogen Limitation in Different Light Conditions for the Improvement of Photosynthesis. Plants 2021, 10, 1932. [Google Scholar] [CrossRef] [PubMed]
- Buchert, F.; Scholz, M.; Hippler, M. Electron Transfer via Cytochrome b 6 f Complex Displays Sensitivity to Antimycin A upon STT7 Kinase Activation. Biochem. J. 2022, 479, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, L.; Liu, J. Proteomic Analysis of Hydrogen Production in Chlorella pyrenoidosa under Nitrogen Deprivation. Algal Res. 2021, 53, 102143. [Google Scholar] [CrossRef]
- Thi Nong, H.; Tateishi, R.; Suriyasak, C.; Kobayashi, T.; Oyama, Y.; Chen, W.J.; Matsumoto, R.; Hamaoka, N.; Iwaya-Inoue, M.; Ishibashi, Y. Effect of Seedling Nitrogen Condition on Subsequent Vegetative Growth Stages and Its Relationship to the Expression of Nitrogen Transporter Genes in Rice. Plants 2020, 9, 861. [Google Scholar] [CrossRef]
- Sanagi, M.; Aoyama, S.; Kubo, A.; Lu, Y.; Sato, Y.; Ito, S.; Abe, M.; Mitsuda, N.; Ohme-Takagi, M.; Kiba, T.; et al. Low Nitrogen Conditions Accelerate Flowering by Modulating the Phosphorylation State of FLOWERING BHLH 4 in Arabidopsis. Proc. Natl. Acad. Sci. USA 2021, 118, e2022942118. [Google Scholar] [CrossRef]
- Liu, H.; Able, A.J.; Able, J.A. Nitrogen Starvation-Responsive MicroRNAs Are Affected by Transgenerational Stress in Durum Wheat Seedlings. Plants 2021, 10, 826. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.d.; Amaral Júnior, A.T.d.; Fritsche-Neto, R.; Kamphorst, S.H.; Ferreira, F.R.A.; Amaral, J.F.T.d.; Vivas, J.M.S.; Santos, P.H.A.D.; Lima, V.J.d.; Khan, S.; et al. Relative Importance of Gene Effects for Nitrogen-Use Efficiency in Popcorn. PLoS ONE 2019, 14, e0222726. [Google Scholar] [CrossRef]
- Hammad, H.M.; Chawla, M.S.; Jawad, R.; Alhuqail, A.; Bakhat, H.F.; Farhad, W.; Khan, F.; Mubeen, M.; Shah, A.N.; Liu, K.; et al. Evaluating the Impact of Nitrogen Application on Growth and Productivity of Maize Under Control Conditions. Front. Plant Sci. 2022, 13, 885479. [Google Scholar] [CrossRef]
- Abubakar, A.W.; Manga, A.A.; Kamara, A.Y.; Tofa, A.I. Physiological Evaluations of Maize Hybrids under Low Nitrogen. Adv. Agric. 2019, 2019, 2624707. [Google Scholar] [CrossRef]
- D’Andrea, K.E.; Parco, M.; Maddonni, G.Á. Maize Prolificacy under Contrasting Plant Densities and N Supplies: II. Growth per Plant, Biomass Partitioning to Apical and Sub-Apical Ears during the Critical Period and Kernel Setting. Field Crops Res. 2022, 284, 108557. [Google Scholar] [CrossRef]
- Ludemann, C.I.; Hijbeek, R.; van Loon, M.P.; Murrell, T.S.; Dobermann, A.; van Ittersum, M.K. Estimating Maize Harvest Index and Nitrogen Concentrations in Grain and Residue Using Globally Available Data. Field Crops Res. 2022, 284, 108578. [Google Scholar] [CrossRef]
- Liu, S.; Qin, F. Genetic Dissection of Maize Drought Tolerance for Trait Improvement. Mol. Breed. 2021, 41, 8. [Google Scholar] [CrossRef]
- Makino, A.; Shimada, T.; Takumi, S.; Kaneko, K.; Matsuoka, M.; Shimamoto, K.; Nakano, H.; Miyao-Tokutomi, M.; Mae, T.; Yamamoto, N. Does Decrease in Ribulose-1,5-Bisphosphate Carboxylase by Antisense RbcS Lead to a Higher N-Use Efficiency of Photosynthesis under Conditions of Saturating CO2 and Light in Rice Plants? Plant Physiol. 1997, 114, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Chen, Q.; Chen, F.; Yuan, L.; Mi, G. Within-Leaf Nitrogen Allocation in Adaptation to Low Nitrogen Supply in Maize during Grain-Filling Stage. Front. Plant Sci. 2016, 7, 699. [Google Scholar] [CrossRef] [PubMed]
- Khamis, S.; Lamaze, T.; Lemoine, Y.; Foyer, C. Adaptation of the Photosynthetic Apparatus in Maize Leaves as a Result of Nitrogen Limitation. Plant Physiol. 1990, 94, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhang, J.; Zhang, Q.; Li, L.; Kuang, T. Modification of Photosystem II Photochemistry in Nitrogen Deficient Maize and Wheat Plants. J. Plant Physiol. 2001, 158, 1423–1430. [Google Scholar] [CrossRef]
- Fu, W.; Wang, Y.; Ye, Y.; Zhen, S.; Zhou, B.; Wang, Y.; Hu, Y.; Zhao, Y.; Huang, Y. Grain Yields and Nitrogen Use Efficiencies in Different Types of Stay-Green Maize in Response to Nitrogen Fertilizer. Plants 2020, 9, 474. [Google Scholar] [CrossRef]
- Lee, E.A.; Tollenaar, M. Physiological Basis of Successful Breeding Strategies for Maize Grain Yield. Crop. Sci. 2007, 47, S-202–S-215. [Google Scholar] [CrossRef]
- Hirel, B.; le Gouis, J.; Ney, B.; Gallais, A. The Challenge of Improving Nitrogen Use Efficiency in Crop Plants: Towards a More Central Role for Genetic Variability and Quantitative Genetics within Integrated Approaches. J. Exp. Bot. 2007, 58, 2369–2387. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, C.; Chen, X.; Li, Q.; Zhang, J.; Chen, F.; Yuan, L.; Mi, G. Characterization of the Plant Traits Contributed to High Grain Yield and High Grain Nitrogen Concentration in Maize. Field Crops Res. 2014, 159, 1–9. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Q.; Chen, F.; Yuan, L.; Mi, G. Dynamic Remobilization of Leaf Nitrogen Components in Relation to Photosynthetic Rate during Grain Filling in Maize. Plant Physiol. Biochem. 2018, 129, 27–34. [Google Scholar] [CrossRef]
- Nasielski, J.; Earl, H.; Deen, B. Luxury Vegetative Nitrogen Uptake in Maize Buffers Grain Yield Under Post-Silking Water and Nitrogen Stress: A Mechanistic Understanding. Front. Plant Sci. 2019, 10, 318. [Google Scholar] [CrossRef]
- Plénet, D.; Lemaire, G. Relationships between Dynamics of Nitrogen Uptake and Dry Matter Accumulation in Maize Crops. Determination of Critical N Concentration. Plant Soil 1999, 216, 65–82. [Google Scholar] [CrossRef]
- Ciampitti, I.A.; Vyn, T.J. Physiological Perspectives of Changes over Time in Maize Yield Dependency on Nitrogen Uptake and Associated Nitrogen Efficiencies: A Review. Field Crops Res. 2012, 133, 48–67. [Google Scholar] [CrossRef]
- Paponov, I.A.; Engels, C. Effect of Nitrogen Supply on Carbon and Nitrogen Partitioning after Flowering in Maize. J. Plant Nutr. Soil Sci. 2005, 168, 447–453. [Google Scholar] [CrossRef]
- De Oliveira Silva, A.; Camberato, J.J.; Coram, T.; Filley, T.; Vyn, T.J. Applicability of a “Multi-Stage Pulse Labeling” 15N Approach to Phenotype N Dynamics in Maize Plant Components during the Growing Season. Front. Plant Sci. 2017, 8, 1360. [Google Scholar] [CrossRef]
- Gao, K.; Chen, F.; Yuan, L.; Zhang, F.; Mi, G. A Comprehensive Analysis of Root Morphological Changes and Nitrogen Allocation in Maize in Response to Low Nitrogen Stress. Plant Cell Environ. 2015, 38, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.M.; Yang, J.T.; Brown, K.M.; Lynch, J.P. Nodal Root Diameter and Node Number in Maize (Zea mays L.) Interact to Influence Plant Growth under Nitrogen Stress. Plant Direct. 2021, 5, e00310. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P. Root Phenes That Reduce the Metabolic Costs of Soil Exploration: Opportunities for 21st Century Agriculture. Plant Cell Environ. 2015, 38, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Hochholdinger, F.; Woll, K.; Sauer, M.; Dembinsky, D. Genetic Dissection of Root Formation in Maize (Zea mays) Reveals Root-Type Specific Developmental Programmes. Ann. Bot. 2004, 93, 359–368. [Google Scholar] [CrossRef]
- Lynch, J.P. Steep, Cheap and Deep: An Ideotype to Optimize Water and N Acquisition by Maize Root Systems. Ann. Bot. 2013, 112, 347–357. [Google Scholar] [CrossRef]
- Wang, Y.; Mi, G.; Chen, F.; Zhang, J.; Zhang, F. Response of Root Morphology to Nitrate Supply and Its Contribution to Nitrogen Accumulation in Maize. J. Plant. Nutr. 2005, 27, 2189–2202. [Google Scholar] [CrossRef]
- Trachsel, S.; Kaeppler, S.M.; Brown, K.M.; Lynch, J.P. Maize Root Growth Angles Become Steeper under Low N Conditions. Field Crops Res. 2013, 140, 18–31. [Google Scholar] [CrossRef]
- Burridge, J.D.; Findeis, J.L.; Jochua, C.N.; Miguel, M.A.; Mubichi-Kut, F.M.; Quinhentos, M.L.; Xerinda, S.A.; Lynch, J.P. A Case Study on the Efficacy of Root Phenotypic Selection for Edaphic Stress Tolerance in Low-Input Agriculture: Common Bean Breeding in Mozambique. Field Crops Res. 2019, 244, 107612. [Google Scholar] [CrossRef]
- Santos, D.L.; Coelho, E.F.; Cunha, F.F.d.; Donato, S.L.R.; Bernado, W.d.P.; Rodrigues, W.P.; Campostrini, E. Partial Root-Zone Drying in Field-Grown Papaya: Gas Exchange, Yield, and Water Use Efficiency. Agric. Water Manag. 2021, 243, 106421. [Google Scholar] [CrossRef]
- Santos, T.d.O.; Amaral Junior, A.T.d.; Bispo, R.B.; Lima, V.J.d.; Kamphorst, S.H.; Leite, J.T.; Santos Júnior, D.R.d.; Santos, P.H.A.D.; Oliveira, U.A.d.; Schmitt, K.F.M.; et al. Phenotyping Latin American Open-Pollinated Varieties of Popcorn for Environments with Low Water Availability. Plants 2021, 10, 1211. [Google Scholar] [CrossRef]
- Favela, A.; Bohn, M.O.; Kent, A.D. Maize Germplasm Chronosequence Shows Crop Breeding History Impacts Recruitment of the Rhizosphere Microbiome. ISME J. 2021, 15, 2454–2464. [Google Scholar] [CrossRef]
- Van Deynze, A.; Zamora, P.; Delaux, P.-M.; Heitmann, C.; Jayaraman, D.; Rajasekar, S.; Graham, D.; Maeda, J.; Gibson, D.; Schwartz, K.D.; et al. Nitrogen Fixation in a Landrace of Maize Is Supported by a Mucilage-Associated Diazotrophic Microbiota. PLoS Biol. 2018, 16, e2006352. [Google Scholar] [CrossRef]
- Jones, P.; Garcia, B.J.; Furches, A.; Tuskan, G.A.; Jacobson, D. Plant Host-Associated Mechanisms for Microbial Selection. Front. Plant Sci. 2019, 10, 862 . [Google Scholar] [CrossRef]
- Bais, H.P.; Park, S.-W.; Weir, T.L.; Callaway, R.M.; Vivanco, J.M. How Plants Communicate Using the Underground Information Superhighway. Trends Plant Sci. 2004, 9, 26–32. [Google Scholar] [CrossRef]
- Roesch, L.F.W.; Camargo, F.A.O.; Bento, F.M.; Triplett, E.W. Biodiversity of Diazotrophic Bacteria within the Soil, Root and Stem of Field-Grown Maize. Plant Soil 2008, 302, 91–104. [Google Scholar] [CrossRef]
- Santoyo, G. How Plants Recruit Their Microbiome? New Insights into Beneficial Interactions. J. Adv. Res. 2022, 40, 45–58. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going Back to the Roots: The Microbial Ecology of the Rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; van Themaat, E.V.L.; Schulze-Lefert, P. Structure and Functions of the Bacterial Microbiota of Plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef] [PubMed]
- De Mello Prado, R. Nitrogen. In Mineral Nutrition of Tropical Plants; Springer International Publishing: Cham, Switzerland, 2021; pp. 69–98. [Google Scholar]
- Bi, Y.-M.; Meyer, A.; Downs, G.S.; Shi, X.; El-kereamy, A.; Lukens, L.; Rothstein, S.J. High Throughput RNA Sequencing of a Hybrid Maize and Its Parents Shows Different Mechanisms Responsive to Nitrogen Limitation. BMC Genom. 2014, 15, 77. [Google Scholar] [CrossRef]
- Cao, H.; Qi, S.; Sun, M.; Li, Z.; Yang, Y.; Crawford, N.M.; Wang, Y. Overexpression of the Maize ZmNLP6 and ZmNLP8 Can Complement the Arabidopsis Nitrate Regulatory Mutant Nlp7 by Restoring Nitrate Signaling and Assimilation. Front. Plant Sci. 2017, 8, 1703. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Turano, F.J. The Putative Glutamate Receptor 1.1 (AtGLR1.1) Functions as a Regulator of Carbon and Nitrogen Metabolism in Arabidopsis Thaliana. Proc. Natl. Acad. Sci. USA 2003, 100, 6872–6877. [Google Scholar] [CrossRef]
- Raddatz, N.; Morales de los Ríos, L.; Lindahl, M.; Quintero, F.J.; Pardo, J.M. Coordinated Transport of Nitrate, Potassium, and Sodium. Front. Plant Sci. 2020, 11, 247. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga, D.L.; Sonnante, G. The Use of Nitrogen and Its Regulation in Cereals: Structural Genes, Transcription Factors, and the Role of MiRNAs. Plants 2019, 8, 294. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Saha, R.; Guillard, L.; Clement, G.; Armengaud, P.; Canas, R.; Maranas, C.D.; Lea, P.J.; Hirel, B. Nitrogen-Use Efficiency in Maize (Zea mays L.): From “omics” Studies to Metabolic Modelling. J. Exp. Bot. 2014, 65, 5657–5671. [Google Scholar] [CrossRef]
- Plett, D.C.; Holtham, L.R.; Okamoto, M.; Garnett, T.P. Nitrate Uptake and Its Regulation in Relation to Improving Nitrogen Use Efficiency in Cereals. Semin. Cell Dev. Biol. 2018, 74, 97–104. [Google Scholar] [CrossRef]
- Engineer, C.B.; Kranz, R.G. Reciprocal Leaf and Root Expression of AtAmt1.1 and Root Architectural Changes in Response to Nitrogen Starvation. Plant Physiol. 2007, 143, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Dong, L.; Lü, W.; Lü, J.; Meng, Q.; Liu, P. Transcriptome Analysis of Maize Seedling Roots in Response to Nitrogen-, Phosphorus-, and Potassium Deficiency. Plant Soil 2020, 447, 637–658. [Google Scholar] [CrossRef]
- Vidal, E.A.; Moyano, T.C.; Krouk, G.; Katari, M.S.; Tanurdzic, M.; McCombie, W.R.; Coruzzi, G.M.; Gutiérrez, R.A. Integrated RNA-Seq and SRNA-Seq Analysis Identifies Novel Nitrate-Responsive Genes in Arabidopsis Thaliana Roots. BMC Genom. 2013, 14, 701. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Naz, M.; Fan, X.; Xuan, W.; Miller, A.J.; Xu, G. Plant Nitrate Transporters: From Gene Function to Application. J. Exp. Bot. 2017, 68, 2463–2475. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Z.; Yang, C.; Yang, Z.; Li, H.; Wu, Y. Physiological Responses and Small RNAs Changes in Maize under Nitrogen Deficiency and Resupply. Genes Genom. 2019, 41, 1183–1194. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, Q.; Zhang, Y.; Zhang, Y.; Xing, J.; Yang, B.; Mi, G.; Li, Z.; Zhang, M. The Role of Gibberellins in Regulation of Nitrogen Uptake and Physiological Traits in Maize Responding to Nitrogen Availability. Int. J. Mol. Sci. 2020, 21, 1824. [Google Scholar] [CrossRef]
- Dechorgnat, J.; Francis, K.L.; Dhugga, K.S.; Rafalski, J.A.; Tyerman, S.D.; Kaiser, B.N. Tissue and Nitrogen-Linked Expression Profiles of Ammonium and Nitrate Transporters in Maize. BMC Plant Biol. 2019, 19, 206. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, Y.; Kuwata, K.; Matsubayashi, Y. A Type 2C Protein Phosphatase Activates High-Affinity Nitrate Uptake by Dephosphorylating NRT2.1. Nat. Plants 2021, 7, 310–316. [Google Scholar] [CrossRef]
- Trevisan, S.; Borsa, P.; Botton, A.; Varotto, S.; Malagoli, M.; Ruperti, B.; Quaggiotti, S. Expression of Two Maize Putative Nitrate Transporters in Response to Nitrate and Sugar Availability. Plant Biol. 2008, 10, 462–475. [Google Scholar] [CrossRef]
- Sinha, S.; Sevanthi, V.A.; Chaudhary, S.; Tyagi, P.; Venkadesan, S.; Rani, M.; Mandal, P. Transcriptome Analysis of Two Rice Varieties Contrasting for Nitrogen Use Efficiency under Chronic N Starvation Reveals Differences in Chloroplast and Starch Metabolism-Related Genes. Genes 2018, 9, 206. [Google Scholar] [CrossRef]
- Goel, P.; Sharma, N.K.; Bhuria, M.; Sharma, V.; Chauhan, R.; Pathania, S.; Swarnkar, M.K.; Chawla, V.; Acharya, V.; Shankar, R.; et al. Transcriptome and Co-Expression Network Analyses Identify Key Genes Regulating Nitrogen Use Efficiency in Brassica juncea L. Sci. Rep. 2018, 8, 7451. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, N.; Liu, S.; Dong, A.; Zenda, T.; Liu, X.; Li, J.; Duan, H. Comparative Proteomic Analysis of Two Contrasting Maize Hybrids’ Responses to Low Nitrogen Stress at the Twelve Leaf Stage and Function Verification of ZmTGA Gene. Genes 2022, 13, 670. [Google Scholar] [CrossRef] [PubMed]
- Jakoby, M.; Weisshaar, B.; Dröge-Laser, W.; Vicente-Carbajosa, J.; Tiedemann, J.; Kroj, T.; Parcy, F. BZIP Transcription Factors in Arabidopsis. Trends Plant Sci. 2002, 7, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.M.; Riveras, E.; Vidal, E.A.; Gras, D.E.; Contreras-López, O.; Tamayo, K.P.; Aceituno, F.; Gómez, I.; Ruffel, S.; Lejay, L.; et al. Systems Approach Identifies TGA1 and TGA4 Transcription Factors as Important Regulatory Components of the Nitrate Response of Arabidopsis Thaliana Roots. Plant J. 2014, 80, 12618. [Google Scholar] [CrossRef]
- Zhong, L.; Chen, D.; Min, D.; Li, W.; Xu, Z.; Zhou, Y.; Li, L.; Chen, M.; Ma, Y. AtTGA4, a BZIP Transcription Factor, Confers Drought Resistance by Enhancing Nitrate Transport and Assimilation in Arabidopsis Thaliana. Biochem. Biophys. Res. Commun. 2015, 457, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 9780123849052. [Google Scholar]
- Taylor, A.R.; Bloom, A.J. Ammonium, Nitrate, and Proton Fluxes along the Maize Root. Plant Cell Environ. 1998, 21, 1255–1263. [Google Scholar] [CrossRef]
- Bloom, A.J.; Meyerhoff, P.A.; Taylor, A.R.; Rost, T.L. Root Development and Absorption of Ammonium and Nitrate from the Rhizosphere. J. Plant Growth Regul. 2002, 21, 416–431. [Google Scholar] [CrossRef]
- Gu, R.; Duan, F.; An, X.; Zhang, F.; von Wirén, N.; Yuan, L. Characterization of AMT-Mediated High-Affinity Ammonium Uptake in Roots of Maize (Zea mays L.). Plant Cell Physiol. 2013, 54, 1515–1524. [Google Scholar] [CrossRef]
- Zhao, S.; Li, C.-I.; Guo, Y.; Sheng, Q.; Shyr, Y. RnaSeqSampleSize: Real Data Based Sample Size Estimation for RNA Sequencing. BMC Bioinform. 2018, 19, 191. [Google Scholar] [CrossRef]
- Meyer, C.; Stitt, M. Nitrate Reduction and Signalling. In Plant Nitrogen; Springer: Berlin/Heidelberg, Germany, 2001; pp. 37–59. [Google Scholar]
- Wang, Y.-Y.; Cheng, Y.-H.; Chen, K.-E.; Tsay, Y.-F. Nitrate Transport, Signaling, and Use Efficiency. Annu. Rev. Plant Biol. 2018, 69, 85–122. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen Uptake, Assimilation and Remobilization in Plants: Challenges for Sustainable and Productive Agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef]
- Asibi, A.E.; Chai, Q.; Coulter, J.A. Mechanisms of Nitrogen Use in Maize. Agronomy 2019, 9, 775. [Google Scholar] [CrossRef]
- Sakakibara, H.; Kawabata, S.; Hase, T.; Sugiyama, T. Differential Effects of Nitrate and Light on the Expression of Glutamine Synthetases and Ferredoxin-Dependent Glutamate Synthase in Maize. Plant Cell Physiol. 1992, 33, 1193–1198. [Google Scholar] [CrossRef]
- Martin, A.; Lee, J.; Kichey, T.; Gerentes, D.; Zivy, M.; Tatout, C.; Dubois, F.; Balliau, T.; Valot, B.; Davanture, M.; et al. Two Cytosolic Glutamine Synthetase Isoforms of Maize Are Specifically Involved in the Control of Grain Production. Plant Cell 2006, 18, 3252–3274. [Google Scholar] [CrossRef]
- Liseron-Monfils, C.; Bi, Y.-M.; Downs, G.S.; Wu, W.; Signorelli, T.; Lu, G.; Chen, X.; Bondo, E.; Zhu, T.; Lukens, L.N.; et al. Nitrogen Transporter and Assimilation Genes Exhibit Developmental Stage-Selective Expression in Maize (Zea mays L.) Associated with Distinct Cis -Acting Promoter Motifs. Plant Signal. Behav. 2013, 8, e26056. [Google Scholar] [CrossRef]
- Suzuki, A.; Knaff, D.B. Glutamate Synthase: Structural, Mechanistic and Regulatory Properties, and Role in the Amino Acid Metabolism. Photosynth. Res. 2005, 83, 191–217. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, T.; Gaufichon, L.; Boutet-Mercey, S.; Christ, A.; Masclaux-Daubresse, C. Enzymatic and Metabolic Diagnostic of Nitrogen Deficiency in Arabidopsis thaliana Wassileskija Accession. Plant Cell Physiol. 2008, 49, 1056–1065. [Google Scholar] [CrossRef]
- Tabuchi, M.; Abiko, T.; Yamaya, T. Assimilation of Ammonium Ions and Reutilization of Nitrogen in Rice (Oryza sativa L.). J. Exp. Bot. 2007, 58, 2319–2327. [Google Scholar] [CrossRef]
- Uauy, C.; Distelfeld, A.; Fahima, T.; Blechl, A.; Dubcovsky, J. A NAC Gene Regulating Senescence Improves Grain Protein, Zinc, and Iron Content in Wheat. Science 2006, 314, 1298–1301. [Google Scholar] [CrossRef]
- Bänziger, M.; Betrán, F.J.; Lafitte, H.R. Efficiency of High-Nitrogen Selection Environments for Improving Maize for Low-Nitrogen Target Environments. Crop Sci. 1997, 37, 1103–1109. [Google Scholar] [CrossRef]
- Bänziger, M.; Lafitte, H.R. Efficiency of Secondary Traits for Improving Maize for Low-Nitrogen Target Environments. Crop Sci. 1997, 37, 1110–1117. [Google Scholar] [CrossRef]
- Ertiro, B.T.; Olsen, M.; Das, B.; Gowda, M.; Labuschagne, M. Efficiency of Indirect Selection for Grain Yield in Maize (Zea mays L.) under Low Nitrogen Conditions through Secondary Traits under Low Nitrogen and Grain Yield under Optimum Conditions. Euphytica 2020, 216, 134. [Google Scholar] [CrossRef]
- Caixeta, D.S.; Fritsche-Neto, R.; Granato, Í.S.C.; Oliveira, L.R.d.; Galvão, J.C.C. Early Indirect Selection for Nitrogen Use Efficiency in Maize. Rev. Ciência Agronômica 2015, 46, 369–378. [Google Scholar] [CrossRef]
- Ertiro, B.T.; Labuschagne, M.; Olsen, M.; Das, B.; Prasanna, B.M.; Gowda, M. Genetic Dissection of Nitrogen Use Efficiency in Tropical Maize Through Genome-Wide Association and Genomic Prediction. Front. Plant Sci. 2020, 11, 474. [Google Scholar] [CrossRef]
- Leite, J.T.; Amaral Junior, A.T.d.; Kamphorst, S.H.; Lima, V.J.d.; Santos Junior, D.R.d.; Schmitt, K.F.M.; Souza, Y.P.d.; Santos, T.d.O.; Bispo, R.B.; Mafra, G.S.; et al. Water Use Efficiency in Popcorn (Zea mays L. Var. Everta): Which Physiological Traits Would Be Useful for Breeding? Plants 2021, 10, 1450. [Google Scholar] [CrossRef] [PubMed]
- Scharf, P.C.; Brouder, S.M.; Hoeft, R.G. Chlorophyll Meter Readings Can Predict Nitrogen Need and Yield Response of Corn in the North-Central USA. Agron. J. 2006, 98, 655–665. [Google Scholar] [CrossRef]
- Yang, Y.; Timlin, D.J.; Fleisher, D.H.; Lokhande, S.B.; Chun, J.A.; Kim, S.-H.; Staver, K.; Reddy, V.R. Nitrogen Concentration and Dry-Matter Accumulation in Maize Crop: Assessing Maize Nitrogen Status with an Allometric Function and a Chlorophyll Meter. Commun. Soil Sci. Plant Anal. 2012, 43, 1563–1575. [Google Scholar] [CrossRef]
- Zhang, J.; Blackmer, A.M.; Ellsworth, J.W.; Koehler, K.J. Sensitivity of Chlorophyll Meters for Diagnosing Nitrogen Deficiencies of Corn in Production Agriculture. Agron. J. 2008, 100, 543–550. [Google Scholar] [CrossRef]
- Hawkins, J.A.; Sawyer, J.E.; Barker, D.W.; Lundvall, J.P. Using Relative Chlorophyll Meter Values to Determine Nitrogen Application Rates for Corn. Agron. J. 2007, 99, 1034–1040. [Google Scholar] [CrossRef]
- Rashid, M.T.; Voroney, P.; Parkin, G. Predicting Nitrogen Fertilizer Requirements for Corn by Chlorophyll Meter under Different N Availability Conditions. Can. J. Soil Sci. 2005, 85, 149–159. [Google Scholar] [CrossRef]
- Blackmer, T.M.; Schepers, J.S. Techniques for Monitoring Crop Nitrogen Status in Corn. Commun. Soil Sci. Plant Anal. 1994, 25, 1791–1800. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Q.; Wang, Z.; Wang, L.; Li, X.; Fan, Z.; Zhang, Y.; Li, J.; Gao, X.; Shi, J.; et al. Effects of Nitrogen Fertilizer on Photosynthetic Characteristics, Biomass, and Yield of Wheat under Different Shading Conditions. Agronomy 2021, 11, 1989. [Google Scholar] [CrossRef]
- Jin, X.; Yang, G.; Tan, C.; Zhao, C. Effects of Nitrogen Stress on the Photosynthetic CO2 Assimilation, Chlorophyll Fluorescence and Sugar-Nitrogen Ratio in Corn. Sci. Rep. 2015, 5, 9311. [Google Scholar] [CrossRef]
- Santos, T.d.O.; de Oliveira, F.T.; Amaral Junior, A.T.d.; de Almeida Filho, J.E.; Bispo, R.B.; de Freitas, M.S.M.; Amaral, J.F.T.d.; Kamphorst, S.H.; de Lima, V.J.; Viana, F.N.; et al. Additive and Non-Additive Effects on the Control of Key Agronomic Traits in Popcorn Lines under Contrasting Phosphorus Conditions. Plants 2022, 11, 2216. [Google Scholar] [CrossRef]
- Mastrodomenico, A.T.; Bohn, M.O.; Lipka, A.E.; Below, F.E. Genomic Selection Using Maize Ex-Plant Variety Protection Germplasm for the Prediction of Nitrogen-Use Traits. Crop Sci. 2019, 59, 212–220. [Google Scholar] [CrossRef]
- Guedes, F.L.; Diniz, R.P.; Balestre, M.; Ribeiro, C.B.; Camargos, R.B.; Souza, J.C. Inheritance of Nitrogen Use Efficiency in Inbred Progenies of Tropical Maize Based on Multivariate Diallel Analysis. Sci. World J. 2014, 2014, 894710. [Google Scholar] [CrossRef] [PubMed]
- DoVale, J.C.; Fritsche-Neto, R.; Bermudez, F.; Miranda, G.V. Efeitos Gênicos de Caracteres Associados à Eficiência No Uso de Nitrogênio Em Milho. Pesqui. Agropecuária Bras. 2012, 47, 385–392. [Google Scholar] [CrossRef]
- Makumbi, D.; Betrán, J.F.; Bänziger, M.; Ribaut, J.-M. Combining Ability, Heterosis and Genetic Diversity in Tropical Maize (Zea mays L.) under Stress and Non-Stress Conditions. Euphytica 2011, 180, 143–162. [Google Scholar] [CrossRef]
- Souza, L.V.d.; Miranda, G.V.; Galvão, J.C.C.; Eckert, F.R.; Mantovani, É.E.; Lima, R.O.; Guimarães, L.J.M. Genetic Control of Grain Yield and Nitrogen Use Efficiency in Tropical Maize. Pesqui. Agropecuária Bras. 2008, 43, 1517–1523. [Google Scholar] [CrossRef]
- Riache, M.; Revilla, P.; Malvar, R.A.; Djemel, A.; Chemlal, A.; Mefti, M. Assessment of Nitrogen Use Efficiency in Algerian Saharan Maize Populations for Tolerance under Drought and No-Nitrogen Stresses. Agronomy 2022, 12, 1123. [Google Scholar] [CrossRef]
- Amegbor, I.K.; Abe, A.; Adjebeng-Danquah, J.; Adu, G.B. Genetic Analysis and Yield Assessment of Maize Hybrids under Low and Optimal Nitrogen Environments. Heliyon 2022, 8, e09052. [Google Scholar] [CrossRef] [PubMed]
- Almeida, V.C.; Viana, J.M.S.; DeOliveira, H.M.; Risso, L.A.; Ribeiro, A.F.S.; DeLima, R.O. Genetic Diversity and Path Analysis for Nitrogen Use Efficiency of Tropical Popcorn (Zea mays Ssp. Everta) Inbred Lines in Adult Stage. Plant Breed. 2018, 137, 839–847. [Google Scholar] [CrossRef]
- Riache, M.; Revilla, P.; Maafi, O.; Malvar, R.A.; Djemel, A. Combining Ability and Heterosis of Algerian Saharan Maize Populations (Zea mays L.) for Tolerance to No-Nitrogen Fertilization and Drought. Agronomy 2021, 11, 492. [Google Scholar] [CrossRef]
- Peterlini, E.; Pinto, R.J.B.; Scapim, C.A.; Rizzardi, D.A.; Bertagna, F.A.B.; Amaral Júnior, A.T. do Diallel Analysis of Popcorn Populations for Yield, Popping Expansion and Resistance to Fall Armyworm. Rev. Ceres 2020, 67, 288–295. [Google Scholar] [CrossRef]
- Schegoscheski Gerhardt, I.F.; Teixeira do Amaral Junior, A.; Ferreira Pena, G.; Moreira Guimarães, L.J.; de Lima, V.J.; Vivas, M.; Araújo Diniz Santos, P.H.; Alves Ferreira, F.R.; Mendonça Freitas, M.S.; Kamphorst, S.H. Genetic Effects on the Efficiency and Responsiveness to Phosphorus Use in Popcorn as Estimated by Diallel Analysis. PLoS ONE 2019, 14, e0216980. [Google Scholar] [CrossRef]
- Schwantes, I.A.; Amaral Júnior, A.T.d.; Vivas, M.; Almeida Filho, J.E.d.; Kamphorst, S.H.; Guimarães, A.G.; Khan, S. Inheritance of Resistance to Fusarium Ear Rot in Popcorn. Crop Breed. Appl. Biotechnol. 2018, 18, 81–88. [Google Scholar] [CrossRef]
- Silva, V.; Amaral JÚnior, A.; Scapim, C.; Freitas Júnior, S.; Gonçalves, L. Inheritance for Economically Important Traits in Popcorn from Distinct Heterotic Groups by Hayman’s Diallel. Cereal Res. Commun. 2010, 38, 272–284. [Google Scholar] [CrossRef]
- Lima, V.J.d.; Amaral Júnior, A.T.d.; Kamphorst, S.H.; Bispo, R.B.; Leite, J.T.; Santos, T.d.O.; Schmitt, K.F.M.; Chaves, M.M.; Oliveira, U.A.d.; Santos, P.H.A.D.; et al. Combined Dominance and Additive Gene Effects in Trait Inheritance of Drought-Stressed and Full Irrigated Popcorn. Agronomy 2019, 9, 782. [Google Scholar] [CrossRef]
- Dofing, S.M.; D’Croz-Mason, N.; Thomas-Compton, M.A. Inheritance of Expansion Volume and Yield in Two Popcorn × Dent Corn Crosses. Crop Sci. 1991, 31, 715–718. [Google Scholar] [CrossRef]
- Coan, M.M.D.; Pinto, R.J.B.; Kuki, M.C.; Amaral Júnior, A.T.; Figueiredo, A.S.T.; Scapim, C.A.; Warburton, M. Inheritance Study for Popping Expansion in Popcorn vs Flint Corn Genotypes. Agron. J. 2019, 111, 2174–2183. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).