Abstract
Background/Objectives: One potential risk factor that remains especially contentious in the anterior cruciate ligament (ACL) injury literature is the role of neuromuscular fatigue in ACL injury risk. Therefore, the purposes of this review are (i) to present the research and practical concepts of lower extremity neuromuscular fatigue; and (ii) to review the literature relating to neuromuscular fatigue as an ACL injury risk factor and mechanism. Methods: A structured review was performed in the Medline database using a search strategy that included terms such as “anterior cruciate ligament injury” and “knee injuries” combined with terms such as “injury” and “fatigue”. Articles were included if they included young healthy participants (18–35) and made a comparison between non-fatigued and fatigued states that were assessed with at least one lower extremity biomechanical variable associated with ACL injury risk. Results: Overall, there were 67 studies included, accounting for 1440 participants (627 male and 813 female) across a variety of sports and activities. Of these, 53 (79%) reported a post-fatigue change in the kinematics, kinetics, neuromuscular, and/or other (e.g., proprioceptive) outcomes that indicate that the participants would be at an increased risk of an ACL injury. The most common argument against fatigue as a risk factor is that ACL injuries do not tend to occur later in a game or season, when it is assumed that athletes would be most fatigued. Conclusions: The evidence presented in this review suggests that localized neuromuscular fatigue is a risk factor, among multiple factors, for ACL injuries, providing another modifiable risk factor that should be considered when developing ACL injury risk reduction interventions.
Keywords:
kinematics; kinetics; injury risk; lower extremity; knee; peripheral fatigue; muscle mechanics 1. Introduction
The anterior cruciate ligament (ACL) is the most injured ligament in the knee, frequently occurring in young, active individuals [1], with incidence rates ranging from 0.03 to 0.42 per 1000 exposures, depending on sex [2], competition level, and environment [3]. Injuries to the ACL have been identified as a multi-factorial injury, and numerous non-modifiable and modifiable ACL injury risk factors have been recognized [4]. Sex [4] and lower extremity anthropometry [5] are two examples of commonly cited non-modifiable risk factors. For example, a systematic review with a meta-analysis indicated that the incidence rate of ACL injuries is statistically different between sexes and is approximately three times higher in females compared to males [6]. With respect to lower extremity morphology and anthropometry, a recent systematic review concluded that more than five different anatomical deviations (small intercondylar notch, offset femoral condyles, and a steep posterior tibial slope) were all significantly associated with an increased risk of ACL injury [7].
While it is important to understand the underlying mechanisms associated with the non-modifiable risk factors, arguably more important is comprehending the modifiable risk factors that would allow clinicians, rehabilitation specialists, and researchers to develop and apply effective injury risk reducing interventions. Although there still remains debate around the association of biomechanical factors and the risk of ACL injury [8], of the most cited and investigated modifiable biomechanical risk factors are those related to an individual’s lower extremity kinematics and kinetics. Boden et al. (2007) [9] analyzed videos of professional basketball players performing decelerations and landing maneuvers to quantify knee joint kinematics and compared them between individuals who suffered an ACL injury and healthy controls. They found that initiating decelerations and landings with the heel increased the risk of ACL injury as the impact could not be adequately absorbed throughout the kinetic chain. Prospective research, such as that conducted by Hewett et al. (2005) [10], showed that an increased knee valgus angle and abduction moment during jump landings at baseline testing increased an athlete’s risk of ACL injury throughout their competitive season. Krosshaug et al. (2016) [11] also identified medial knee displacement as a risk factor in individuals who had previously experienced an ACL injury. Although Leppanen et al. (2017) [12] did not identify knee valgus as a risk factor for ACL injury, they did identify that increases in vertical ground forces increased injury risk.
Further to this, strain on the ACL caused by anterior translation has been shown to be greater than the strain caused by isolated knee valgus forces or internal rotation moments. For example, 100 N of anterior translation resulted in 3.7% ACL strain, while 200 N of valgus and 10 Nm of internal rotation produced only 2.5% and 2.0% ACL strain, respectively [13]. However, combinations of anterior translation, valgus, internal rotation, and near end-range extension have also been implicated as a common ACL injury mechanism [14].
There appears to be a relative consensus that some patterns of lower extremity joint kinematics and kinetics have a role in inducing ACL injuries. A potential modulator of this is neuromuscular fatigue that occurs to the musculature of the lower extremity. Neuromuscular fatigue, defined as a change in the force generating capacity of the muscle along with alterations in the firing patterns at the neuromuscular junction [15], may result in altered motor unit recruitment (i.e., fatigue affects both the magnitude and timing of muscle force production), which in turn may lead to changes in the kinematics at the affected joint(s). It is the altered kinematics that are suggested to be responsible for an increase in ACL injury risk.
In the most general sense, neuromuscular fatigue can be referred to as a change in the force-generating capacity of the muscle along with alterations in the firing patterns at the neuromuscular junction and incorporates both central and peripheral processes [15]. However, it has been argued that this definition alone does not fully encapsulate the range of fatiguing processes and the effect these have on all types of performance [16]. Alternatively, Enoka and Duchateau (2016) [16] proposed a taxonomy of fatigue that addresses the two main attributes of fatigue: (i) performance fatigability and (ii) perceived fatigability. In the simplest form, however, performance fatigability includes those processes and outcomes that result in a decline in objective measures of fatigue such as contractile function and muscle activation. Perceived fatigability, however, relates to the sensations of fatigue that affect performance and includes both homeostasis and the psychological state of the individual. While it is important to understand this process, these taxonomies [16,17] are still governed by central and peripheral processes of fatigue.
While the Enoka and Duchateau provides a mechanism to categorize different types of fatigue, this framework was further updated by Behrens et al. (2023) [17] to parse out the differences between motor and cognitive performance and the perception of fatigue in acute fatigue settings. Here, they suggest that while motor performance fatigue is defined by the traditional neuromuscular markers of fatigue, the onset and measurement of cognitive motor fatigue (e.g., decreased reaction time) is less well agreed upon but likely depends on the performance of the central nervous system. Finally, a major strength of the updated framework is the acknowledgment that the measurement of state fatigue should include objective measures and that the onset and measurement of motor and cognitive fatigue can be affected by perceived fatigue.
1.1. Central Fatigue
Central fatigue refers to fatigue-related processes proximal to and at the neuromuscular junction (NMJ). In their review, Taylor et al. (2016) [18] categorized central fatigue primarily as the decreased stimulatory output of motor neurons caused by either a decrease in their excitatory output or an increase in their inhibitory output. This is similar to Potvin and Fuglvand’s [15] description of firing-rate adaptation, where over time and with a constant excitatory drive, the neural inhibition of muscle activity rises exponentially to limit force production before the signal reaches the muscle. These aspects of central fatigue could be categorized as performance fatigability [16] or perceived motor and cognitive fatigue [17].
Cognitive or mental fatigue is another type of centralized fatigue and was defined by Marcora et al. (2009) [19] as “a psychobiological state caused by prolonged exposure to a cognitively demanding task where individuals may report feelings of tiredness or lack of energy”; or, according to the previously defined taxonomy, aspects of perceived fatigability. To induce this type of fatigue, researchers have typically asked participants to complete a difficult cognitive task; however, this type of fatigue is difficult to measure and quantify. Cutsem et al. (2017) [20] performed a systematic review that included 11 studies describing and attempting to quantify the effect of mental and/or cognitive fatigue on aerobic performance. Overall, they reported that cognitive fatigue led to a consistent decrease in endurance performance, accompanied by an increase in the athletes’ rate of perceived exertion. However, this was not accompanied by any physiological markers of endurance fatigue (e.g., increased heart rate, increased lactate concentration) or changes in strength and power. This suggests that psychological or cognitive fatigue may be different, and elicit different responses, compared to neuromuscular fatigue.
1.2. Peripheral Fatigue
Peripheral [16], or motor performance [17], fatigue primarily refers to fatigue at and distal to the NMJ [15]. This often involves the force-generating capabilities and intrinsic fatiguability of motor units (MUs) within a muscle and can be categorized as performance fatigability [16,17]. Burke et al. (1973) [21] described this as a decrease in force capacity within a muscle after repetitive stimulation. Potvin and Fuglevand (2017) [15] also examined this principle in their muscle fatigue model. This model simulates peripheral fatigue following sustained contractions with marked decreases in contractile speed where decreases were greatest in the stronger, most fatigable MUs. It is this type of localized fatigue that we hypothesize primarily contributes to ACL injuries.
Despite the current understanding of the types and process of fatigue, fatigue as an injury risk factor or fatigue as an injury mechanism has remained contentious in the literature for a variety of reasons. Therefore, the purposes of this review are (i) to present the research and practical concepts of lower extremity neuromuscular fatigue; and (ii) to review the literature related to neuromuscular fatigue as an ACL injury risk factor and mechanism.
2. A Review: Fatigue and ACL Injuries
Search and Analysis Methods
A structured approach to the literature search was taken to ensure that a thorough, broad, and representative sample of literature was collected and reviewed. The Medline database was searched on 23 June 2023, using a structured search strategy that included terms such as “anterior cruciate ligament injury” and “knee injuries” combined with terms such as “injury” and “fatigue”. Details of the full search strategy can be found in the Supplemental Materials. Initially, 577 articles were included (567 from the initial search and 10 after searching reference lists) with 30 duplicates removed (Figure 1). Articles were included if (1) they involved young healthy participants (18–35); (2) made a comparison between non-fatigued and fatigued states; (3) measured at least one lower extremity biomechanical variable associated with ACL injury risk; and ((4) was published within the last 30 years (after 1993). Articles were excluded if they (1) included pediatric participants; (2) studied participants post-ACLR; and/or (3) specifically studied runners with a focus on running biomechanics. Systematic reviews were also excluded. After screening, a total of 60 articles were included for data extraction.
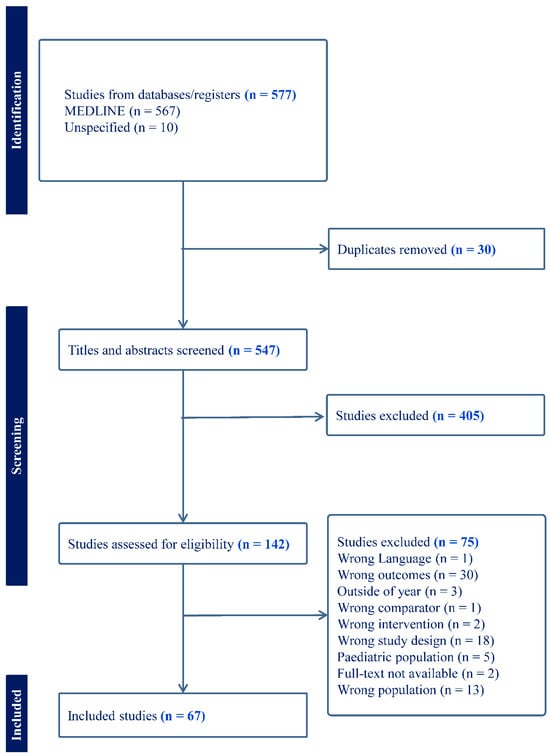
Figure 1.
PRISMA flowchart showing the review process.
Sample demographics, including the number of participants, age, and sex, were extracted, in addition to the activity level and/or the sport participation of the participants (Table 1). Methodological information was then extracted and summarized, which included the fatiguing protocol, the way in which fatigue was identified/assessed, and the tasks that were used to test the effect of fatigue on lower extremity biomechanics (Table 1).

Table 1.
Summary of the included studies detailing the demographics of the participants included, the methods used to induce and assess the inset of fatigue, and the tasks used to compare between pre-fatigue and fatigued conditions.
Following the extraction of the data, the results of each included study were summarized as kinematic, kinetic, neuromuscular, or other (Table 2 and Supplementary Table S1). We qualitatively assessed the results and further classified as to whether they indicated an increased or decreased risk of ACL injury post-fatigue (Table 2 and Supplementary Table S1). The kinematic risk factors identified with increasing the risk of ACL injury were decreased knee flexion [88,89], increased knee abduction [10,88], internal rotation [89], and anterior translation [90]. Increases in trunk lateral position following fatigue were also considered as an increased risk of ACL injury [91]. With respect to the kinetic factors, an increased risk of ACL injury was assessed as an increase in the knee abduction moment [10] and anterior tibial shear [91] and increases in vertical ground reaction force [10]. We assessed quadriceps and hamstring strength and activation variables as increasing the risk of ACL injury post-fatigue if the quadriceps to hamstring ratios decreased (i.e., indicating a larger contribution of the quadriceps post-fatigue) [92,93,94]. Finally, the primary variable that was included in the “other category” was related to measures of proprioception. In these instances, an increased risk of ACL injury post-fatigue was assessed as an increase in the absolute or relative angle or force sense errors.

Table 2.
Summary of the outcomes of each included study.
3. Results
Overall, there were 1440 participants across the 67 included articles with 627 male and 813 female participants with a mean (SD) age of 27.2 (2.2) years. The studies included participants from a variety of sports and activities including soccer (n = 14), dance (n = 3), handball (n = 3), basketball (n = 3) volleyball (n = 2), Australian football (n = 1), and jumping athletes (n = 1) (Table 1). In addition, forty studies included healthy physically active participants, one recruited reserve officer’s training corps (ROTC) participants, and four included division one college athletes (the sport was not specified). There was generally no consistency in the specific fatigue-inducing protocols, but these did include aerobic, anaerobic, and isometric or cyclical concentric methods (Table 1). While the specific parameters associated with the different tasks differed between studies, these could be generally categorized as landing (n = 36), jumping (n = 23), cutting (n = 14), proprioception (n = 5), muscle strength (n = 7), and clinical anterior translation testing (n = 4) (Table 1). Finally, the specific methods used to indicate fatigue onset were highly heterogenous across the included studies. The majority of the studies utilized a volitional failure method (n = 53) that could be described as either an RPE criteria (n = 7) or methods that used a measured decrease in effort (n = 39); seven of the studies did not include any additional measures of fatigue. A second group of studies (n = 14) stopped the fatigue testing when the participants had completed the fatigue protocol of a pre-determined time or number of cycles (Table 1). Only one of the included studies [50] objectively measured neuromuscular fatigue during the fatigue protocol via the EMG mean power frequency.
Of the 67 included studies, 53 (79%) reported a post-fatigue change in the kinematics, kinetics, neuromuscular, and/or other (e.g., proprioceptive) outcomes that would indicate that the participants would be at an increased risk of an ACL injury (Table 2).
4. Discussion
The aim of this review was to determine the effect of neuromuscular fatigue on the biomechanical risk factors for ACL injury. The main finding of this study was that, although the methods used to induce and measure fatigue are highly variable across studies, the majority of those included in this review found that fatigue increased at least one biomechanical risk factor for ACL injury.
Evidence was provided in this review that supports the notion that neuromuscular fatigue may increase the risk of ACL injury. For example, in 20 professional and elite female soccer players, Zago et al. (2021) [86] demonstrated post-fatigue kinematic and kinetic changes in 100% and 85% of their sample, respectively, in response to a change in direction task. The specific changes that were quantified are consistent with an increased risk of ACL injury including decreases in knee flexion angles and increases in knee valgus moments. Tsai et al. (2009) [80] reported significant increases in both knee internal rotation (4.9º) and knee valgus (2.4º) angles in a cutting task after a fatiguing protocol. Benjaminse et al. (2008) [26] reported significantly less knee flexion (2.1º) when landing from a single-leg drop jump–vertical jump after a fatiguing protocol, and Moran and Marshall (2006) [62] noted significant increases from the baseline measures in tibial impact acceleration (24%) during a drop jump after inducing fatigue. McLean and Samorezov (2009) [58] found significant decreases in knee flexion upon initial contact and significant increases in knee abduction and the knee abduction moment during the descent landing phase of a single-leg jump landing when fatigued. Cortes et al. (2012) [34] found significant increases in knee abduction angles (3º) and decreases in knee flexion angles (10º) at initial contact during a crossover task compared to pre-fatigue values. Bedo et al. (2022) [24], using statistical parametric mapping, reported significant increases in knee abduction angles at initial ground contact from a single-leg jump landing and significant decreases in knee flexion angles in the first third of a cutting task and the first 75% of a drop vertical jump task after a fatigue protocol. Wojtys et al. (1996) [83] found a significant 32.5% post-fatigue increase in anterior tibial translation during a tibial translation stress test when compared to the non-fatigued state. This body of evidence that includes a range of tasks and biomechanical outcome measures suggests that fatigue is a contributor to ACL injuries.
While the majority of evidence suggests that fatigue is likely a contributor to ACL injuries, there were still 21% of the included studies that did not find a difference in the biomechanical outcomes following a fatigue protocol. Bourne et al. (2019) [95] and Doyle et al. (2019) [96] provide the strongest arguments against fatigue as a risk factor for ACL injuries. They argue that neuromuscular fatigue results in athletes moving slower, which leads to less force production, thus decreasing the risk of injury. This argument counters previous research, which has demonstrated that fatigued athletes may land from jumps with stiffer legs (less knee flexion upon landing), which requires more eccentric force from the quadriceps muscles and requires more non-contractile tissues to bear significant load, resulting in a higher risk of injury [26]. Kim et al. (2021) [49] found that when the gluteus medius was fatigued, there was no significant increase in ACL load but a significant decrease in the knee abduction moment in response to a single-leg landing task. This is relevant, as it has often been assumed that control of hip ab/adduction reduces the likelihood of valgus collapse, which has been cited as an indicator of increased ACL injury risk [97]; although recently, the role of valgus collapse as an injury risk factor and mechanism has been debated [98]. Even so, there were significant increases in trunk lateral flexion and excursion, which have been identified as increasing the risk of ACL injury. Furthermore, in their clinical commentary, Bourne et al. (2019) [95] include several studies [26] that demonstrate small to no effect of fatigue on frontal plane lower extremity kinematics. However, these studies still demonstrate sagittal plane kinematic changes (e.g., decreases in knee flexion upon ground contact) that are consistent with ACL injury mechanisms.
Another major argument against fatigue as a risk factor of ACL injury is that ACL injuries do not appear to occur more frequently at the end of a game or season compared to earlier time-points. For example, Bourne et al. (2019) [95] and Doyle et al. (2019) [96] argued that since the odds ratios of ACL injuries were not different between the first and second halves of individual competitions (odds ratio = 0.43) or full seasons (odds ratio = 1.27), fatigue cannot be associated with these injuries. This theory has also been supported by other research groups who found that more ACL injuries occurred earlier in the season [99] or that the distribution of ACL injuries was not different across timepoints [100,101]. However, a limitation of this approach is that none of these studies included a physiological measure of fatigue. While it would be a difficult task, experimentally, to collect in-game physiological and/or biomechanical data, recent advances in wearable technologies suggest that this may be feasible and should be explored in future research of this kind. Additionally, lower extremity neuromuscular fatigue can occur at any point within a shift, series, game, or other unit of playing time and is not exclusive to end-of-game scenarios or later in the season. The studies that have used time of season or game as a surrogate of fatigue have not adequately considered the specific physical circumstances that occurred prior to the injury. For example, although the injury may have occurred in the first half of a game, the injured athlete could have performed a maximal effort task, leading to localized neuromuscular fatigue, just prior to the injury. Furthermore, medial collateral ligament (MCL) injury research in professional soccer players has demonstrated a significant increase in the proportion of MCL injuries in the last 15 min of each half compared to other 15 min intervals throughout a soccer match [102]. Although Berns et al. (1992) [13] demonstrated that knee valgus alone (the primary mechanism for MCL injury) is not enough to cause an ACL rupture and that a combination of loading patterns and/or anterior tibial translation is required. Many of the studies presented above arguing in favor of fatigue as a risk factor for ACL injury do demonstrate multiple kinematic changes.
A consistent criticism of the literature surrounding the effects of fatigue on lower extremity kinematics is that protocols to induce and quantify fatigue are highly variable, making it difficult to reach a consensus or conduct an impactful meta-analysis [103]. Three primary methods of inducing lower extremity fatigue have been presented in the literature: aerobic, aerobic-anaerobic, and isometric or cyclical concentric methods. These methods all induce different underlying physiological processes and may fatigue different musculature depending on the selection of fatiguing tasks. Therefore, the following paragraphs will describe these fatigue methods in detail, with the goal of moving toward more standardized approaches for future research.
Aerobic fatiguing methods have commonly required a participant to perform a treadmill or bike test until volitional failure, at which point neuromuscular fatigue is assumed to have occurred [62]. The tests commence with the participant running or cycling at a baseline intensity that increases linearly every 1–3 min until the test is terminated [104]. To quantify fatigue with this method, researchers typically rely on the rate of perceived exertion (RPE), a subjective measure reported by the participant based on their effort level. Participants may also be instructed to stop the test when they have reached volitional failure (i.e., when they feel fatigued). There are several limitations associated with the aerobic approach that may influence the desired outcomes. First, this approach includes the lack of objective quantification, as RPE, by definition, is a subjective rating. As such, these subjective measures do not indicate if fatigue has occurred at the neuromuscular level or if participants have simply lost motivation to continue. Evidence suggests that an individual’s tolerance to suffering may affect their overall performance and therefore their volitional indication of being fatigued [105]. Another limitation associated with aerobic fatigue methods is the type of fatigue that is being induced. Given that this is aerobic fatigue with sustained, low-intensity muscular efforts, it is likely that the cardiovascular system is the limiting factor and that maximal changes at the neuromuscular level have not or will not occur. Finally, there is limited external validity associated with utilizing aerobic fatigue to induce changes consistent with ACL injury risk. There are few sport scenarios that require one long and sustained aerobic effort, and different protocols can result in a different time to failure for the same individual [104].
Aerobic-anaerobic methods rely on participants performing a combination of exercises in a cyclic manner. For example, some studies have used a whole-body sawing task [106], while others have incorporated sport-specific and/or agility-based tasks in circuits [24,32,34,86,107,108,109]. There are also protocols that implement a combination of sprints, squats, and/or (sub)maximal vertical jumps to induce fatigue [30,84,110]. Regardless of the specific tasks, these methods all attempt to emulate sport settings, where participants are fatigued with a combination of short-duration high-intensity bouts over longer durations. Fatigue is often defined/measured using a more quantitative approach to volitional failure, often defined as a decrease in the force produced or performance (e.g., a 10% decrease in maximal jump height). A limitation with these protocols, however, is the arbitrary selection of criteria that defines when fatigue has occurred. For example, Lessi et al. (2018) [111] defined fatigue as when participants’ maximal single-leg vertical jump height decreased by 20% relative to baseline measures, while Chappell et al. (2005) [30] defined fatigue as volitional failure (terminating task) in a circuit involving sprints and submaximal (115% reach height) double-leg vertical jumps. While this does add a quantitative element to measuring fatigue, it still relies on the assumption that the participant is truly fatigued and has not simply lost motivation to continue performing at a near-maximal level.
Finally, isometric or cyclical concentric fatigue protocols include either repeated or a single sustained contraction where movement at the designated joint is mechanically blocked or controlled such that it is purely uniplanar (e.g., isolated knee flexion and extension). Within this method, fatigue is often defined as a decrease in force and/or torque production as measured with a load cell [54,83]. This method also requires an arbitrarily defined cut-off point. For example, Wojtys et al. (1996) [83] defined fatigue as a 50% decrease in work, but Longpre et al. (2013) [55] used either a decrease in peak knee extension or flexion torque of at least 25%. These methods also have less external validity than the other methods as neuromuscular fatigue in sport and exercise settings is reached dynamically, with multiple joints and movements being involved in inducing fatigue [30]. More specifically, there are very few instances in sport where athletes are tasked with a maximal isometric or controlled cyclical concentric effort to fatigue. However, this method is useful in computer models of fatigue as it is easiest to simulate and can provide valuable insights into neuromuscular fatigue [15].
Within the neuromuscular fatigue research, there are methods available to objectively quantify fatigue, such as an analysis of the EMG signal by quantifying the mean power frequency (MnPF) and assessment of evoked potentials. However, the subsequent sections will focus on the MnPF, as it offers the most feasible method of objectively quantifying fatigue in real-time in response to dynamic ecologically valid tasks. Calculating the MnPF involves computing the mean power by converting the electromyography (EMG) signal from the time domain to the frequency domain and analyzing the power spectrum [112,113]. As EMG records the electrical activity at the neuromuscular junction, this method quantifies localized or peripheral fatigue [15]. This requires performing a fast Fourier transformation on the EMG signal and calculating the mean power (amplitude squared) over predetermined time intervals of data [113,114]. A decrease in the MnPF of 10–20% has been associated with the onset of neuromuscular fatigue. This reduction occurs because of a progressive reduction in muscle motor unit spike amplitude and firing frequency, both of which are physiological indicators of neuromuscular fatigue [115,116]. Fatigue-induced changes in MnPF are greater for higher intensity muscle contractions (>30% maximum voluntary isometric contraction [MVIC]) compared to lower intensities (<20% MVIC) (Arendt-Nielsen et al., 1989). Additionally, Hummel et al. (2005) [117] demonstrated that decreases in MnPF were correlated with increases in participants’ RPE, suggesting that the metric closely aligns with participants’ subjective rating of their fatigue. As such, the use of MnPF is warranted for the objective quantification of neuromuscular fatigue.
5. Conclusions
ACL injury is a well-researched phenomenon that is complex and multi-factorial. The research related to the potential effect that fatigue has on ACL injuries remains a controversial topic, given the difficulties in defining, measuring, and inducing fatigue in a repeatable and standardized manner. While the research remains a controversial topic, localized neuromuscular fatigue is likely a risk factor, among multiple factors, for ACL injuries. This is another modifiable risk factor that should be considered when developing ACL injury risk reduction interventions. Although further work is needed to best identify how fatigue can be integrated into risk reduction and rehabilitation programs, introducing assessments of individuals in the fatigued state is a possible first step. Implementing controlled training while fatigued may also allow individuals to develop movement strategies in the fatigued state. Finally, including a focus on neuromuscular endurance training may improve an individual’s resiliency to the factors that induce fatigue (e.g., greater force-generating capacity prior to reaching the point of fatigue). Future work should also aim to develop standardized methods of defining, measuring, and inducing neuromuscular fatigue to address current limitations and move toward a consensus.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biomechanics5010011/s1, Table S1: Search terms and strategy used in the Medline database; Table S2: Detailed summary of the outcome of each of the included studies.
Author Contributions
J.L.I.T. was involved in the conceptualization of the review, the design of the methodology, the interpretation of the findings, and the writing and editing of the submitted manuscript. T.A.B. was involved in the conceptualization of the review, the design of the methodology, the interpretation of the findings, and the writing and editing of the submitted manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially funded by a MITACS accelerate grant, in partnership with OSSUR Canada awarded to Joshua L. I. Taylor.
Conflicts of Interest
Joshua Taylor received salary support from OSSUR Canada through a MITACS acceleration grant. The authors declare no other conflicts of interest.
References
- Campbell, C.J.; Carson, J.D.; Diaconescu, E.D.; Celebrini, R.; Rizzardo, M.R. Canadian academy of sport and exercise medicine position statement: Neuromuscular training programs can decrease anterior cruciate ligament injuries in youth soccer Players. Clin. J. Sport Med. 2014, 24, 263–267. [Google Scholar] [CrossRef]
- Gornitzky, A.L.; Lott, A.; Yellin, J.L.; Fabricant, P.D.; Lawrence, J.T.; Ganley, T.J. Sport-specific yearly risk and incidence of anterior cruciate ligament tears in high school athletes. Am. J. Sports Med. 2016, 44, 2716–2723. [Google Scholar] [CrossRef]
- Grassi, A.; Macchiarola, L.; Filippini, M.; Lucidi, G.A.; Della Villa, F.; Zaffagnini, S. Epidemiology of anterior cruciate ligament injury in italian first division soccer players. Sports Health 2020, 12, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Shultz, S.J. ACL Injury in the female athlete: A multifactorial problem that remains poorly understood. J. Athl. Train. 2008, 43, 455. [Google Scholar] [CrossRef]
- Park, J.S.; Nam, D.C.; Kim, D.H.; Kim, H.K.; Hwang, S.C. Measurement of knee morphometrics using MRI: A comparative study between ACL-injured and non-injured knees. Knee Surg. Relat. Res. 2012, 24, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Chia, L.; De Oliveira Silva, D.; Whalan, M.; McKay, M.J.; Sullivan, J.; Fuller, C.W.; Pappas, E. Non-contact anterior cruciate ligament injury epidemiology in team-ball sports: A systematic review with meta-analysis by sex, age, sport, participation level, and exposure type. Sports Med. 2022, 52, 2447–2467. [Google Scholar] [CrossRef]
- Bayer, S.; Meredith, S.J.; Wilson, K.; de Sa, D.; Pauyo, T.; Byrne, K.; McDonough, C.M.; Musahl, V. Knee morphological risk factors for anterior cruciate ligament injury: A systematic review. J. Bone Jt. Surg. Am. Vol. 2020, 102, 703–718. [Google Scholar] [CrossRef]
- Sharir, R.; Rafeeuddin, R.; Staes, F.; Dingenen, B.; George, K.; Vanrenterghem, J.; Robinson, M.A. Mapping current research trends on anterior cruciate ligament injury risk against the existing evidence: In vivo biomechanical risk factors. Clin. Biomech. 2016, 37, 34–43. [Google Scholar] [CrossRef]
- Boden, B.P.; Torg, J.S.; Knowles, S.B.; Hewett, T.E. Video analysis of anterior cruciate ligament injury: Abnormalities in hip and ankle kinematics. Am. J. Sports Med. 2009, 37, 252–259. [Google Scholar] [CrossRef]
- Hewett, T.E.; Myer, G.D.; Ford, K.R.; Heidt, R.S., Jr.; Colosimo, A.J.; McLean, S.G.; Van Den Bogert, A.J.; Paterno, M.V.; Succop, P. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: A prospective study. Am. J. Sports Med. 2005, 33, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Krosshaug, T.; Steffen, K.; Kristianslund, E.; Nilstad, A.; Mok, K.-M.; Myklebust, G.; Andersen, T.E.; Holme, I.; Engebretsen, L.; Bahr, R. The vertical drop jump is a poor screening test for ACL injuries in female elite soccer and handball players. Am. J. Sports Med. 2016, 44, 874–883. [Google Scholar] [CrossRef]
- Leppänen, M.; Pasanen, K.; Kujala, U.M.; Vasankari, T.; Kannus, P.; Äyrämö, S.; Krosshaug, T.; Bahr, R.; Avela, J.; Perttunen, J.; et al. Stiff Landings Are Associated with Increased ACL Injury Risk in Young Female Basketball and Floorball Players. Am. J. Sports Med. 2017, 45, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Berns, G.S.; Hull, M.L.; Patterson, H.A. Strain in the anteromedial bundle of the anterior cruciate ligament under combined loading conditions. J. Orthop. Res. 1992, 10, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Neumann, D.A. Lower Extremity: Knee, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Potvin, J.R.; Fuglevand, A.J. A motor unit-based model of muscle fatigue. PLoS Comput. Biol. 2017, 13, e1005581. [Google Scholar] [CrossRef] [PubMed]
- Enoka, R.M.; Duchateau, J. Translating Fatigue to Human Performance. Med. Sci. Sports Exerc. 2016, 48, 2228–2238. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.; Gube, M.; Chaabene, H.; Prieske, O.; Zenon, A.; Broscheid, K.-C.; Schega, L.; Husmann, F.; Weippert, M. Fatigue and Human Performance: An Updated Framework. Sports Med. 2023, 53, 7–31. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Amann, M.; Duchateau, J.; Meesuen, R.; Rice, C. Neural contributions to muscle fatigue: From the brain to the muscle and back again. Med. Sci. Sports Exerc. 2016, 48, 2294–2306. [Google Scholar] [CrossRef]
- Marcora, S.M.; Staiano, W.; Manning, V. Mental fatigue impairs physical performance in humans. J. Appl. Physiol. 2009, 106, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, J.; Marcora, S.; De Pauw, K.; Bailey, S.; Meeusen, R.; Roelands, B. The effects of mental fatigue on physical performance: A systematic review. Sports Med. 2017, 47, 1569–1588. [Google Scholar] [CrossRef]
- Burke, R.E.; Levine, D.N.; Tsairis, P.; Zajac, F.E. Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J. Physiol. 1973, 234, 723–748. [Google Scholar] [CrossRef]
- Abergel, R.E.; Tuesta, E.; Jarvis, D.N. The effects of acute physical fatigue on sauté jump biomechanics in dancers. J. Sports Sci. 2021, 39, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.F.; Sell, T.C.; Benjaminse, A.; Lephart, S.M. Force sense of the knee not affected by fatiguing the knee extensors and flexors. J. Sport. Rehabil. 2016, 25, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Bedo, B.L.S.; Catelli, D.S.; Lamontagne, M.; Moraes, R.; Pereira, D.R.; Graça, J.B.; Santiago, P.R.P. Fatigue modifies hip and knee kinematics during single- and double-leg dynamic tasks: An investigation with female handball players. J. Sports Sci. 2022, 40, 1964–1972. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.; Mau-Moeller, A.; Wassermann, F.; Plewka, A.; Bader, R.; Bruhn, S. Repetitive jumping and sprinting until exhaustion alters hamstring reflex responses and tibial translation in males and females. J. Orthop. Res. 2015, 33, 1687–1692. [Google Scholar] [CrossRef]
- Benjaminse, A.; Habu, A.; Sell, T.C.; Abt, J.P.; Fu, F.H.; Myers, J.B.; Lephart, S.M. Fatigue alters lower extremity kinematics during a single-leg stop-jump task. Knee Surg. Sports Traumatol. Arthrosc. 2008, 16, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Borotikar, B.S.; Newcomer, R.; Koppes, R.; McLean, S.G. Combined effects of fatigue and decision making on female lower limb landing postures: Central and peripheral contributions to ACL injury risk. Clin. Biomech. 2008, 23, 81–92. [Google Scholar] [CrossRef]
- Bossuyt, F.M.; García-Pinillos, F.; Raja Azidin, R.M.F.; Vanrenterghem, J.; Robinson, M.A. The Utility of a High-intensity Exercise Protocol to Prospectively Assess ACL Injury Risk. Int. J. Sports Med. 2015, 37, 125–133. [Google Scholar] [CrossRef]
- Brazen, D.M.; Kent Todd, M.; Ambegaonkar, J.P.; Wunderlich, R.; Peterson, C. The Effect of Fatigue on Landing Biomechanics in Single-Leg Drop Landings. Clin. J. Sport Med. 2010, 20, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Chappell, J.D.; Herman, D.C.; Knight, B.S.; Kirkendall, D.T.; Garrett, W.E.; Yu, B. Effect of fatigue on knee kinetics and kinematics in stop-jump tasks. Am. J. Sports Med. 2005, 33, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Coratella, G.; Bellin, G.; Beato, M.; Schena, F. Fatigue affects peak joint torque angle in hamstrings but not in quadriceps. J. Sports Sci. 2015, 33, 1276–1282. [Google Scholar] [CrossRef]
- Cortes, N.; Greska, E.; Ambegaonkar, J.P.; Kollock, R.O.; Caswell, S.V.; Onate, J.A. Knee kinematics is altered post-fatigue while performing a crossover task. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 2202–2208. [Google Scholar] [CrossRef]
- Cortes, N.; Greska, E.; Kollock, R.; Ambegaonkar, J.; Onate, J.A. Changes in lower extremity biomechanics due to a short-term fatigue protocol. J. Athl. Train. 2013, 48, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Cortes, N.; Quammen, D.; Lucci, S.; Greska, E.; Onate, J. A functional agility short-term fatigue protocol changes lower extremity mechanics. J. Sports Sci. 2012, 30, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Dickin, D.C.; Johann, E.; Wang, H.; Popp, J.K. Combined effects of drop height and fatigue on landing mechanics in active females. J. Appl. Biomech. 2015, 31, 237–243. [Google Scholar] [CrossRef] [PubMed]
- El-Ashker, S.; Allardyce, J.M.; Carson, B.P. Sex-related differences in joint-angle-specific hamstring-to-quadriceps function following fatigue. Eur. J. Sport. Sci. 2019, 19, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Gehring, D.; Melnyk, M.; Gollhofer, A. Gender and fatigue have influence on knee joint control strategies during landing. Clin. Biomech. 2009, 24, 82–87. [Google Scholar] [CrossRef]
- Geiser, C.F.; O’Connor, K.M.; Earl, J.E. Effects of isolated hip abductor fatigue on frontal plane knee mechanics. Med. Sci. Sports Exerc. 2010, 42, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Gillot, T.; L’Hermette, M.; Garnier, T.; Tourny-Chollet, C. Effect of Fatigue on Functional Stability of the Knee: Particularities of Female Handball Players. Int. J. Sports Med. 2019, 40, 468–476. [Google Scholar] [CrossRef]
- Greco, C.C.; Da Silva, W.L.; Camarda, S.R.A.; Denadai, B.S. Fatigue and rapid hamstring/quadriceps force capacity in professional soccer players. Clin. Physiol. Funct. Imaging 2013, 33, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Greig, M. Concurrent changes in eccentric hamstring strength and knee joint kinematics induced by soccer-specific fatigue. Phys. Ther. Sport 2019, 37, 21–26. [Google Scholar] [CrossRef]
- Harato, K.; Morishige, Y.; Niki, Y.; Kobayashi, S.; Nagura, T. Fatigue and recovery have different effects on knee biomechanics of drop vertical jump between female collegiate and recreational athletes. J. Orthop. Surg. Res. 2021, 16, 739. [Google Scholar] [CrossRef] [PubMed]
- Hassanlouei, H.; Arendt-Nielsen, L.; Kersting, U.G.; Falla, D. Effect of exercise-induced fatigue on postural control of the knee. J. Electromyogr. Kinesiol. 2012, 22, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M.A.; Hatfield, G.L. Ankle and knee biomechanics during normal walking following ankle plantarflexor fatigue. J. Electromyogr. Kinesiol. 2017, 35, 24–29. [Google Scholar] [CrossRef]
- Iguchi, J.; Tateuchi, H.; Taniguchi, M.; Ichihashi, N. The effect of sex and fatigue on lower limb kinematics, kinetics, and muscle activity during unanticipated side-step cutting. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 41–48. [Google Scholar] [CrossRef]
- Kellis, E.; Kouvelioti, V. Agonist versus antagonist muscle fatigue effects on thigh muscle activity and vertical ground reaction during drop landing. J. Electromyogr. Kinesiol. 2009, 19, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Kernozek, T.W.; Torry, M.R.; Iwasaki, M. Gender differences in lower extremity landing mechanics caused by neuromuscular fatigue. Am. J. Sports Med. 2008, 36, 554–565. [Google Scholar] [CrossRef]
- Khalid, A.J.; Ian Harris, S.; Michael, L.; Joseph, H.; Qu, X. Effects of neuromuscular fatigue on perceptual-cognitive skills between genders in the contribution to the knee joint loading during side-stepping tasks. J. Sports Sci. 2015, 33, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Lee, S.Y.; Lee, S.C.; Rosen, A.B.; Grindstaff, T.L.; Knarr, B.A. Effect of isolated hip abductor fatigue on single-leg landing mechanics and simulated ACL loading. Knee 2021, 31, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Youm, C.; Son, M.; Kim, J.; Lee, M. The effect of knee flexor and extensor fatigue on shock absorption during cutting movements after a jump landing. Knee 2017, 24, 1342–1349. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, P.J.; Petrella, R.J.; Sproule, J.R.; Fowler, P.J. Effects of fatigue on knee proprioception. Clin. J. Sports Med. 1997, 7, 22–27. [Google Scholar] [CrossRef]
- Lessi, G.C.; dos Santos, A.F.; Batista, L.F.; de Oliveira, G.C.; Serrão, F.V. Effects of fatigue on lower limb, pelvis and trunk kinematics and muscle activation: Gender differences. J. Electromyogr. Kinesiol. 2017, 32, 9–14. [Google Scholar] [CrossRef]
- Liederbach, M.; Kremenic, I.J.; Orishimo, K.F.; Pappas, E.; Hagins, M. Comparison of landing biomechanics between male and female dancers and athletes, part 2. Am. J. Sports Med. 2014, 42, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Longpre, H.S.; Acker, S.M.; Maly, M.R. Muscle activation and knee biomechanics during squatting and lunging after lower extremity fatigue in healthy young women. J. Electromyogr. Kinesiol. 2015, 25, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Longpré, H.S.; Potvin, J.R.; Maly, M.R. Biomechanical changes at the knee after lower limb fatigue in healthy young women. Clin. Biomech. 2013, 28, 441–447. [Google Scholar] [CrossRef]
- McEldowney, K.M.; Hopper, L.S.; Etlin-Stein, H.; Redding, E. Fatigue Effects on Quadriceps and Hamstrings Activation in Dancers Performing Drop Landings. J. Danc. Med. Sci. 2013, 17, 109–114. [Google Scholar] [CrossRef]
- McLean, S.G.; Felin, R.E.; Suedekum, N.; Calabrese, G.; Passerallo, A.; Joy, S. Impact of fatigue on gender-based high-risk landing strategies. Med. Sci. Sports Exerc. 2007, 39, 502–514. [Google Scholar] [CrossRef]
- McLean, S.G.; Samorezov, J.E. Fatigue-induced ACL injury risk stems from a degradation in central control. Med. Sci. Sports Exerc. 2009, 41, 1661–1672. [Google Scholar] [CrossRef] [PubMed]
- Mejane, J.; Faubert, J.; Romeas, T.; Labbe, D.R. The combined impact of a perceptual–cognitive task and neuromuscular fatigue on knee biomechanics during landing. Knee 2019, 26, 52–60. [Google Scholar] [CrossRef]
- Miura, K.; Ishibashi, Y.; Tsuda, E.; Okamura, Y.; Otsuka, H.; Toh, S. The Effect of Local and General Fatigue on Knee Proprioception. Arthrosc. J. Arthrosc. Relat. Surg. 2004, 20, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Moran, K.A.; Clarke, M.; Reilly, F.; Wallace, E.S.; Brabazon, D.; Marshall, B. Does endurance fatigue increase the risk of injury when performing drop jumps? J. Strength. Cond. Res. 2009, 23, 1448–1455. [Google Scholar] [CrossRef]
- Moran, K.A.; Marshall, B.M. Effect of fatigue on tibial impact accelerations and knee kinematics in drop jumps. Med. Sci. Sports Exerc. 2006, 38, 1836–1842. [Google Scholar] [CrossRef] [PubMed]
- Murdock, G.H.; Hubley-Kozey, C.L. Effect of a high intensity quadriceps fatigue protocol on knee joint mechanics and muscle activation during gait in young adults. Eur. J. Appl. Physiol. 2012, 112, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Nyland, J.A.; Shapiro, R.; Rebecca Stine, P.I. Relationship of fatigued run and rapid stop to ground reaction forces, lower extremity kinematics, and muscle activation. J. Orthop. Sports Phys. Therpay 1994, 20, 132–137. [Google Scholar] [CrossRef]
- Sanna, G.; O’Connor, K.M. Fatigue-related changes in stance leg mechanics during sidestep cutting maneuvers. Clin. Biomech. 2008, 23, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Orishimo, K.F.; Kremenic, I.J. Effect of fatigue on single-leg hop landing biomechanics. J. Appl. Biomech. 2006, 22, 245–254. [Google Scholar] [CrossRef]
- Ortiz, A.; Olson, S.L.; Etnyre, B.; Trudelle-Jackson, E.E.; Bartlett, W.; Venegas-Rios, H.L. Fatigue effects on knee joint stability during two jump tasks in women. J. Strength. Cond. Res. 2010, 24, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Patrek, M.F.; Kernozek, T.W.; Willson, J.D.; Wright, G.A.; Doberstein, S.T. Hip-abductor fatigue and single-leg landing mechanics in women athletes. J. Athl. Train. 2011, 46, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Quammen, D.; Cortes, N.; Van Lunen, B.L.; Lucci, S.; Ringleb, S.I.; Onate, J. Two different fatigue protocols and lower extremity motion patterns during a stop-jump task. J. Athl. Train. 2012, 47, 32–41. [Google Scholar] [CrossRef]
- Qu, X.; Jiang, J.; Hu, X. Effects of subsensory noise and fatigue on knee landing and cross-over cutting biomechanics in male athletes. J. Appl. Biomech. 2018, 34, 205–210. [Google Scholar] [CrossRef]
- Radzak, K.N.; Stickley, C.D. Fatigue-induced hip-abductor weakness and changes in biomechanical risk factors for running-related injuries. J. Athl. Train. 2020, 55, 1270–1276. [Google Scholar] [CrossRef]
- Rahnama, N.; Reilly, T.; Lees, A.; Graham-Smith, P. Muscle fatigue induced by exercise simulating the work rate of competitive soccer. J. Sports Sci. 2003, 21, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.; Venâncio, J.; Quintas, P.; Oliveira, J. The effect of fatigue on knee position sense is not dependent upon the muscle group fatigued. Muscle Nerve 2011, 44, 217–220. [Google Scholar] [CrossRef]
- Salgado, E.; Ribeiro, F.; Oliveira, J. Joint-position sense is altered by football pre-participation warm-up exercise and match induced fatigue. Knee 2015, 22, 243–248. [Google Scholar] [CrossRef]
- Savage, R.J.; Lay, B.S.; Wills, J.A.; Lloyd, D.G.; Doyle, T.L.A. Prolonged running increases knee moments in sidestepping and cutting manoeuvres in sport. J. Sci. Med. Sport. 2018, 21, 508–512. [Google Scholar] [CrossRef]
- Schmitz, R.J.; Kim, H.; Shultz, S.J. Neuromuscular fatigue and tibiofemoral joint biomechanics when transitioning from non-weight bearing to weight bearing. J. Athl. Train. 2015, 50, 23–29. [Google Scholar] [CrossRef]
- Smeets, A.; Vanrenterghem, J.O.S.; Staes, F.; Verschueren, S. Match play-induced changes in landing biomechanics with special focus on fatigability. Med. Sci. Sports Exerc. 2019, 51, 1884–1894. [Google Scholar] [CrossRef]
- Thomas, A.C.; Mclean, S.G.; Palmieri-Smith, R.M. Quadriceps and hamstrings fatigue alters hip and knee mechanics. J. Appl. Biomech. 2010, 2, 159–170. [Google Scholar] [CrossRef]
- Thomas, A.C.; Palmieri-Smith, R.M.; Mclean, S.G. Isolated hip and ankle fatigue are unlikely risk factors for anterior cruciate ligament injury. Scand. J. Med. Sci. Sports. 2011, 21, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.C.; Sigward, S.M.; Pollard, C.D.; Fletcher, M.J.; Powers, C.M. Effects of fatigue and recovery on knee mechanics during side-step cutting. Med. Sci. Sports Exerc. 2009, 41, 1952–1957. [Google Scholar] [CrossRef] [PubMed]
- Weeks, B.K.; Carty, C.P.; Horan, S.A. Effect of sex and fatigue on single leg squat kinematics in healthy young adults Rehabilitation, physical therapy and occupational health. BMC Musculoskelet. Disord. 2015, 16, 271. [Google Scholar] [CrossRef]
- Weinhandl, J.T.; Smith, J.D.; Dugan, E.L. The effects of repetitive drop jumps on impact phase joint kinematics and kinetics. J. Appl. Biomech. 2011, 27, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Wojtys, E.M.; Wylie, B.B.; Huston, L.J. The effects of muscle fatigue on neuromuscular function and anterior tibial translation in healthy knees. Am. J. Sports Med. 1996, 24, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.L.; Huang, C.F.; Chen, P.C. Effects of lower extremity muscle fatigue on knee loading during a forward drop jump to a vertical jump in female athletes. J. Hum. Kinet. 2020, 72, 5–13. [Google Scholar] [CrossRef]
- Xia, R.; Zhang, X.; Wang, X.; Sun, X.; Fu, W. Effects of two fatigue protocols on impact forces and lower extremity kinematics during drop landings: Implications for noncontact anterior cruciate ligament injury. J. Healthc. Eng. 2017, 2017, 5690519. [Google Scholar] [CrossRef]
- Zago, M.; David, S.; Bertozzi, F.; Brunetti, C.; Gatti, A.; Salaorni, F.; Tarabini, M.; Galvani, C.; Sforza, C.; Galli, M. Fatigue induced by repeated changes of direction in élite female football (soccer) players: Impact on lower limb biomechanics and implications for ACL injury prevention. Front. Bioeng. Biotechnol. 2021, 9, 666841. [Google Scholar] [CrossRef]
- Zebis, M.K.; Bencke, J.; Andersen, L.L.; Alkjær, T.; Suetta, C.; Mortensen, P.; Kjær, M.; Aagaard, P. Acute fatigue impairs neuromuscular activity of anterior cruciate ligament-agonist muscles in female team handball players. Scand. J. Med. Sci. Sports 2011, 21, 833–840. [Google Scholar] [CrossRef]
- Boden, B.P.; Sheehan, F.T.; Torg, J.S.; Hewett, T.E. Non-contact ACL injuries: Mechanisms and risk factors. J. Am. Acad. Orthop. Surg. 2010, 18, 520–527. [Google Scholar] [CrossRef]
- Koga, H.; Muneta, T.; Bahr, R.; Engebretsen, L.; Krosshaug, T. ACL injury mechanisms: Lessons learned from video analysis. In Rotatory Knee Instability: An Evidence Based Approach; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 27–36. [Google Scholar] [CrossRef]
- Ni, Q.K.; Song, G.Y.; Zhang, Z.J.; Zheng, T.; Feng, Z.; Cao, Y.-W.; Feng, H.; Zhang, H. Steep posterior tibial slope and excessive anterior tibial translation are predictive risk factors of primary anterior cruciate ligament reconstruction failure: A case-control study with prospectively collected data. Am. J. Sports Med. 2020, 48, 2954–2961. [Google Scholar] [CrossRef]
- Hewett, T.E.; Ford, K.R.; Hoogenboom, B.J.; Myer, G.D. Understanding and preventing ACL injuries: Current biomechanical and epidemiologic considerations-update 2010. N. Am. J. Sports Phys. Ther. 2010, 5, 234–251. [Google Scholar] [PubMed]
- Myer, G.D.; Ford, K.R.; Barber Foss, K.D.; Liu, C.; Nick, T.G.; Hewett, T.E. The relationship of hamstrings and quadriceps strength to anterior cruciate ligament injury in female athletes. Clin. J. Sport Med. 2009, 19, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Wetters, N.; Weber, A.E.; Wuerz, T.H.; Schub, D.L.; Mandelbaum, B.R. Mechanism of injury and risk factors for anterior cruciate ligament injury. Oper. Tech. Sports Med. 2016, 24, 2–6. [Google Scholar] [CrossRef]
- Willigenburg, N.W.; McNally, M.P.; Hewett, T.E. Quadriceps and hamstrings strength in athletes. In Hamstring and Quadriceps Injuries in Athletes; Springer: Berlin/Heidelberg, Germany, 2014; pp. 15–28. [Google Scholar] [CrossRef]
- Bourne, M.N.; Webster, K.E.; Hewett, T.E. Is fatigue a risk factor for anterior cruciate ligament rupture? Sports Med. 2019, 49, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
- Doyle, T.L.; Schilaty, N.; Webster, K.; Hewett, T. Time of season and game segment is not related to likelihood of lower-limb injuries: A meta-analysis. Clin. J. Sport Med. 2021, 31, 304–312. [Google Scholar] [CrossRef]
- Hewett, T.E.; Webster, K.E.; Hurd, W.J. Systematic selection of key logistic regression variables for risk prediction analyses: A five factor maximum model. Clin. J. Sport Med. 2019, 29, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Nilstad, A.; Petushek, E.; Mok, K.M.; Bahr, R.; Krosshaug, T. Kiss goodbye to the ‘kissing knees’: No association between frontal plane inward knee motion and risk of future non-contact ACL injury in elite female athletes. Sports Biomech. 2023, 22, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Mouton, C.; Gokeler, A.; Urhausen, A.; Nuhrenborger, C.; Seil, R. High incidence of anterior cruciate ligament injuries within the first 2 months of the season in amateur. Sports Health 2022, 14, 183–187. [Google Scholar] [CrossRef]
- Della Villa, F.; Buckthorpe, M.; Grassi, A.; Nabiuzzi, A.; Tosarelli, F.; Zaffagnini, S.; Della Villa, S. Systematic video analysis of ACL injuries in professional male football (soccer): Injury mechanisms, situational patterns and biomechanics study on 134 consecutive cases. Br. J. Sports Med. 2020, 54, 1423–1432. [Google Scholar] [CrossRef]
- Zhou, J.; Schilaty, N.D.; Hewett, T.E.; Bates, N.A. Analysis of timing of secondary ACL injury in professional athletes does not support game timing or season timing as a contributor to injury risk. Int. J. Sports Phys. Ther. 2020, 15, 254–262. [Google Scholar] [CrossRef]
- Lundblad, M.; Waldén, M.; Magnusson, H.; Karlsson, J.; Ekstrand, J. The UEFA injury study: 11-year data concerning 346 MCL injuries and time to return to play. Br. J. Sports Med. 2013, 47, 759–762. [Google Scholar] [CrossRef]
- Barber-Westin, S.D.; Noyes, F.R. Effect of fatigue protocols on lower limb neuromuscular function and implications for anterior cruciate ligament injury prevention training: A systematic review. Am. J. Sports Med. 2017, 45, 3388–3396. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Chaloupka, E.C.; Mastrangelo, M.A.; Biren, G.B.; Robertson, R.J. Physiological comparisons among three maximal treadmill exercise protocols in trained and untrained individuals. Eur. J. Appl. Physiol. 2001, 84, 291–295. [Google Scholar] [CrossRef]
- Zeller, L.; Shimoni, N.; Vodonos, A.; Sagy, I.; Barski, L.; Buskila, D. Pain sensitivity and physical fitness. J. Sports Med. Phys. Fit. 2019, 59, 1635–1639. [Google Scholar]
- Gates, D.H.; Dingwell, J.B. The effects of neuromuscular fatigue on task performance during repetitive goal-directed movements. Exp. Brain Res. 2008, 187, 573–585. [Google Scholar] [CrossRef]
- De Ste Croix, M.B.A.; Priestley, A.M.; Lloyd, R.S.; Oliver, J.L. ACL injury risk in elite female youth soccer: Changes in neuromuscular control of the knee following soccer-specific fatigue. Scand. J. Med. Sci. Sports 2015, 25, e531–e538. [Google Scholar] [CrossRef] [PubMed]
- Thorlund, J.B.; Michalsik, L.B.; Madsen, K.; Aagaard, P. Acute fatigue-induced changes in muscle mechanical properties and neuromuscular activity in elite handball players following a handball match. Scand. J. Med. Sci. Sports 2008, 18, 462–472. [Google Scholar] [CrossRef]
- Jordan, M.J.; Aagaard, P.; Herzog, W. Asymmetry and Thigh Muscle Coactivity in Fatigued Anterior Cruciate Ligament-Reconstructed Elite Skiers. Med. Sci. Sports Exerc. 2017, 49, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Lessi, G.C.; Silva, R.S.; Serrao, F.V. Comparison of the effects of fatigue on kinematics and muscle activation between men and women after anterior cruciate ligament reconstruction. Phys. Ther. Sport 2018, 31, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Lessi, G.; Carvalho, C.; Serrao, F. Association of hip and trunk strength with three-dimensional trunk, hip, and knee kinematics during a single-leg drop veritcal jump. J. Strength Cond. Res. 2018, 33, 1902–1908. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.A. Biomechanics and Motor Control of Human Movement, 4th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- McManus, L.; Lowery, M.; Merletti, R.; Søgaard, K.; Besomi, M.; Clancy, E.A.; van Dieën, J.H.; Hug, F.; Wrigley, T.; Besier, T.; et al. Consensus for experimental design in electromyography (CEDE) project: Terminology matrix. J. Electromyogr. Kinesiol. 2021, 59, 102565. [Google Scholar] [CrossRef] [PubMed]
- Öberg, T.; Sandsjö, L.; Kadefors, R. Variability of the EMG mean power frequency: A study on the trapezius muscle. J. Electromyogr. Kinesiol. 1991, 1, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Moritani, T.; Muro, M.; Nagata, A. Intramuscular and surface electromyogram changes during muscle fatigue. J. Appl. Physiol. 1986, 60, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.R.; Potvin, J.R. Fatigue-related EMG responses of trunk muscles to a prolonged, isometric twist exertion. Clin. Biomech. 1997, 12, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Hummel, A.; Läubli, T.; Pozzo, M.; Schenk, P.; Spillmann, S.; Klipstein, A. Relationship between perceived exertion and mean power frequency of the EMG signal from the upper trapezius muscle during isometric shoulder elevation. Eur. J. Appl. Physiol. 2005, 95, 321–326. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).