Abstract
Excretory/secretory products from parasites (ESPs) can act as pathogen-associated molecular patterns (PAMPs) to activate innate immunity. Parasites may achieve immune evasion by modulating the interaction between PAMPs and the nucleotide-binding oligomerization domain-like receptor family, pyrin domain-containing three (NLRP3) inflammasome. Previous studies have suggested that some components of ESPs from Clonorchis sinensis (CsESPs) can induce the host’s immune responses, but the components that balance immunopathology and maintain chronic infection in chronic Clonorchis sinensis (C. sinensis) remain unclear. We previously found that the iNOS-interacting protein from C. sinensis (CsNOSIP), a component of CsESP, stimulates macrophages to produce reactive oxygen species (ROS) and nitric oxide (NO), both of which inhibit NLRP3 inflammasome activation. Therefore, this study investigated the effects of CsESP and CsNOSIP on inflammasome activation using RT-PCR, Western blot, and ELISA. This study showed that CsESPs promoted NLRP3 inflammasome activation in RAW264.7 cells, while CsNOSIP inhibited LPS-induced IL-1β secretion through an NLRP3-caspase-1-dependent pathway and reversed the CsESPs-induced activation through the iNOS/NO–NF-κB pathway. These results reveal the antagonistic effects of CsESPs and CsNOSIP in inflammasome regulation, suggesting that this balance contributes to the regulation of the host’s immunity and the promotion of chronic infection of C. sinensis, providing potential targets for prevention and treatment.
1. Background
Clonorchiasis, a prevalent foodborne parasitic disease caused by Clonorchis sinensis (C. sinensis), is predominantly endemic in East Asia, affecting countries such as China, South Korea, Thailand, and northern Vietnam. Globally, it is estimated that around 13.24 million people are currently infected [1,2,3]. Individuals infected with C. sinensis in the early stages typically show no symptoms. However, repeated and chronic infections may eventually lead to various liver- and biliary-related symptoms and complications. Multiple studies have shown that C. sinensis continuously release excretory/secretory products (CsESPs). Some components of CsESPs can induce the host’s immune response, which may be closely associated with the occurrence of cholangitis, liver fibrosis, and cholangiocarcinoma (CCA) [4,5]. However, the specific components of CsESPs that regulate immune pathology and contribute to the establishment of chronic C. sinensis infection remain unidentified.
Molecules in ESPs as pathogen-associated molecular patterns (PAMPs) can activate innate immune responses [6,7]. Recent studies have shown that molecules secreted or presented on the surface of parasites (such as glycolipids, glycoproteins, DNA, and RNA) can activate innate immune responses via Pattern Recognition Receptors (PRRs). For example, the circumsporozoite protein of Plasmodium falciparum enhance inflammatory responses in the host [8,9], while glycoproteins released by Trypanosoma induce a strong Th1-type immune response [10], thereby strengthening the host’s ability to clear parasites [11]. Conversely, the glycosylated antigens of Schistosoma shift the host’s immune response toward a Th2-type, thereby avoiding excessive Th1-mediated cytotoxicity [12,13,14]. Similarly, the lipophosphoglycan of Leishmania could disrupt macrophage activation, facilitating its intracellular survival [15]. These findings indicate that ESPs have dual roles in parasitic infections: they may assist in parasite clearance or be exploited by parasites for immune evasion and long-term survival.
Recent studies further suggest that parasites might regulate the interaction between PAMPs and the NLRP3 inflammasome to promote immune evasion. For instance, Leishmania can evade the host’s immune response by targeting NLRP3 inflammasome activation, leading to the suppression of the inflammatory response [16,17]. During chronic infection, Toxoplasma gondii products can activate the NLRP3 inflammasome. However, the absence of the inflammasome or any of its components leads to enhanced parasitic growth, suggesting that NLRP3 activation plays a protective role in controlling the infection [18]. However, whether similar mechanisms are involved in C. sinensis infections remains unclear. Our previous studies identified the nitrite oxide synthase-interacting protein from C. sinensis (CsNOSIP) as being predominantly localized in the gut, eggs, and vitelline glands of C. sinensis adults. It showed that the CsNOSIP stimulates macrophages to produce a reactive oxygen species (ROS) and NO, and NO/nitrite oxide synthase (iNOS) has been shown to suppress NLRP3 inflammasome activation through multiple pathways in vitro. Thus, it remains to be determined whether the CsNOSIP facilitates chronic infection by regulating NLRP3 inflammasome activity and the specific mechanisms involved.
The present study aims to investigate the activation of the NLRP3 inflammasome during a C. sinensis infection, providing a theoretical basis for understanding the interactions between parasites and their hosts and the mechanisms maintaining long-term homeostasis.
2. Materials and Methods
2.1. Expression and Purification of Recombinant CsNOSIP
A purified CsNOSIP protein and CsESPs were produced, as previously described [19,20]. Briefly, pET-30a (+)-CsNOSIP was constructed and transformed into Escherichia coli (DE3) (Promega, Shanghai, China). Induced by isopropyl-β-D-thiogalactopyranoside (IPTG), the protein was expressed at 30 °C for 3 h in Luria–Bertani medium and purified with a His Bind Purification kit (Novagen, Darmstadt, Germany). The purified CsNOSIP was analyzed by 12% SDS-PAGE, stained with Coomassie brilliant blue G-250, and the final recombinant soluble protein concentration was detected by the bicinchoninic acid (BCA) protein assay kit (Cwbiotech, Beijing, China). The protein was treated by AffinityPak™ Detoxi-Gel™ Endotoxin Removing Gel (Thermo Fisher Scientific, Waltham, MA, USA) to remove the endotoxin. Then, the concentration of CsNOSIP was determined by a BCA protein assay kit again, to avoid the loss of protein during the process of endotoxin elimination.
2.2. Culture and Treatment of Macrophage Cell Line RAW264.7 Cells
RAW264.7 cells are a mouse macrophage-like cell line that is widely used to study immune function, inflammation, and cancer biology. RAW264.7 cells were purchased from Experimental Animal Center of Sun Yat-sen University. RAW264.7 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Carlsbad, CA, USA), which was supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C in an atmosphere of 5% CO2. Cells were seeded at 5 × 105 cells/well in a 12-well plate or 1 × 106 in a 6-well plate and firstly, primed with LPS from Escherichia coli (055: B5) (Sigma-Alrich, Steinheim, Germany) (500 ng/mL) for 3 h. To investigate the effects of CsESPs and CsNOSIP, LPS-primed RAW264.7 were co-incubated with CsESPs 10 μg/mL for 12 h or/and different concentrations of CsNOSIP (5, 10, 20 μg/mL) for 6 or 12 h, in the presence or absence of 100nM N-Nitro-L-Arginine Methyl Ester (L-NAME), an inhibitor of iNOS (Sigma-Alrich, Steinheim, Germany). Finally, the NLRP3 inflammasome activator ATP (Sigma-Alrich, Steinheim, Germany) (5 mM) was added for 1 h before the cells were harvested.
2.3. Reverse Transcription and Quantitative Real-Time PCR
Total RNA was isolated from cells by using TRIzol reagent according to the manufacturer’s protocol, and cDNA was synthesized using a TransScript All-in-One First-Strand cDNA Synthesis SuperMix for qPCR Kit (TransGen Biotech, Beijing, China). Quantitative real-time PCR were performed to evaluate the transcriptional levels of NLPR3 inflammasome-related genes (NLPR3, ASC, caspase-1, IL-1β, etc.). mRNA levels of the target proteins were calculated against that of β-actin, according to the 2−ΔΔCt method. All experiments were performed independently at least three times and were replicated by independent researchers. Primer sequences are listed in Table 1.

Table 1.
Primer sequences for quantitative real-time PCR.
2.4. ELISA
IL-1β levels in supernatants of cell cultures were measured according to the manufacturer’s protocol in ELISA kits (Elabscience, Wuhan, China). The concentration of IL-1β was determined using a standard curve according to the kit instructions. All experiments were performed in triplicate wells and repeated in at least three independent experiments.
For immunofluorescence staining, RAW264.7 cells cultured on slides were fixed with 4% paraformaldehyde for 20 min at room temperature (RT), then washed with PBS and permeabilized in PBS containing 0.2% Triton X-100 for 5 min. The slides were blocked with PBS containing 5% bovine serum albumin for 30 min and incubated with a primary monoclonal antibody against NLRP3 (Cell signaling technology, Boston, MA, USA), caspase-1 p10 (M-20) (Santa Cruz, Santa Cruz, CA, USA) or ASC (ABclonal, Boston, MA, USA) overnight at 4 °C. The slides were then incubated with a Cy3-conjugated or/and FITC conjugated secondary antibody (Proteintech, Rosemont, IL, USA) for 1 h in darkness, and finally stained with 4′,6-diamidino-2-phenylindole (DAPI, SouthernBiotech, Homewood, AL, USA). Images were obtained using a microscope (Olympus BX63) and cell Sens Dimension (Version1.8) software (Olympus, Tokyo, Japan).
2.5. Western Blot Analysis
Protein lysates for immunoblotting from treated cells, as described above, were prepared with RIPA buffer (Cwbiotech, Beijing, China) supplemented with protease inhibitors (Beyotime, Shanghai, China). Protein lysates for Western blotting from treated cells as described above were prepared with RIPA buffer (Cwbiotech, Beijing, China) supplemented with phosphatase inhibitors (Beyotime, Shanghai, China). Cells lysates were denatured in loading buffer containing SDS and 100 mM DTT and boiled for 5 min. Equal amounts of total protein (40 µg) were loaded onto SDS-PAGE-separated gels and then transferred to 0.22 µM PVDF membranes (Millipore, Burlington, MA, USA), followed by incubation in blocking buffer (25 mM Tris, pH 7.4, 0.15 M NaCl, 0.1% Tween-20, 5% nonfat milk) for 1 h at RT. The membranes were incubated with primary antibodies against NLRP3 (ABclonal, Boston, MA, USA), CASP1 (ABclonal, Boston, MA, USA), iNOS (DB Biotech, Košice, Slovak), Phospho-NF-κB p65 (Ser536) (Cell signaling technology, Boston, MA, USA), or GADPH (Ray antibody Biotech, Beijing, China) at 4 °C overnight. After washing, the membranes were incubated with HRP-conjugated secondary antibodies for 1 h on RT. Proteins were visualized with an ECLkit (Advansta, San Jose, CA, USA). GADPH was used as a loading control. All experiments were performed independently at least three times and were replicated by independent researchers. The intensity of Western blotting bands was analyzed with Image J software (NIH, Bethesda, MD, USA, 2022).
2.6. Measurement of NO Level
The Griess reactions were used to detect the accumulation of NO in the culture medium. RAW264.7 cells were treated as previously described and the culture medium was collected to evaluate the NO according to the protocol of the Nitric Oxide Detection Kit (Beyotime, Shanghai, China).
2.7. Statistical Analysis
All data are presented as the mean ± SEM. Data were analyzed by performing independent Student’s t-tests and ANOVA, followed by a Bonferroni multiple comparisons test using SPSS software for Windows (version 16.0; SPSS, Inc., Chicago, IL, USA). p < 0.05 was considered statistically significant.
3. Results
3.1. CsESPs Facilitated Activation of NLRP3 Inflammasome in RAW264.7 Cells
IL-1β is a key product of NLRP3 inflammasome activation and plays an important role during numerous inflammatory processes. Compared with the positive group, 10 μg/mL of CsESPs stimulation increased IL-1β transcription levels (p < 0.01, Figure 1a) in 500 ng/mL lipopolysaccharide (LPS)-primed RAW264.7 cells for 12 h, followed by treatment with 5 mM ATP (an activator of the NLRP3 inflammasome). Consistent with its facilitation effect on IL-1β, CsESPs could facilitate the immunofluorescence intensity of NLRP3 inflammasome-related components—NLRP3 and caspase-1, but not ASC (Figure 1b).
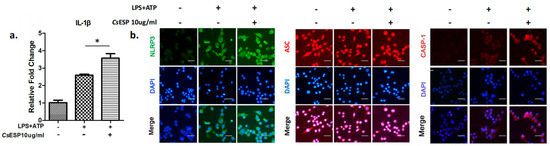
Figure 1.
The activation of NLRP3 inflammasome in the treated cells. A total of 500 ng/mL of LPS-primed RAW264.7 cells were treated with 10 μg/mL CsESPs for 12 h, followed by treatment with 5 mM ATP for 1 h. (a) Quantitative real-time PCR analysis of IL-1β levels. Data are shown as mean ± SEM. * p < 0.01. (b) Immunofluorescence staining analysis. NLRP3 (green), ASC (red) and Caspase-1 (red). Nuclei were stained with DAPI (blue). The images were magnified at 200× (scale bar: 25 μm).
3.2. CsNOSIP Suppressed NLRP3 Inflammasome Mediated IL-1β Secretion in LPS-Primed RAW264.7 via NLRP3- and Caspase-1-Dependent Manner
CsNOSIP dramatically inhibited transcription levels of IL-1β in LPS-primed RAW264.7 cells in a dose-dependent manner, whether treating cells for 6 h (p < 0.01, Figure 2a) or 12 h (p < 0.001, Figure 2a). Consistent with the results at the mRNA levels, the expression of IL-1β in the culture supernatants (p < 0.0001, Figure 2b) and the total cell lysates (Figure 2c) were also decreased. However, CsNOSIP only suppressed the transcriptional levels of NLRP3 in a dose-dependent manner at the 6 h (p < 0.001) and 12 h (p < 0.001) treating points, but this had no effect on those of caspase-1 and ASC (Figure 2d–f). The protein levels of NLRP3 in stimulated RAW264.7 cells were consistent with Figure 2d (Figure 2g). However, although CsNOSIP had no inhibitory effect on caspase-1, a high concentration (20 μg/mL) of CsNOSIP could dramatically suppress the expression of cleaved caspase-1 (p20) (Figure 2h), a regulating component of inflammasome to facilitate the secretion of IL-1β.
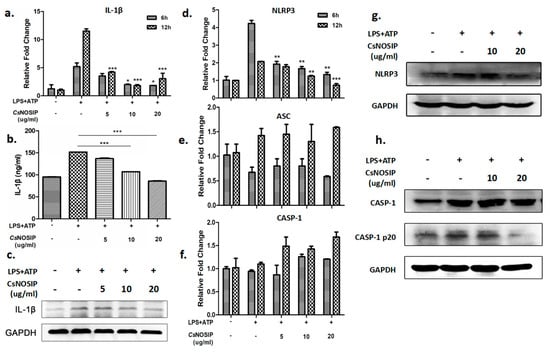
Figure 2.
CsNOSI-inhibited NLRP3 inflammasome mediated caspase-1 activation and IL-1β secretion. A total of 500 ng/mL of LPS-primed RAW264.7 cells were treated with different concentration of CsNOSIP (5, 10, 20 μg/mL) for 6 h and 12 h, followed by treatment with 5 mM ATP for 1 h. (a) Quantitative real-time PCR analysis of IL-1β expression. ELISA detection (b) and Western blotting (c) analysis of IL-1β expression in culture supernatant of treated cells for 12 h. (d–f) Quantitative real-time PCR analysis of mRNA levels of NLRP3 inflammasome-related genes (NLRP3, ASC, caspase-1) in the treated cells. Western blotting analysis of NLRP3 (g), caspase-1 and caspase-1 p20 (h) in 12 h treated cells. In panel (a,b,d–f), the levels were, respectively, compared to those in LPS+ATP-alone-treated positive control cells. Data are shown as mean ± SEM. In panel (d,g,h), GAPDH was a loading control. * p < 0.05, ** p < 0.01, *** p < 0.001.
3.3. CsNOSIP Reversed the Facilitation Effect of NLRP3 Inflammasome Activation Induced by CsESPs in LPS Primed-RAW264.7
The immunofluorescence staining results showed that, when compared with CsNOSIP treatment alone, the immunofluorescence intensity of NLRP3 and caspase-1 dramatically decreased in LPS-primed RAW264.7 cells treated with CsESPs and CsNOSIP in response to ATP treatment (Figure 3).
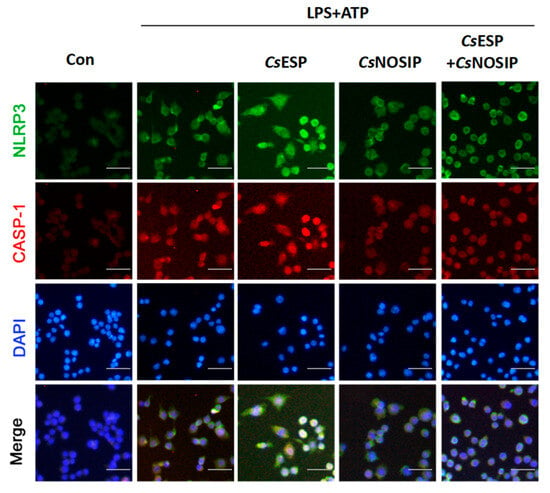
Figure 3.
CsNOSIP dampened the NLRP3 inflammasome activation induced by CsESPs in RAW264.7 cell. Immunofluorescence staining analysis of NLRP3 (green) and total caspase-1 (red) expression in 500 ng/mL LPS primed-RAW264.7 cells treated with 10 μg/mL CsESPs or/and 20 μg/mL CsNOSIP for 12 h followed by treatment with 5mM ATP for 1 h. Nuclei were stained with DAPI (blue). The images were magnified at 200× (scale bar: 25 μm).
3.4. Cellular Mechanisms Involved in CsNOSI-Mediated Reverse Effect on NLRP3 Inflammasome Activation Induced by CsESPs
Upon activation with LPS and ATP, the NO secretion was significantly increased when the RAW264.7 cells were additionally co-cultured with CsNOSIP for 6 h (p < 0.001, Figure 4a) and 12 h (p < 0.001, Figure 4a). The effect was accentuated with increasing doses of CsNOSIP and longer stimulation time. CsNOSIP stimulation facilitated the expression of iNOS in LPS-primed RAW264.7 cells in a concentration-dependent manner at different time points—6 h (p < 0.001) and 12 h (p < 0.001) (Figure 4b,c)—in both transcription and translation levels. The CsNOSIP prevention effect was reversed in the presence of L-NAME, an inhibitor of iNOS (Figure 4e). Furthermore, NF-κB p65 expression was suppressed in the stimulated RAW264.7 cells in a dose-dependent manner (Figure 4d).
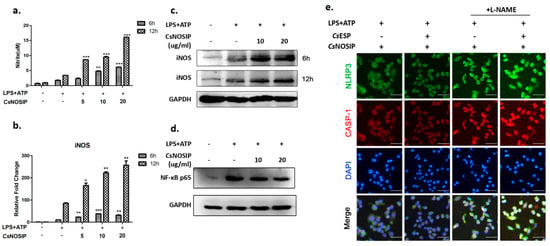
Figure 4.
The expression of iNOS, NO and NF-κB in treated cells. A total of 500 ng/mL LPS primed-RAW264.7 cells were treated with different concentrations of CsNOSIP (5, 10, 20 μg/mL) for 6 h and 12 h, followed by treatment with 5mM ATP for 1 h. (a) Griess reaction analysis of NO expression in culture supernatant of the cells. Data are shown as mean ± SEM. Quantitative real-time PCR (b) and Western blotting (c) analysis of iNOS expression levels were compared to that in positive control. Data are shown as mean ± SEM. (d) Western blotting analysis of NF-κB p65 expression. The GAPDH was a loading control for Western blotting. (e) Immunofluorescence staining analysis of NLRP3 (green) and total caspase-1 expression in 500 ng/mL LPS-primed RAW264.7 cells treated with CsESPs (10 μg/mL) or/and CsNOSIP (20 μg/mL) for 12 h, followed by treatment with 5 mM ATP for 1 h in the presence or absence of 100 uM L-NAME. Nuclei were stained with DAPI (blue). The images were magnified at 200× (scale bar: 25 μm). * p < 0.05, ** p < 0.01, *** p < 0.001.
4. Discussion
The inflammasome is a type of intracellular multi-protein complex and an important component of the innate immune system [21]. Among them, NLRP3 inflammasome is the most extensively studied, and plays a central role in regulating the inflammatory response during the host’s defense against various pathogens [17]. In the host’s immune response to infection, inflammation must be balanced within an appropriate range: excessive inflammation can lead to cell and tissue damage in the host, while insufficient inflammation may provide favorable conditions for pathogen survival and spread.
As an important secretion of the parasite that directly interacts with the host, ESP plays a crucial role in the interaction between the parasite and the host. CsESPs is also involved in liver damage and the development of cholangiocarcinoma (CCA) triggered by C. sinensis in the bile ducts [22]. In the present study, CsESPs could induce the activation of the NLRP3 inflammasome in vitro (Figure 1). In the endemic area, chronic infections of C. sinensis are more common. This suggests that CsESPs might regulate the host’s inflammatory response by activating the NLRP3 inflammasome during the chronic infection.
Activation of the NLRP3 inflammasome triggered by PAMPs from pathogens plays an important role in the immune defense against parasitic infections [23]. Related reports have indicated that in some parasitic infections, activation of the NLRP3 inflammasome may have dual effects. For example, in Trypanosoma cruzi [24] and Leishmania [25] infections, activation of the NLRP3 inflammasome helps control the parasite load and reduce tissue damage in the host. However, in malaria, excessive activation of the NLRP3 inflammasome may exacerbate disease severity and decrease the likelihood of host survival [26,27]. Moreover, in Leishmania infections, NLRP3 inflammasome activation not only promotes the maturation and secretion of inflammation-related factors, such as IL-1β and IL-18, but may also enhance local inflammation through the secretion of interferon-γ (IFN-γ), thus helping to clear the infection [28]. However, excessive activation of the inflammasome can also lead to tissue damage and inflammatory diseases. Studies have found that overactivation of NLRP3 limits the spread of Toxoplasma gondii within the host, but by regulating this pathway, T. gondii can persist long term within the host while avoiding fatal pathological responses [29,30].
These findings suggest that the role of the NLRP3 inflammasome in parasitic infections is complex. On the one hand, it plays a protective role in the host’s immune response; on the other hand, dysregulated activation may suppress immune clearance and delay the host’s response to the parasite. C. sinensis adults can keep living in the host’s body long term: even for 20–30 years. CsESPs activated the NLRP3 inflammasome, raising the possibility that there may be molecules that could maintain a stable parasitic environment to balance the immune response activated by NLRP3 inflammasome.
CsESPs consist of various substances secreted or excreted at different stages of the parasite’s life cycle, including enzymes, toxins, and other functional molecules with different functions during the interaction between the worm and the host [20]. Among them, calcium-binding protein [31] and alkaline phosphatase [32] enhance the function of the host’s immune cells, promoting the immune response and helping to eliminate the parasite. In contrast, trypsin, elastase, and matrix metalloproteinases [20,33] degrade the host’s tissues, assisting the parasite in migrating, parasitizing, and developing within the host.
Research data suggest that an increase in NO generation stabilized intracellular potassium levels and inhibits the activity of NLRP3 [34]. Typically, a high expression of iNOS is accompanied by the release of large amounts of NO [35,36]. Our previous studies have confirmed that CsNOSIP could stimulate macrophages to produce ROS and NO in vitro [37]. CsNOSIP predominantly localized in the gut and vitelline glands of C. sinensis adults [37]. CsNOSIP could appear in CsESPs and act as a molecule playing a role in the interaction between the worm and host, accompanied by renewal or degradation of the worm. Thus, we proposed that CsNOSIP might also have an inhibitory effect on NLRP3 activation. The activation of the NLRP3 inflammasome depends on the oligomerization of ASC and activation of Caspase-1, with IL-1β being a hallmark product of NLRP3 inflammasome activation, whose secretion relies on Caspase-1 cleavage [38]. Our data showed that CsSONIP significantly reduced the expression of ASC, IL-1β, and Caspase-1 when stimulating macrophages in vitro, with a concentration-dependent effect (Figure 2), suggesting that CsSONIP interferes with the formation of the NLRP3 inflammasome.
Since C. sinensis may persist in the host for 20–30 years, it raises the question of whether the interference of CsSONIP with NLRP3 inflammasome activation contributes to the long-term chronic infection. We found that when CsSONIP and CsESPs were co-stimulated in macrophages, CsSONIP attenuated the activation effect of CsESPs on the inflammasome (Figure 3). It suggested that CsSONIP might play a negative feedback regulatory role, preventing excessive activation of the inflammasome. We hypothesized that during a C. sinensis infection, when inflammasome activation reached a certain threshold, CsSONIP might suppress NLRP3 activation to protect the parasite from the host’s immune responses, ensuring its long-term chronic infection.
Moreover, similar interactions have been observed in other parasitic infections. For instance, Toxoplasma gondii secretes effector molecules such as GRA15, which activate NF-κB to upregulate NLRP3 expression [30], but ROP16 limits excessive activation of NLRP3 by inhibiting ROS generation [29,39]. Eggs or adult worm’s antigens from Schistosoma or adult worms secrete glycoproteins that trigger the initial activation of NLRP3 inflammasome through the toll-like receptor four and other pathways, while also limiting the complete activation of NLRP3 by inhibiting potassium ion efflux and ROS generation [40]. These findings suggest that during parasitic infections, such interactions can trigger mild inflammation that helps maintain host survival, while avoiding excessive inflammation that could lead to pathogen clearance.
The mechanism by which CsSONIP weakened the activation of the NLRP3 inflammasome in a C. sinensis infection deserves further investigation. The activation of the NLRP3 inflammasome typically requires initial activation of the NF-κB signaling pathway [41], and NO has been shown to inhibit NF-κB activity through multiple mechanisms, including the suppression of IκB kinase activation [42]. Research also shows that inhibition of the NF-κB pathway reduces the expression levels of NLRP3 and its downstream effectors, thereby reducing the inflammatory response [43]. Our data also indicate that CsSONIP significantly inhibited the expression of NF-κB in macrophages, suggesting that CsSONIP might suppress NLRP3 activation by inhibiting NF-κB activity (Figure 4). This further implied that C. sinensis might utilize this strategy to suppress the host’s immune killing effects, establish chronic infection, and maintain long-term survival.
In summary, our findings indicate that CsESP, as a major pathogenic component, promotes NLRP3 inflammasome activation and IL-1β secretion in LPS-primed RAW264.7 cells. We also uncovered that CsNOSIP could suppress the NLRP3 inflammasome activation and IL-1β secretion, which was probably through upregulating the expression of iNOS and NO and downregulating the NF-κB signaling pathway. All these results indicated that CsNOSIP was attributable, at least in part, to the inhibition of inflammatory responses induced by a C. sinensis infection, which in turn, conferred the chronicity of C. sinensis infections. However, further studies should be investigated in vivo models to validate our in vitro data.
Thus, the opposing effects of CsESPs and CsSONIP on the NLRP3 inflammasome during a C. sinensis infection help the parasite persist long-term within the host, but it was well worth investigating the involved mechanisms of how the worm regulates or renews the generation of CsSONIP in CsESPs, and then initiates a system of checks and balances related to activating the NLRP3 inflammasome. This complex immune regulation mechanism not only helps the pathogen survive but may also provide potential targets for parasitic infection research and the design of immunotherapies.
Author Contributions
Conceptualization, X.Y. (Xuran Yang), H.D., X.Y. (Xinbing Yu), Y.H.; Methodology, X.Y. (Xuran Yang), X.K., X.L.; Investigation, X.Y. (Xuran Yang), X.K., X.C., Q.L.; Data curation, H.D., X.K., X.L.; Formal analysis, X.Y. (Xuran Yang), H.D., X.K., X.L., X.C., Q.L.; Writing—original draft, X.Y. (Xuran Yang), H.D., X.K.; Writing—review & editing, W.L., Y.H.; Funding acquisition, X.Y. (Xinbing Yu), W.L., Y.H.; Supervision, X.L., X.Y. (Xinbing Yu), Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the National Key Research and Development Program of China (No. 2021YFC2300800, 2021YFC2300803), and the Basic and Applied Basic Research Foundation of Guangdong Province (2023A1515140139, 2019A1515110300).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Control of foodborne trematode infections: Report of a WHO study group. World Health Organ. Tech. Rep. Ser. 1995, 849, 1–157. [Google Scholar]
- Xiao, H.-Y.; Chai, J.-Y.; Fang, Y.-Y.; Lai, Y.-S. The spatial-temporal risk profiling of Clonorchis sinensis infection over 50 years implies the effectiveness of control programs in South Korea: A geostatistical modeling study. Lancet Reg. Health–West. Pac. 2023, 33, 100697. [Google Scholar] [CrossRef]
- Qian, M.-B.; Keiser, J.; Utzinger, J.; Zhou, X.-N. Clonorchiasis and opisthorchiasis: Epidemiology, transmission, clinical features, morbidity, diagnosis, treatment, and control. Clin. Microbiol. Rev. 2024, 37, e0000923. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Tian, Y.; Chen, W.; Wang, X.; Li, X.; Mao, Q.; Sun, J.; Li, R.; Xu, Y.; Liang, C.; et al. The biochemical and immunological characterization of two serpins from Clonorchis sinensis. Mol. Biol. Rep. 2013, 40, 3977–3985. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.B.; Chen, Y.D.; Fang, Y.Y.; Xu, L.Q.; Zhu, T.J.; Tan, T.; Zhou, C.H.; Wang, G.F.; Jia, T.W.; Yang, G.J.; et al. Disability weight of Clonorchis sinensis infection: Captured from community study and model simulation. PLoS Negl. Trop. Dis. 2011, 5, e1377. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Mazmanian, S.K. Innate immune recognition of the microbiota promotes host-microbial symbiosis. Nat. Immunol. 2013, 14, 668–675. [Google Scholar] [CrossRef]
- Tsubokawa, D. Immunomodulators secreted from parasitic helminths act on pattern recognition receptors. Front. Parasitol. 2023, 1, 1091596. [Google Scholar] [CrossRef]
- Singh, A.P.; Buscaglia, C.A.; Wang, Q.; Levay, A.; Nussenzweig, D.R.; Walker, J.R.; Winzeler, E.A.; Fujii, H.; Fontoura, B.M.; Nussenzweig, V. Plasmodium circumsporozoite protein promotes the development of the liver stages of the parasite. Cell 2007, 131, 492–504. [Google Scholar] [CrossRef]
- Singer, M.; Kanatani, S.; Castillo, S.G.; Frischknecht, F.; Sinnis, P. The Plasmodium circumsporozoite protein. Trends Parasitol. 2024, 40, 1124–1134. [Google Scholar] [CrossRef]
- Silverman, J.M.; Clos, J.; Horakova, E.; Wang, A.Y.; Wiesgigl, M.; Kelly, I.; Lynn, M.A.; McMaster, W.R.; Foster, L.J.; Levings, M.K.; et al. Leishmania exosomes modulate innate and adaptive immune responses through effects on monocytes and dendritic cells. J. Immunol. 2010, 185, 5011–5022. [Google Scholar] [CrossRef]
- Rodrigues, M.M.; Oliveira, A.C.; Bellio, M. The Immune Response to Trypanosoma cruzi: Role of Toll-Like Receptors and Perspectives for Vaccine Development. J. Parasitol. Res. 2012, 2012, 507874. [Google Scholar] [CrossRef]
- Schramm, G.; Haas, H. Th2 immune response against Schistosoma mansoni infection. Microbes Infect. 2010, 12, 881–888. [Google Scholar] [CrossRef]
- Houlder, E.L.; Stam, K.A.; Koopman, J.P.R.; König, M.H.; Langenberg, M.C.C.; Hoogerwerf, M.A.; Niewold, P.; Sonnet, F.; Janse, J.J.; Partal, M.C.; et al. Early symptom-associated inflammatory responses shift to type 2 responses in controlled human schistosome infection. Sci. Immunol. 2024, 9, eadl1965. [Google Scholar] [CrossRef]
- van Die, I.; van Vliet, S.J.; Nyame, A.K.; Cummings, R.D.; Bank, C.M.; Appelmelk, B.; Geijtenbeek, T.B.; van Kooyk, Y. The dendritic cell-specific C-type lectin DC-SIGN is a receptor for Schistosoma mansoni egg antigens and recognizes the glycan antigen Lewis x. Glycobiology 2003, 13, 471–478. [Google Scholar] [CrossRef]
- Mazumder, S.; Sinha, A.; Ghosh, S.; Sharma, G.C.; Prusty, B.M.; Manna, D.; Pal, D.; Pal, C.; Dasgupta, S. Leishmania LPG interacts with LRR5/LRR6 of macrophage TLR4 for parasite invasion and impairs the macrophage functions. Pathog. Dis. 2023, 81, ftad019. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, R.V.H.; Lima-Júnior, D.S.; de Oliveira, C.V.; Zamboni, D.S. Endosymbiotic RNA virus inhibits. iScience 2021, 24, 102004. [Google Scholar] [CrossRef] [PubMed]
- Alonaizan, R. Molecular regulation of NLRP3 inflammasome activation during parasitic infection. Biosci. Rep. 2024, 44, BSR20231918. [Google Scholar] [CrossRef] [PubMed]
- Bergsbaken, T.; Fink, S.L.; Cookson, B.T. Pyroptosis: Host cell death and inflammation. Nat. Rev. Microbiol. 2009, 7, 99–109. [Google Scholar] [CrossRef]
- Bian, M.; Li, S.; Wang, X.; Xu, Y.; Chen, W.; Zhou, C.; Chen, X.; He, L.; Xu, J.; Liang, C.; et al. Identification, immunolocalization, and immunological characterization of nitric oxide synthase-interacting protein from Clonorchis sinensis. Parasitol. Res. 2014, 113, 1749–1757. [Google Scholar] [CrossRef]
- Zheng, M.; Hu, K.; Liu, W.; Li, H.; Chen, J.; Yu, X. Proteomic analysis of different period excretory secretory products from Clonorchis sinensis adult worms: Molecular characterization, immunolocalization, and serological reactivity of two excretory secretory antigens-methionine aminopeptidase 2 and acid phosphatase. Parasitol. Res. 2013, 112, 1287–1297. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. 2020, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.L.; Huang, Y.; Yu, X.B. Current status and perspectives of Clonorchis sinensis and clonorchiasis: Epidemiology, pathogenesis, omics, prevention and control. Infect. Dis. Poverty 2016, 5, 71. [Google Scholar] [CrossRef]
- Celias, D.P.; Motrán, C.C.; Cervi, L. Helminths Turning on the NLRP3 Inflammasome: Pros and Cons. Trends Parasitol. 2020, 36, 87–90. [Google Scholar] [CrossRef]
- Paroli, A.F.; Gonzalez, P.V.; Díaz-Luján, C.; Onofrio, L.I.; Arocena, A.; Cano, R.C.; Carrera-Silva, E.A.; Gea, S. NLRP3 Inflammasome and Caspase-1/11 Pathway Orchestrate Different Outcomes in the Host Protection Against Trypanosoma cruzi Acute Infection. Front. Immunol. 2018, 9, 913. [Google Scholar] [CrossRef]
- Lima-Junior, D.S.; Costa, D.L.; Carregaro, V.; Cunha, L.D.; Silva, A.L.; Mineo, T.W.; Gutierrez, F.R.; Bellio, M.; Bortoluci, K.R.; Flavell, R.A.; et al. Inflammasome-derived IL-1β production induces nitric oxide-mediated resistance to Leishmania. Nat. Med. 2013, 19, 909–915. [Google Scholar] [CrossRef]
- Dunst, J.; Kamena, F.; Matuschewski, K. Cytokines and Chemokines in Cerebral Malaria Pathogenesis. Front. Cell Infect. Microbiol. 2017, 7, 324. [Google Scholar] [CrossRef]
- Strangward, P.; Haley, M.J.; Albornoz, M.G.; Barrington, J.; Shaw, T.; Dookie, R.; Zeef, L.; Baker, S.M.; Winter, E.; Tzeng, T.C.; et al. Targeting the IL33-NLRP3 axis improves therapy for experimental cerebral malaria. Proc. Natl. Acad. Sci. USA 2018, 115, 7404–7409. [Google Scholar] [CrossRef]
- Andargie, T.E.; Ejara, E.D. Pro-and anti-inflammatory cytokines in visceral leishmaniasis. J. Cell Sci. Ther. 2015, 6, 1. [Google Scholar] [CrossRef]
- Chen, L.; Christian, D.A.; Kochanowsky, J.A.; Phan, A.T.; Clark, J.T.; Wang, S.; Berry, C.; Oh, J.; Chen, X.; Roos, D.S.; et al. The Toxoplasma gondii virulence factor ROP16 acts in cis and trans, and suppresses T cell responses. J. Exp. Med. 2020, 217, e20181757. [Google Scholar] [CrossRef]
- Wang, P.; Li, S.; Zhao, Y.; Zhang, B.; Li, Y.; Liu, S.; Du, H.; Cao, L.; Ou, M.; Ye, X.; et al. The GRA15 protein from Toxoplasma gondii enhances host defense responses by activating the interferon stimulator STING. J. Biol. Chem. 2019, 294, 16494–16508. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.; Sun, H.; Wu, Y.; Gong, Y.; Tang, Z.; Meng, F.; He, L.; Yu, X.; Huang, Y.; Li, X. In vivo and in vitro studies using Clonorchis sinensis adult-derived total protein (CsTP) on cellular function and inflammatory effect in mouse and cell model. Parasitol. Res. 2020, 119, 1641–1652. [Google Scholar] [CrossRef]
- Qiu, Y.Y.; Chang, Q.C.; Gao, J.F.; Bao, M.J.; Luo, H.T.; Song, J.H.; Hong, S.J.; Mao, R.F.; Sun, Y.Y.; Chen, Y.Y.; et al. Multiple biochemical indices and metabolomics of Clonorchis sinensis provide a novel interpretation of biomarkers. Parasit. Vectors 2022, 15, 172. [Google Scholar] [CrossRef]
- Zheng, M.; Hu, K.; Liu, W.; Hu, X.; Hu, F.; Huang, L.; Wang, P.; Hu, Y.; Huang, Y.; Li, W.; et al. Proteomic analysis of excretory secretory products from Clonorchis sinensis adult worms: Molecular characterization and serological reactivity of a excretory-secretory antigen-fructose-1,6-bisphosphatase. Parasitol. Res. 2011, 109, 737–744. [Google Scholar] [CrossRef]
- Tapia-Abellán, A.; Angosto-Bazarra, D.; Alarcón-Vila, C.; Baños, M.C.; Hafner-Bratkovič, I.; Oliva, B.; Pelegrín, P. Sensing low intracellular potassium by NLRP3 results in a stable open structure that promotes inflammasome activation. Sci. Adv. 2021, 7, eabf4468. [Google Scholar] [CrossRef]
- Mao, K.; Chen, S.; Chen, M.; Ma, Y.; Wang, Y.; Huang, B.; He, Z.; Zeng, Y.; Hu, Y.; Sun, S.; et al. Nitric oxide suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock. Cell Res. 2013, 23, 201–212. [Google Scholar] [CrossRef]
- Yoon, S.J.; Park, J.Y.; Choi, S.; Lee, J.B.; Jung, H.; Kim, T.D.; Yoon, S.R.; Choi, I.; Shim, S.; Park, Y.J. Ginsenoside Rg3 regulates S-nitrosylation of the NLRP3 inflammasome via suppression of iNOS. Biochem. Biophys. Res. Commun. 2015, 463, 1184–1189. [Google Scholar] [CrossRef]
- Bian, M.; Xu, Q.; Xu, Y.; Li, S.; Wang, X.; Sheng, J.; Wu, Z.; Huang, Y.; Yu, X. Investigation on oxidative stress of nitric oxide synthase interacting protein from Clonorchis sinensis. Parasitol. Res. 2016, 115, 77–83. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, W.; Zhou, R. NLRP3 inflammasome activation and cell death. Cell. Mol. Immunol. 2021, 18, 2114–2127. [Google Scholar] [CrossRef] [PubMed]
- Bando, H.; Lee, Y.; Sakaguchi, N.; Pradipta, A.; Ma, J.S.; Tanaka, S.; Cai, Y.; Liu, J.; Shen, J.; Nishikawa, Y.; et al. Inducible Nitric Oxide Synthase Is a Key Host Factor for Toxoplasma GRA15-Dependent Disruption of the Gamma Interferon-Induced Antiparasitic Human Response. mBio 2018, 9, e01738-18. [Google Scholar] [CrossRef] [PubMed]
- Sanches, R.C.O.; Souza, C.; Oliveira, S.C. Schistosoma antigens as activators of inflammasome pathway: From an unexpected stimulus to an intriguing role. Microbes Infect. 2020, 22, 534–539. [Google Scholar] [CrossRef]
- Zhong, Z.; Umemura, A.; Sanchez-Lopez, E.; Liang, S.; Shalapour, S.; Wong, J.; He, F.; Boassa, D.; Perkins, G.; Ali, S.R.; et al. NF-κB Restricts Inflammasome Activation via Elimination of Damaged Mitochondria. Cell 2016, 164, 896–910. [Google Scholar] [CrossRef] [PubMed]
- Zhen, D.; Xuan, T.Q.; Hu, B.; Bai, X.; Fu, D.N.; Wang, Y.; Wu, Y.; Yang, J.; Ma, Q. Pteryxin attenuates LPS-induced inflammatory responses and inhibits NLRP3 inflammasome activation in RAW264.7 cells. J. Ethnopharmacol. 2022, 284, 114753. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.F.; Ouyang, Z.J.; Feng, L.L.; Chen, G.; Guo, W.J.; Shen, Y.; Wu, X.D.; Sun, Y.; Xu, Q. Suppression of NF-κB signaling and NLRP3 inflammasome activation in macrophages is responsible for the amelioration of experimental murine colitis by the natural compound fraxinellone. Toxicol. Appl. Pharmacol. 2014, 281, 146–156. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).