Recent Developments on the Performance of Algal Bioreactors for CO2 Removal: Focusing on the Light Intensity and Photoperiods
Abstract
1. Introduction
2. The Role of Algae in CO2 Removal
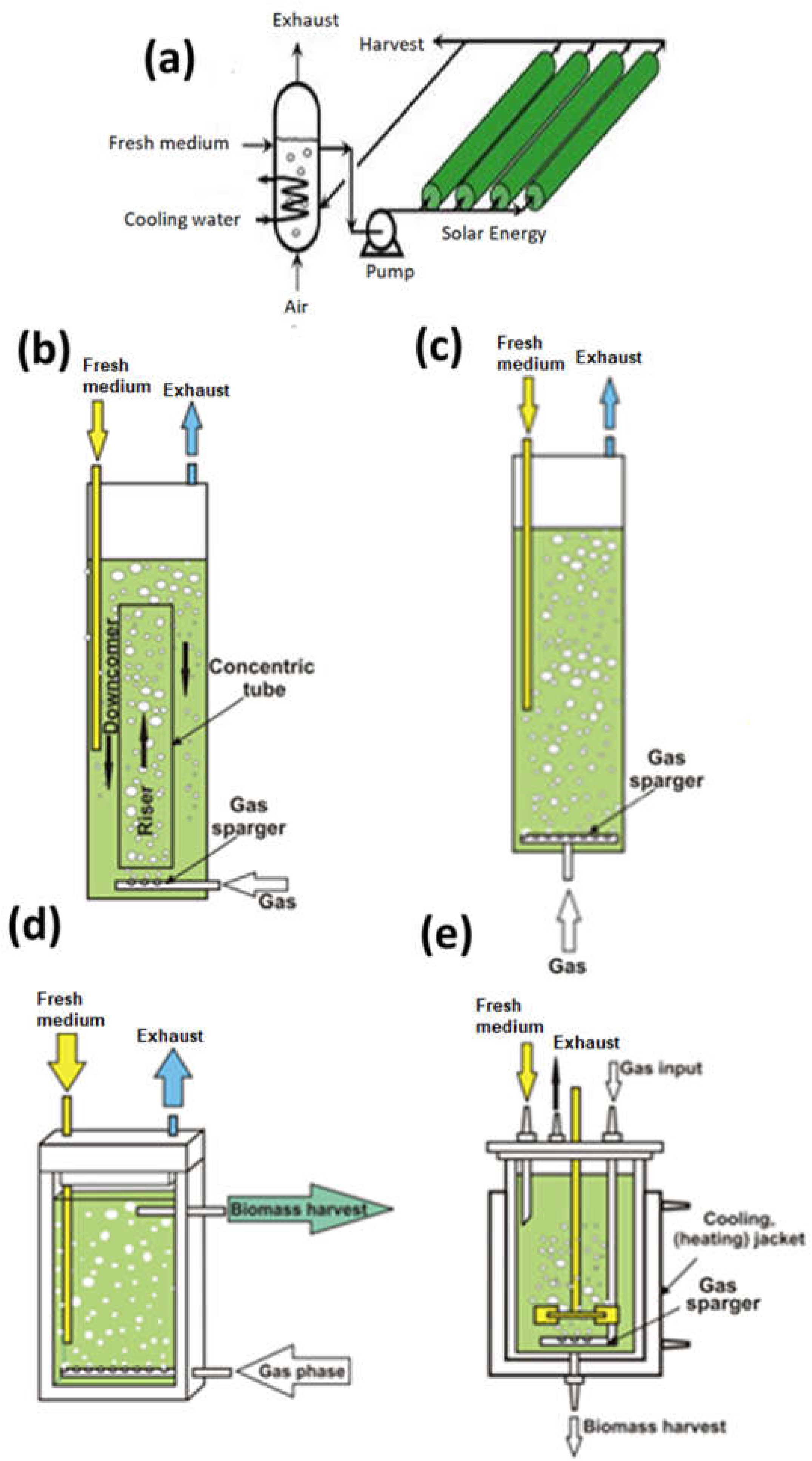
3. Types of Algae Bioreactors
3.1. Tubular Bioreactor
3.2. Vertical Airlift Reactor
3.3. Bubble Column Reactor
3.4. Flat Panel or Plate Reactor
3.5. Stirred Tank Reactor
3.6. Other Types of Bioreactors
| Type of Bioreactor | Algal Species | Invention Year & Country | Biomass Yield a (%) | CO2 Fixation Efficiency (%) | CO2 Removal Rates (gCO2 L−1 day−1) | References |
|---|---|---|---|---|---|---|
| Tubular | Spirulina sp. b | 2016 & Brazil | 550 | 0.197 ± 0.061 | da Rosa et al. [28] | |
| Bubble column | Parachlorella kessleri | 2022 & Canada | 0.49% | 0.211 | Beigbeder et al. [20] | |
| Vertical Column | Chlorophyta | 2021 & Philippines | 43.3 c 31.3 d | 15.6 | 3.91–22.04 3.55–15.66 | Alarde et al. [22] |
| Flat plate | Chlorella vulgaris | 2011 & China | 42 e | Feng et al. [38] | ||
| Hollow fiber membrane | Spirulina platensis | 2009 & USA | 63.9 | 80 | 1.44 | Kumar et al. [35] |
| Airlift Bioreactor | Chlorella vulgaris | 2020 & India | 0.32 ± 0.01 | Madhubalaji et al. [11] |
4. Factors Controlling CO2 Removal: Focusing on Light Intensity
4.1. Effect of Incident Light Intensity
| Type of Bioreactor | Algal Species | Light Source | Light Intensity (µmol m−2s−1) | Photoperiods | Biomass Productivity (mg L−1 day−1) | References |
|---|---|---|---|---|---|---|
| Photobioreactor (Volume: 10 L, Diameter: 22 cm, Height: 29 cm) | Arthospira (Spiralina) plantesis | White LED lamp | 635, 980, 1300, 2300 | Light-dark cycles (12 h:12 h) | 620 | Chaiklahan et al. [13] |
| Flat plat (Volume: 4 L, Length: 33 cm Diameter: 22 cm, Height: 10 cm) | Oscillatoria sp. | Fluorescent lamps | 160 µE m−2s−1 | Light-dark cycles (12 h:12 h) | 75 (Once supply frequency) 80 (Twice supply frequency) 92 (Thrice supply frequency) | Nithiya et al. [22] |
| Photobioreactor (Volume: 30 L, Diameter: 40 cm, Height: 50 cm) | Mixed culture | LED lights (red, blue, and white) | 110 | Light-dark cycles (16 h:8 h) | 47.75 (UQ-Lake A at 10% CO2) 50.04 (UQ-Lake F at 10% CO2) 54.87 (Strom water A at 10% CO2) 54.5 (Strom water F at 10% CO2) | Aslam et al. [44] |
| Sintered disk glass bubble column | S. Platensis | White LED light | Light-dark cycles (14 h:10 h) | 457.5 (Maximum with CO2 and NaOH for culture medium of Z(20)3) | Kumari et al. [45] | |
| In-vertical tubular photobioreactor (Volume: 2.5 L) | Chlorella vulgaris (BA 002) | High pressure sodium (HPS) light LED lights (red, blue, and white) | 13.5 µmol s−1 | Light-dark cycles (12 h:12 h, 18 h:6 h, 24 h:0 h) | 27.08 ± 7.80 (under optimum HPS) 24.21 ± 8.89 (under optimum LED) | Ratomski and Hawrot-Paw, [46] |
| Scenedesmus Abundans | White tube light | 27 (BG-11 media) 40.5 (Fogg’s media) 54 (CHU-13 media) | Light-dark cycles (16 h:8 h) | 47.4 ± 0.002 (BG-11 media) 70.23 ± 0.001 (Fogg’s media) 52.46 ± 0.002 (CHU-13 media) | Rai and Gupta, [47] | |
| Photobioreactor (Volume: 40 L) | Chlorella vulgaris | LED light | 0–80 | Geiman et al. [48] | ||
| Sequential Column (Working volume: 300 mL Diameter: 56 mm Height: 160 mm) | Chlorella PY-ZU1 | White lights and plant lights | 4500–6000 lux | 950 (at 10 min of Empty Bed Residence Time) | Cheng et al. [49] |
4.2. Photoinhibition and Incident Light Intensity
4.3. Effect of Light Provision Scheme
4.4. Effect of Photoperiod
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, W.; Wang, J.; Chen, P.; Ji, C.; Kang, Q.; Lu, B.; Li, K.; Liu, J.; Ruan, R. Bio-mitigation of carbon dioxide using microalgal systems: Advances and perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1163–1175. [Google Scholar] [CrossRef]
- Znad, H.; Naderi, G.; Ang, H.; Tade, M. CO2 Biomitigation and Biofuel Production Using Microalgae: Photobioreactors Developments and Future Directions, In Advances in Chemical Engineering; Nawaz, Z., Naveed, S., Eds.; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Vunjak-Novakovic, G.; Kim, Y.; Wu, X.; Berzin, A.I.; Merchuk, J.C. Air-Lift Bioreactors for Algal Growth on Flue Gas: Mathematical Modeling and Pilot-Plant Studies. Ind. Eng. Chem. Res. 2005, 44, 6154–6163. [Google Scholar] [CrossRef]
- Singh, A.; Nigam, P.S.; Murphy, J.D. Renewable fuels from algae: An answer to debatable land based fuels. Bioresour. Technol. 2011, 102, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Bahadar, A.; Khan, M.B. Progress in energy from microalgae: A review. Renew. Sustain. Energy Rev. 2013, 27, 128–148. [Google Scholar] [CrossRef]
- Jalilian, N.; Najafpour, G.D.; Khajouei, M. Macro and Micro Algae in Pollution Control and Biofuel Production—A Review. ChemBioEng Rev. 2020, 7, 18–33. [Google Scholar] [CrossRef]
- Ge, Y.; Liu, J.; Tian, G. Growth characteristics of Botryococcus braunii 765 under high CO2 concentration in photobioreactor. Bioresour. Technol. 2011, 102, 130–134. [Google Scholar] [CrossRef]
- Endo, H.; Sansawa, H.; Nakajima, K. Studies on Chlorella regularis, heterotrophic fast-growing strain II. Mixotrophic growth in relation to light intensity and acetate concentration. Plant Cell Physiol. 1977, 18, 199–205. [Google Scholar] [CrossRef]
- Ghayal, M.S.; Pandya, M.T. Microalgae biomass: A renewable source of energy. Energy Procedia 2013, 32, 242–250. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Yoo, C.; Jun, S.-Y.; Ahn, C.-Y.; Oh, H.-M. Comparison of several methods for effective lipid extraction. Bioresour. Technol. 2010, 101, S75–S77. [Google Scholar] [CrossRef]
- Madhubalaji, C.K.; Chandra, T.S.; Chauhan, V.S.; Sarada, R.; Mudliar, S.N. Chlorella vulgaris cultivation in airlift photobioreactor with transparent draft tube: Effect of hydrodynamics, light and carbon dioxide on biochemical profile particularly ω-6/ω-3 fatty acid ratio. J. Food Sci. Technol. 2019, 57, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Duan, D.; Chang, H.; Guo, C. Optimizing light distributions in a membrane photobioreactor via optical fibre to enhance CO2 photo biochemical conversion by a Scenedesmus obliquus biofilm. Ind. Eng. Chem. Res. 2020, 59, 21654–21662. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Srinorasing, T.; Attasat, S.; Nopharatana, A.; Bunnag, B. Enhanced biomass and phycocyanin production of Arthrospira (Spirulina) platensis by a cultivation management strategy: Light intensity and cell concentration. Bioresour. Technol. 2021, 343, 126077. [Google Scholar] [CrossRef] [PubMed]
- Al Haboubi, N. CO2 sequestration using a novel Belt Conveyor Reactor with rotating sieve trays compared with Airlift Bubble Column as photobioreactors. J. King Saud Univ.-Eng. Sci. 2021, in press. [Google Scholar] [CrossRef]
- Do, C.V.T.; Dinh, C.T.; Dang, M.T.; Tran, T.D.; Le, T.G. A novel flat-panel photobioreactor for simultaneous production of lutein and carbon sequestration by Chlorella sorokiniana. Bioresour. Technol. 2021, 345, 126552. [Google Scholar] [CrossRef]
- Alarde, H.P.; Bartolabac, K.J.; Acut, D. Development of an Arduino-based Photobioreactor (PBR) to investigate Algae growth rate and Carbon dioxide (CO2) removal efficiency. IAES Int. J. Robot. Autom. (IJRA) 2022, 11, 141–160. [Google Scholar] [CrossRef]
- Senatore, V.; Buonerba, A.; Zarra, T.; Oliva, G.; Belgiorno, V.; Boguniewicz-Zablocka, J.; Naddeo, V. Innovative membrane photobioreactor for sustainable CO2 capture and utilization. Chemosphere 2021, 273, 129682. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Li, Y.; Latimer, B.; Zhang, C.; Nair, S.S.; Hu, Z. Prediction of maximum algal productivity in membrane bioreactors with a light-dependent growth model. Sci. Total Environ. 2021, 753, 141922. [Google Scholar] [CrossRef]
- Ratomski, P.; Hawrot-Paw, M.; Koniuszy, A. Utilization of CO2 from Sodium Bicarbonate to Produce Chlorella Vulgaris Biomass in Tubular Photobioreactors for Biofuel Purposes. Sustainability 2021, 13, 9118. [Google Scholar] [CrossRef]
- Beigbeder, J.-B.; Lavoie, J.-M. Effect of photoperiods and CO2 concentration on the cultivation of carbohydrate-rich P. kessleri microalgae for the sustainable production of bioethanol. J. CO2 Util. 2022, 58, 101937. [Google Scholar] [CrossRef]
- Cui, J.; Purton, S.; Baganz, F. Characterization of a simple ‘hanging bag’ photobioreactor for low-cost cultivation of microalgae. J. Chem. Technol. Biotechnol. 2021, 97, 608–619. [Google Scholar] [CrossRef]
- Nithiya, E.M.; Tamilmani, J.; Vasumathi, K.K.; Premalatha, M. Improved CO2 fixation with Oscillatoria sp. in response to various supply frequencies of CO2 supply. J. CO2 Util. 2017, 18, 198–205. [Google Scholar] [CrossRef]
- Rinanti, A.; Dewi, K.; Kardena, E.; Astuti, D.I. Biotechnology Carbon Capture and Storage (CCS) by Mix-culture Green Microalgae to Enhancing Carbon Uptake Rate and Carbon Dioxide Removal Efficiency with Variation Aeration Rates in Closed System Photobioreactor. J. Teknol. 2014, 69. [Google Scholar] [CrossRef]
- Chae, S.; Hwang, E.; Shin, H. Single cell protein production of Euglena gracilis and carbon dioxide fixation in an innovative photo-bioreactor. Bioresour. Technol. 2006, 97, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Algae Market Potentially Worth $320 Billion Draws Honda, Eneos. 2022. Available online: https://www.bloomberg.com/news/articles/2022-01-23/algae-market-potentially-worth-320-billion-draws-honda-eneos (accessed on 31 October 2022).
- Patel, A.; Matsakas, L.; Rova, U.; Christakopoulos, P. A perspective on biotechnological applications of thermophilic microalgae and cyanobacteria. Bioresour. Technol. 2019, 278, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, H.; Chu, H.; Yu, S. A batch study on the bio-fixation of carbon dioxide in the absorbed solution from a chemical wet scrubber by hot spring and marine algae. Chemosphere 2007, 66, 878–886. [Google Scholar] [CrossRef]
- da Rosa, G.M.; Moraes, L.; Souza, M.D.R.A.Z.D.; Costa, J.A.V. Spirulina cultivation with a CO2 absorbent: Influence on growth parameters and macromolecule production. Bioresour. Technol. 2016, 200, 528–534. [Google Scholar] [CrossRef]
- Kazbar, A.; Cogne, G.; Urbain, B.; Marec, H.; Le-Gouic, B.; Tallec, J.; Takache, H.; Ismail, A.; Pruvost, J. Effect of dissolved oxygen concentration on microalgal culture in photobioreactors. Algal Res. 2019, 39, 101432. [Google Scholar] [CrossRef]
- Kunjapur, A.M.; Eldridge, R.B. Photobioreactor Design for Commercial Biofuel Production from Microalgae. Ind. Eng. Chem. Res. 2010, 49, 3516–3526. [Google Scholar] [CrossRef]
- Junying, Z.; Junfeng, R.; Baoning, Z. Factors in mass cultivation of microalgae for biodiesel. Chin. J. Catal. 2013, 34, 80–100. [Google Scholar]
- Growing Algae. Available online: https://energyeducation.ca/encyclopedia/Growing_algae (accessed on 6 December 2022).
- Płaczek, M.; Patyna, A.; Witczak, S. Technical evaluation of photobioreactors for microalgae cultivation. E3S Web Conf. 2017, 19, 02032. [Google Scholar] [CrossRef]
- Singh, R.N.; Sharma, S. Development of suitable photobioreactor for algae production—A review. Renew. Sustain. Energy Rev. 2012, 16, 2347–2353. [Google Scholar] [CrossRef]
- Kumar, A.; Yuan, X.; Sahu, A.K.; Dewulf, J.; Ergas, S.J.; Van Langenhove, H. A hollow fiber membrane photo-bioreactor for CO2 sequestration from combustion gas coupled with wastewater treatment: A process engineering approach. J. Chem. Technol. Biotechnol. 2010, 85, 387–394. [Google Scholar] [CrossRef]
- Fan, L.H.; Zhang, Y.T.; Cheng, L.H.; Zhang, L.; Tang, D.S.; Chen, H.L. Optimization of carbon dioxide fixation by Chlorella vulgaris cultivated in a membrane-photobioreactor. Chem. Eng. Technol. Ind. Chem.-Plant Equip.-Process Eng.-Biotechnol. 2007, 30, 1094–1099. [Google Scholar]
- Zhang, M.; Leung, K.-T.; Lin, H.; Liao, B. The biological performance of a novel microalgal-bacterial membrane photobioreactor: Effects of HRT and N/P ratio. Chemosphere 2020, 261, 128199. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Li, C.; Zhang, D. Lipid production of Chlorella vulgaris cultured in artificial wastewater medium. Bioresour. Technol. 2011, 102, 101–105. [Google Scholar] [CrossRef]
- Sebestyén, P.; Blanken, W.; Bacsa, I.; Tóth, G.; Martin, A.; Bhaiji, T.; Dergez, Á.; Kesserű, P.; Koós, Á.; Kiss, I. Upscale of a laboratory rotating disk biofilm reactor and evaluation of its performance over a half-year operation period in outdoor conditions. Algal Res. 2016, 18, 266–272. [Google Scholar] [CrossRef]
- Sivasangari, S.; Rajan, T.V.; Nandhini, J. Aspects of photobioreactor and algadisk in CO2 sequestration and biomass production. Energy Sources Part A Recover. Util. Environ. Eff. 2019, 1–8. [Google Scholar] [CrossRef]
- Li, M.; Zhou, M.; Luo, J.; Tan, C.; Tian, X.; Su, P.; Gu, T. Carbon dioxide sequestration accompanied by bioenergy generation using a bubbling-type photosynthetic algae microbial fuel cell. Bioresour. Technol. 2019, 280, 95–103. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, Y.; Zhao, G.; Zhang, H. Nutrient removal and biogas upgrading by integrating freshwater algae cultivation with piggery anaerobic digestate liquid treatment. Appl. Microbiol. Biotechnol. 2015, 99, 6493–6501. [Google Scholar] [CrossRef]
- Singh, H.M.; Kothari, R.; Gupta, R.; Tyagi, V. Bio-fixation of flue gas from thermal power plants with algal biomass: Overview and research perspectives. J. Environ. Manag. 2019, 245, 519–539. [Google Scholar] [CrossRef]
- Aslam, A.; Thomas-Hall, S.R.; Mughal, T.A.; Schenk, P.M. Selection and adaptation of microalgae to growth in 100% unfiltered coal-fired flue gas. Bioresour. Technol. 2017, 233, 271–283. [Google Scholar] [CrossRef]
- Kumari, A.; Kumar, A.; Pathak, A.K.; Guria, C. Carbon dioxide assisted Spirulina platensis cultivation using NPK-10:26:26 complex fertilizer in sintered disk chromatographic glass bubble column. J. CO2 Util. 2014, 8, 49–59. [Google Scholar] [CrossRef]
- Ratomski, P.; Hawrot-Paw, M. Production of Chlorella vulgaris Biomass in Tubular Photobioreactors during Different Culture Conditions. Appl. Sci. 2021, 11, 3106. [Google Scholar] [CrossRef]
- Rai, M.P.; Gupta, S. Effect of media composition and light supply on biomass, lipid content and FAME profile for quality biofuel production from Scenedesmus abundans. Energy Convers. Manag. 2017, 141, 85–92. [Google Scholar] [CrossRef]
- Geiman, C.B.; Taub, F.B.; Garbini, J.L. Improving Algae Photobioreactor Efficiency through Active Irradiance Control for Dynamic Carbon Dioxide Fixation. In Proceedings of the 50th International Conference on Environmental Systems, Lisbon, Portugal, 12–15 July 2021. [Google Scholar]
- Cheng, J.; Huang, Y.; Feng, J.; Sun, J.; Zhou, J.; Cen, K. Improving CO2 fixation efficiency by optimizing Chlorella PY-ZU1 culture conditions in sequential bioreactors. Bioresour. Technol. 2013, 144, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Tyystjärvi, E. Photoinhibition of photosystem II. Int. Rev. Cell Mol. Biol. 2013, 300, 243–303. [Google Scholar] [PubMed]
- Platt, T.; Gallegos, C.L. Modelling primary production. In Primary Productivity in the Sea; Springer: Boston, MA, USA, 1980; pp. 339–362. [Google Scholar]
- Keren, N.; Berg, A.; van Kan, P.J.M.; Levanon, H.; Ohad, I. Mechanism of photosystem II photoinactivation and D1 protein degradation at low light: The role of back electron flow. Proc. Natl. Acad. Sci. USA 1997, 94, 1579–1584. [Google Scholar] [CrossRef]
- Förster, B.; Osmond, C.B.; Pogson, B. Improved survival of very high light and oxidative stress is conferred by spontaneous gain-of-function mutations in Chlamydomonas. Biochim. Biophys. Acta (BBA)-Bioenerg. 2005, 1709, 45–57. [Google Scholar] [CrossRef]
- Duarte, J.H.; Costa, J.A.V. Synechococcus nidulans from a thermoelectric coal power plant as a potential CO2 mitigation in culture medium containing flue gas wastes. Bioresour. Technol. 2017, 241, 21–24. [Google Scholar] [CrossRef]
- Ai, W.; Guo, S.; Qin, L.; Tang, Y. Development of a ground-based space micro-algae photo-bioreactor. Adv. Space Res. 2008, 41, 742–747. [Google Scholar] [CrossRef]
| Algal Species | Growth Rate (mg L−1 day−1) | Biomass Concentration (g L−1) | CO2 Concentration v/v (%) | CO2 Removal Rate (gCO2 L−1 day−1) | CO2 Removal Efficiency (%) | References |
|---|---|---|---|---|---|---|
| Botryococcus brauna 765 | 2.31 on 25th day | 20 | Ge et al. [7] | |||
| Chlorella vulgaris | 242 | 9 | Ghayal [9] | |||
| Chlorella vulgaris | 171 | 1.13 ± 0.03 | 15 | 0.32 ± 0.01 | Madhubalaji et al. [11] | |
| Scenedesmus obliquus | 4.36 g m−2day−1 | 10 | 40 | Sun et al. [12] | ||
| Arthospira (Spiralina) plantesis | 620 | Chaiklahan et al. [13] | ||||
| Chlorella vulgaris | 2.12 (BCR) a 1.42 (ALR) b | 40 25 | Alhaboubi et al. [14] | |||
| Chlorella sorokiniana TH01 | 284–469 | 5 | 63–100 | Do et al. [15] | ||
| Chlorophyta | 0.56 g in−2 day−1,c 0.51 g in−2 day−1,d | 21.5 g in 7 days c 19.7 g in 7 days d | 22.04 | Alrade et al. [16] | ||
| Chlorella vulgaris | 1.01 | 15 | 80 | Senatore et al. [17] | ||
| Chlorella sp. | 6.7 g m−2day−1,e 28.0 g m−2day−1,f | Feng et al. [18] | ||||
| Chlorella vulgaris | 7 ± 1 | 0.572 ± 0.04 | 0.927 ± 0.073 | Ratomski et al. [19] | ||
| Parachlorella kessleri | 104 | 5 | 0.211 | Beigbeder et al. [20] | ||
| Chlorella sorokinaina | 0.292 ± 1 g 313 ± 5 h | Cui et al. [21] | ||||
| Chlaymydomonas reinhardtii | 369 ± 10 g 329 ± 12 h | Cui et al. [21] | ||||
| Mixture of Chlorella sp., Scenedesmus sp., and Ankistrodesmus sp. | 0.979 | 59.8 | Rinanti et al. [23] | |||
| Spirulina sp. i | 110.2 ± 4.2 | 1.30 ± 0.07 | 0.197 ± 0.061 | 29.8 ± 0.9 | Da Rosa et al. [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shareefdeen, Z.; Elkamel, A.; Babar, Z.B. Recent Developments on the Performance of Algal Bioreactors for CO2 Removal: Focusing on the Light Intensity and Photoperiods. BioTech 2023, 12, 10. https://doi.org/10.3390/biotech12010010
Shareefdeen Z, Elkamel A, Babar ZB. Recent Developments on the Performance of Algal Bioreactors for CO2 Removal: Focusing on the Light Intensity and Photoperiods. BioTech. 2023; 12(1):10. https://doi.org/10.3390/biotech12010010
Chicago/Turabian StyleShareefdeen, Zarook, Ali Elkamel, and Zaeem Bin Babar. 2023. "Recent Developments on the Performance of Algal Bioreactors for CO2 Removal: Focusing on the Light Intensity and Photoperiods" BioTech 12, no. 1: 10. https://doi.org/10.3390/biotech12010010
APA StyleShareefdeen, Z., Elkamel, A., & Babar, Z. B. (2023). Recent Developments on the Performance of Algal Bioreactors for CO2 Removal: Focusing on the Light Intensity and Photoperiods. BioTech, 12(1), 10. https://doi.org/10.3390/biotech12010010