Abstract
Butterflies have nectar-feeding preferences based on various floral characteristics, including flower shape, size, color, fragrance, and nectar composition, which in turn affect their survival, reproduction, and roles in pollination. The National Botanical Garden (NBG) in Lalitpur, Nepal, holds a variety of flowering plants and butterfly populations, providing a suitable study site to test the hypotheses on floral preferences of butterflies. This study assessed the floral preferences of the butterfly community in the NBG based on flower color, the origin of flowering plants (native and alien), and the type of plants (herbs and shrubs). It also tested the association between butterfly proboscis lengths and corolla tube lengths of flowers. Data were collected from 10 blocks (each 5 × 5 m2) through direct observation during the spring and autumn seasons, from March to October 2022. A total of 24 species of butterflies were recorded during the study period, with the chocolate pansy (Junonia iphita) being the most abundant. The relative abundance of pink flowers was higher in the NBG, but the butterflies’ visitation frequency was significantly higher on yellow flowers (p < 0.05) than on other colors. The visitation frequencies of butterflies significantly varied with the flowers’ origin and types. Butterflies visited flowers of alien origin more frequently than native ones (p < 0.05) and those of herbs over shrubs (p < 0.05). Flowers from alien plants, such as Calluna vulgaris and Viola tricolor, were among the most frequently visited. The proboscis length of butterflies showed a significantly strong positive correlation with the corolla tube length of flowers (τ = 0.74, p < 0.001). These results can inform conservation practices and garden management strategies aimed at supporting butterfly diversity through the intentional selection of floral resources.
1. Introduction
Butterflies (Lepidoptera: Rhopalocera) are important pollinators that also serve as useful indicators of biodiversity [1,2]. They are among highly developed insects with remarkable diversity, with over 28,000 species being recorded worldwide [3]. These are opportunistic foragers, frequently visiting a broad spectrum of flowering plants, thereby functioning as pollinators in the ecosystems [4,5]. Some butterflies are flower generalists, and others are flower specialists. However, research in this area has been minimal and primarily focused only on treatments of individual species or any specific groups of species rather than taking a broader approach [6].
Currently, 692 species of butterflies have been documented in Nepal [7]. The species richness of butterflies is influenced by the elevational gradients; towards higher elevations, especially above 3000 m, floral and butterfly diversity decreases [8]. Nepal is divided into 10 butterfly zones: Western Terai, Eastern Terai, Western, Karnali, Gandaki, Trans-Himalayan, Kaski, Kathmandu Valley, Bagmati (Bagmati Province except Kathmandu Valley), and East [9]. These divisions are based on geographical regions and river basins across the country. The geographic categorization was performed to make the data more precise and gather information from different ecological areas, especially those that haven’t been studied as much as others. In the southern region of the Kathmandu Valley, stretching from Godawari to Phulchowki Mountain, a high richness of butterfly species can be found. With over 150 species, the majority of them being forest dwellers, this area is recognized for its rich butterfly diversity [10]. The National Botanical Garden, Godawari, Lalitpur, Nepal (NBG), a managed botanical park at the base of the Phulchoki Mountain, also holds a high diversity of butterflies [11]. Butterflies are one of the major attractions for the visitors of the NBG.
Various butterfly species demonstrate zonal distribution that is closely tied to strict seasonality and specific habitat preferences, including factors such as color, shape, nectar, size, and scent. Additionally, terrain complexity, climate, and types of vegetation are believed to significantly influence butterfly distribution [12]. Their sensory modalities, for instance, visual and olfactory signals, help to differentiate between unique flower species with colors, morphologies, or fragrances [13]. Studies concluded that the frequency with which butterflies visit flowers is significantly affected by particular attributes of the flowers themselves, such as flower colors, which are a crucial component for the various types of pollinators [14,15]. The study from Rupa Wetland of Nepal recorded that butterfly visits were considerably influenced by diverse plant categories, as well as color preference, with yellow, white, and purple colors over pink ones [16]. Another study from Xiama Forest Farm, Gansu, China, reported that the preference for yellow flowers was higher compared to red, pink, purple, and white flowers, indicating that butterflies show distinct preferences for floral colors [17]. The diversity of floral color is the result of the evolutionary history of the signaling mechanisms between plants and pollinators [18]. Additionally, flower color plays a crucial role in helping pollinators detect flowers, making it an important attribute among floral traits [19]. Multiple studies conclude that butterflies prefer mostly bright-colored flowers, i.e., yellow, in comparison to other colors [20,21].
The foraging patterns of butterflies are characterized by a variety of behaviors, including flower selection, time spent at a nectar source, and the total number of flowers visited in a foraging session [22]. The foraging behavior of insects is shaped by the chemical and physical traits of plants. Additionally, the secondary metabolites present in them are believed to play a significant role in determining the quality and quantity of foraging resources [23]. It is an ecologically important behavior exhibited by butterflies during pollination [24]. For this, several researchers have studied butterfly–plant interactions [16,25,26,27]. The proboscis is an essential and highly adaptive organ in pollinators. Butterflies have a long and coiled proboscis, which they use to feed on nectar [28]. Species with long proboscises feed on long corolla flowers, while those with a short proboscis feed on short corolla flowers [29]. Insects rely on visual and olfactory signals to find suitable food sources with compatible flower morphology with the length of the corolla that suits its proboscis [30,31]. Different species of butterfly prefer different types of flowers [32]. Numerous interactions between insects and flora for the purpose of pollination set perfect examples of coevolution in nature [33]. Studies show that butterflies visit tree flowers less frequently in comparison to their visits to flowers of herbs and shrubs [26]. Alien flowers are mostly preferred by butterflies with extended proboscis, as a high correlation was found between preference for alien flowering plants and proboscis length [30].
Managed parks and botanical gardens are known to support a large number of pollinating insects, including butterflies [34]. It is essential to understand the plant traits that determine butterfly diversity and affect the butterfly visitation frequencies to the flowers. This study explores the butterflies’ floral preferences, relying on traits like flower colors, origin, and shapes in the National Botanical Garden, Godawari, Lalitpur, Nepal. We hypothesized that (i) the light-colored flowers are visited more frequently by the butterflies than the dark ones; (ii) alien plants, being bright colored, are visited more frequently; and (iii) a positive association exists between the proboscis length of butterflies and the corolla tube length of flowers. We tested these hypotheses by direct observation of butterflies visiting the flowers in the National Botanical Garden, Lalitpur, Nepal. Such studies are crucial to protect biodiversity by providing valuable insights into butterfly foraging behavior and the relationship between butterfly proboscis length and flower corolla tube length. Additionally, enhancing our understanding of pollination dynamics and ecological interactions is instrumental to conserving butterfly diversity and habitat.
2. Materials and Methods
2.1. Study Area
The National Botanical Garden (NBG) lies in Lalitpur District in the central mid-hills of Nepal. It is situated between 83°55′56″ and 83°58′50″ E longitude and 28°11′40″ and 28°12′25″ N latitude at the foothills of Phulchowki Mountain at 2765 m above sea level (asl) [11]. It was established on 28 October 1962, under the Department of Plant Resources (DPR) of the Ministry of Forests and Soil Conservation (MoFSC) of the Nepal government. The NBG has been a nationally and internationally renowned botanical garden and a member of Botanical Gardens Conservation International (BGCI) since 2015. This botanical garden’s natural landscape is subdivided into 30 thematic gardens (subgardens) that extend between 1480 and 2000 m of elevational range (Figure 1). Godawari, a natural stream, flows through the center of the garden, enhancing its scenic beauty [11].

Figure 1.
Map of Lalitpur District showing National Botanical Garden and the study blocks within the garden.
2.2. Data Collection
The Special Flower Garden of the NBG on its northern periphery was selected as the study site, harboring flower varieties along with the abundant butterfly population. The preliminary field visit was conducted to explore the abundance of butterflies and different attributes of flowers (color, origins, and types) in NBG during March 2022. The data were collected during two flowering seasons of 2022, i.e., spring (April and May) and autumn (September and October), excluding the winter season due to temperature limitations—butterflies’ presence is very sensitive to cold temperatures [35]—and the summer season because of frequent and heavy rainfall in the study area from the South Asian summer monsoon. The observation was carried out for 32 days and 6 h a day between 10 A.M. and 4 P.M. in each season. A total of ten study blocks of 5 × 5 m2 were laid randomly, and the availability of different colors of flowers was counted every three alternate days. Within each block, three plots of each 1 × 1 m2 were randomly laid, and the flowers of different origins and colors were counted to enumerate their relative abundance. Butterfly visitation frequency was recorded over a specified 30 min observation period in each block. The frequency of butterfly visits to specific flowers was recorded, and the flowering plants were categorized by their flower color (yellow, red, blue, white, orange, pink, and purple), origin (native and alien), and type (shrub and herb). Throughout the study period, 320 observation sessions were completed (160 observation sessions in each season). During the study period, a total of 5507 butterfly visits were recorded across different flower colors, origin, and types in both autumn and spring seasons.
To investigate the association between proboscis and corolla tube length, we conducted 50 measurements. During each measurement, live butterflies were immediately caught after they were observed visiting certain flowers. A sweep net (33.03 cm mouth opening diameter, 40.64–149.86 cm retractable handle, 58.42 cm net depth) was used to catch the butterfly. The length of the proboscis was measured by gripping the unfurled proboscis tip with a needle or forceps and measuring the length from the base to the tip [33]. Moreover, the flowers on which the observed butterflies were feeding were gathered, and the length of their corolla tubes was measured. The corolla’s depth was determined by measuring from the base to the point at which the butterfly could access the nectar. All the length measurements were recorded in centimeters (cm) at an accuracy of ±0.1 cm.
2.3. Data Analysis
This study explored the association between butterfly visitation frequency (the dependent variable) and several independent variables, including seasons (autumn and spring) and flower colors (yellow, red, blue, white, orange, pink, and purple), origins (native and alien), and types (shrub and herb) of the flowering plant. As the dependent variable did not violate the assumption of overdispersion and followed the Poisson distribution, the analysis employed Poisson regression as the primary statistical method to model the relationships between butterfly visitation and season, color, origin, and type of flowers. We developed a Poisson regression model by using the ‘glm’ function in the ‘MASS’ package, with visitation frequency as the dependent variable and seasons, flower colors, origin, and type of flowering plants as independent variables. The summarized model used in the analysis was expressed as follows:
here, μ represents the expected count of butterfly visits; β0 is the intercept; and β1, β2, β3, and β4 are the model coefficients.
Model fit was assessed using goodness-of-fit statistics such as the deviance statistic, through AIC (Akaike Information Criterion), and plotting the residual vs. expected values. Residual analysis was conducted to evaluate the model assumptions, including a check for the presence of overdispersion. Overdispersion occurs when the variance is greater than the mean in a Poisson model. To detect overdispersion, we used the Pearson chi-squared test (p > 0.05) and the dispersion statistic (p > 0.05) and found that the models did not violate the assumption of overdispersion in the analysis. To identify differences in butterfly visitation between flower colors, we conducted two post hoc analyses using the “emmeans” package in R. The first analysis compared visitation frequencies across colors, while the second controlled the seasonal effects on visitation frequencies and color. Furthermore, we employed the Holm–Bonferroni method for adjusting p-values to control the family-wise error rate during the test. Due to the violation of an assumption of normal distribution and multiple ties in the dataset, Kendall’s tau correlation coefficient (τ) was calculated to identify the possible association between the length of butterfly proboscis and the corolla tube length of flowers. All the analyses were performed in R version 4.2.1 (https://www.r-project.org/, accessed on 24 June 2024).
3. Results
3.1. Butterfly Diversity in the National Botanical Garden
A total of 24 species of butterflies belonging to five families were recorded in the NBG during the study period (Table 1). Family Nymphalidae had the highest species richness (n = 13), and the chocolate pansy (Junonia iphita) was the most abundant species (Figure 2).

Table 1.
Butterfly species recorded from the National Botanical Garden during the study period. The identification of butterflies was performed using Smith [36].

Figure 2.
Some representative species of butterflies foraging on the flowers in the National Botanical Garden. (A) Junonia almanac; (B) Junonia atlites; (C) Junonia iphita; (D) Aglais caschmirensis; (E) Eurema hecabe; (F) Colias croceus (photos by Ujjawala KC).
3.2. Color- and Season-Based Variation in Flower Visitation Frequency of Butterflies
Pink flowers (30.66%) were the most abundant, followed by yellow (22.85%) and purple (21%) ones (Table 2). The expected log count of visitation frequency during the spring season was higher than in the autumn (Figure 3a). Yellow flowers had statistically significant higher visitation frequencies by the butterflies over the other colors (Figure 3b).

Table 2.
Flower availability based on colors and their relative abundance in the National Botanical Garden.

Figure 3.
Box plots showing flower visitation frequency of butterflies in the National Botanical Garden: (a) based on seasons and (b) based on flower colors. In each box plot, the central line indicates the median, while the upper and lower whiskers extending from the box represent the range of the data beyond the interquartile range (IQR). The individual points beyond the upper and lower whiskers represent outliers. Data points are denoted as black dots. The letters above each boxplot’s upper whisker (Figure 3b) denote outcomes of post hoc statistical tests. The groups with different letters differ significantly (p < 0.05) with post hoc tests.
The Poisson regression analysis between the flower availability and butterfly visitation frequency revealed butterfly visitation to the flower significantly differed between the seasons and among the colors of the flowers. There was a higher chance of butterfly visitation to the yellow color in comparison to other color categories (Table 3). The post hoc analysis showed that yellow flowers generally had the highest visitation frequency (p < 0.001), followed by white (p < 0.001) and orange (p < 0.001) flowers. Blue flowers consistently had the lowest visitation frequency (p < 0.001) across all comparisons. The differences were statistically significant for most pairs, except for the comparisons between orange and white flowers (p > 0.05) (Table A1, Figure 3b). Post hoc analysis by controlling the effect of season on visitation frequencies on different color flowers revealed a strong preference for yellow flowers across both seasons, with significantly higher visitation frequencies compared to all other colors. Blue flowers consistently had the lowest visitation frequencies. The detailed post hoc test results from the Poisson regression analysis are presented in Appendix A: Table A1 and Table A2. These results provide further insight into the differences in butterfly visitation frequencies among various flower colors, controlling for seasonal effects.

Table 3.
Results from Poisson regression analysis (β: coefficient, SE: standard error, ∆ frequency: differences in visitation frequency).
3.3. Butterfly Floral Preference Based on Flower Origin and Plant Types
Through Poisson regression analysis, we found the origin of the plants (Table 4) has a significant effect on butterfly visitation frequency. The native flower species had a lower visitation frequency (β ± SE = −1.0447 ± 0.0367, Z = −28.43, p < 0.001) compared to the alien flower species. Flowering plant types also exhibited a significant effect on butterfly visitation; shrubs (β ± SE = −0.6795 ± 0.0320, Z = −21.26, p < 0.001) were less frequently visited by the butterflies than the herbs. This suggests that native and shrub-type flowering plants were associated with lower visitation frequencies by butterflies compared to aliens and herbs after controlling for the season in both cases. Regardless of the origin and type of the flowering plants, in both cases, butterflies visited more flowers during the spring season than the autumn season (Figure 4a,b).

Table 4.
Available flowering plants (alien and native) visited by butterflies and the relative abundance of flowers in the National Botanical Garden. Plants were identified according to Parmar et al. [37].

Figure 4.
Visitation frequencies of butterflies in response to (a) the origin of the flowering plants and (b) the type of the flowering plants in the National Botanical Garden. In each box plot, the central line indicates the median, while the upper and lower whiskers extending from the box represent the range of the data beyond the interquartile range (IQR). The individual points beyond the upper and lower whiskers represent outliers. Data points are denoted as black dots.
3.4. Association Between Corolla Tube Length and Butterfly Proboscis
The proboscis length of the observed butterflies ranged between 1.668 ± 0.565 cm (mean ± SD), and corolla tube length ranged between 1.788 ± 0.627 cm (mean ± SD). The Kendall tau correlation analysis revealed a robust and positive correlation between proboscis length and corolla length (τ = 0.74, p < 0.001, Figure 5).
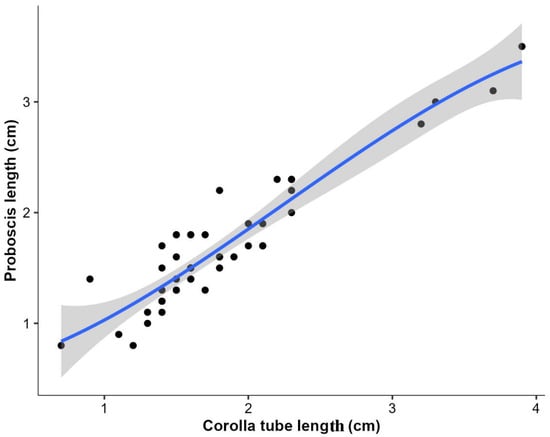
Figure 5.
Association between proboscis length of butterflies and corolla tube length of flowers in the National Botanical Garden. Black dots represent the individual data points, and the line is the polynomial regression best-fit line.
4. Discussion
The study highlighted that the multiple plant characteristics, such as flower color, origin, and type, significantly influence butterfly visitation frequencies to the flowers. There was a significant difference between butterfly visitation in two different seasons, i.e., spring and autumn, in different flower colors. Butterflies visited yellow flowers more frequently, followed by white, orange, pink, purple, red, and blue flowers. This might be because butterflies prefer light-colored flowers more than dull-colored ones. Butterflies in Rupa Wetland of Nepal exhibited a significant inclination towards flowers with yellow, white, and purple, while they were less likely to visit pink flowers [16]. Color is the most important visual signal that attracts butterflies during foraging [17]. A study conducted in India suspected that butterflies visit yellow color more often, which might be attributed to the possibility that yellow flowers might have offered more nectar to visiting pollinators than other color flowers [21]. Tiple et al. [5] reported the frequency of butterflies’ visitation was found significantly higher in yellow, red, blue, and purple when compared to white and pink flowers. Blackiston et al. [20] showed that monarch butterflies selected yellow flowers more frequently than blue flowers, with red being the least preferred color. Briscoe et al. [38] also observed that there was an association between the color preferences of closely related butterfly species when foraging for nectar and indicated that color preference may be due to the phylogenetic traits of their color vision and its evolution may have played a significant role in shaping the foraging behavior of butterflies. In this study, we found that butterflies exhibited the lowest preference for blue flowers among the colors examined. This result contrasts with previous studies that identified a higher preference for blue flowers in certain butterfly species. For example, Swihart [39] and Scherer and Kolb [40] reported a preference for blue in heliconiid and satyrid butterflies. Additionally, research on the green-eyed white butterfly (Leptophobia aripa) indicated a strong preference for red flowers over pink, white, and yellow ones [41], making red the second least preferred color in our study. The variations in flower color preferences across different studies may be influenced by factors such as species-specific visual sensitivities to different colors [17] and the relative abundance of various flower colors. In our study, the lower preference for blue flowers may be explained by their reduced abundance compared to other colors, while red flowers were the least abundant. Furthermore, our research evaluates cumulative foraging preferences across multiple butterfly species, whereas studies with differing results often focused on a single species or family of butterflies. The study revealed that butterflies prefer alien over native flowers, which might be related to the higher resource availability (nectar) or more compatible with the alien flowers’ floral characteristics. Bergerot et al. [30] found that butterflies possessing long proboscises more frequently visited alien plants, as their flowers tend to be deeper than the native flowers. Additionally, alien flowers were taller and had larger floral diameters than their native flowers. The choice of native and alien plants was found to be strongly correlated with the length of the proboscis. Furthermore, butterflies are known to be rapid learners who prefer highly rewarding flowers over natural color preferences [42]. Similarly, a high floral preference for butterflies’ visitation was found in herbs than shrubs which might be associated with the quality and quantity of the food produced by the different types of flowering plants. Tiple et al. [5] investigated the visitation frequency of butterflies to flowers of diverse plant categories and showed that butterfly visitation frequency was higher to herbs than to shrubs. As butterflies often require frequent broods and need a constant supply of nectar from flowering plants. In this context, herbs and shrubs may play a crucial role by providing nectar more consistently than trees do. In rainy seasons butterflies obtain more floral nutrients from herbs and shrubs and mostly depend on trees during dry seasons [43].
This study found that a significant association exists between the length of butterflies’ proboscises and the depth of flower corollas. In particular, butterflies with shorter proboscises generally prefer flowers with shorter corolla tubes, whereas those with longer proboscises are more likely to visit flowers with deeper corollas. Proboscis and corolla lengths were significantly associated with each other among butterflies in Rupa Wetland, Nepal [16]. According to Corbet [44], the depth of a flower’s corolla limits the consumption of nectar by the unsuitable butterfly species and stores nectar for those with proboscises of sufficient length. Consequently, a butterfly with a shorter proboscis is unable to access nectar from flowers with deep corollas. Ranta and Lundberg [45] reported that in bumblebees, when proboscis length matched the depth of the flower’s corolla, the foraging efficiency was maximum. This suggests that bumblebees with longer proboscises are more efficient at accessing nectar from flowers with deeper and more diverse corollas. A similar correlation was observed between the proboscis length of clouded apollo (Parnassius mnemosyne) butterflies and their flower visitation to flowers with deeper corollas [46]. Coevolution between butterflies and flowers is the major factor that contributes to the association between corolla length and proboscis length [47]. Another study reached the same conclusion that butterflies can only be able to feed from flowers whose corolla depth falls within their proboscis length range. Similarly, Tiple et al. [31] studied butterflies at the family level and demonstrated that butterflies belonging to Papilionids more frequently fed on the flowers with deep corolla tubes in central India. Mertens et al. [48] detected a positive relationship between the lengths of the proboscis of hesperoid butterflies and tubes of visited flowers in the tropical rainforests of Mount Cameroon. These associations between butterflies and plant traits enable us to understand the ecology of butterflies, and hence such knowledge could be applicable in managing botanical gardens with diverse floral plants and a higher diversity of pollinators such as butterflies.
This study provides valuable insights into butterfly floral preferences at the National Botanical Garden; however, several limitations should be considered when interpreting the results. First, the observations were limited to just two seasons, which may restrict our understanding of year-round variations in butterfly visitation patterns. Second, because the study examined a multi-species butterfly population, the findings may be biased towards the most abundant species, potentially obscuring the distinct or contrasting floral preferences of rarer species. Third, we tested the floral preferences of butterflies and found that they favored alien flowers over native ones; however, we cannot conclude that the plants preferred by butterflies equally support their immature stages. Additionally, while we explored the relationship between butterfly proboscis length and corolla tube length, we did not account for other morphological or ecological factors that could influence these interactions. Future studies that include data from all seasons, focus on species-specific behaviors, and incorporate additional ecological variables would enhance our understanding of pollinator–plant interactions.
5. Conclusions
This study highlights the significant role of floral traits, including the flower colors, origin, and plant types, in butterfly visitation patterns. The multi-species population of butterflies showed a higher preference for yellow-colored flowers while exhibiting a lesser preference for blue-colored flowers. Alien plants were more frequently visited by butterflies compared to native plants. Furthermore, butterflies exhibited a preference for herbs over shrubs. A strong positive association was observed between the length of the butterflies’ proboscis and the length of the corolla tubes in the flowers that the butterflies visited. This suggests that the proboscis lengths of butterflies influence their ability to access nectar resources with the length of the floral tubes. Despite inherent limitations such as seasonal restrictions and potential dominance of abundant species in a multi-species context, this study offers valuable evidence on how butterfly visitation patterns relate to floral traits. These results can inform conservation practices and garden management strategies aimed at supporting butterfly diversity through the intentional selection of floral resources.
Author Contributions
Conceptualization, L.K.; methodology, U.K. and S.S.; software, U.K. and S.S.; validation, U.K., S.S. and L.K.; formal analysis, U.K. and S.S.; investigation, U.K.; resources, U.K.; data curation, U.K. and S.S.; writing—original draft preparation, U.K. and S.S.; writing—review and editing, A.S., N.P., A.K.S., N.P.K. and L.K.; visualization, S.S. and A.S.; supervision, L.K.; project administration, U.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [L.K.], upon reasonable request.
Acknowledgments
We are thankful to the National Botanical Garden, Godawari, for granting permission to carry out this research inside the garden’s premises and to its officials for their cooperation throughout the study period.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| NBG | National Botanical Garden |
| BGCI | Botanical Gardens Conservation International |
| MoFSC | Ministry of Forests and Soil Conservation |
| DPR | Department of Plant Resources |
Appendix A

Table A1.
Post hoc pairwise comparison results from a Poisson regression analysis, using the Holm–Bonferroni method to adjust p-values for multiple testing. Bold p-values indicate statistically significant differences in butterfly visitation frequencies among the flower colors compared.
Table A1.
Post hoc pairwise comparison results from a Poisson regression analysis, using the Holm–Bonferroni method to adjust p-values for multiple testing. Bold p-values indicate statistically significant differences in butterfly visitation frequencies among the flower colors compared.
| Color Contrast | Differences in Estimated Marginal Means | Standard Error | df | Z-Statistics | p-Value |
|---|---|---|---|---|---|
| Blue–Orange | −2.16 | 0.11 | Inf | −19.41 | <0.0001 |
| Blue–Pink | −1.80 | 0.11 | Inf | −15.81 | <0.0001 |
| Blue–Purple | −1.59 | 0.12 | Inf | −13.76 | <0.0001 |
| Blue–Red | −1.34 | 0.12 | Inf | −11.32 | <0.0001 |
| Blue–White | −2.21 | 0.11 | Inf | −19.86 | <0.0001 |
| Blue–Yellow | −3.32 | 0.11 | Inf | −30.94 | <0.0001 |
| Orange–Pink | 0.36 | 0.06 | Inf | 6.48 | <0.0001 |
| Orange–Purple | 0.57 | 0.06 | Inf | 9.56 | <0.0001 |
| Orange–Red | 0.82 | 0.06 | Inf | 12.67 | <0.0001 |
| Orange–White | −0.05 | 0.05 | Inf | −0.90 | >0.05 |
| Orange–Yellow | −1.16 | 0.04 | Inf | −28.26 | <0.0001 |
| Pink–Purple | 0.21 | 0.06 | Inf | 3.24 | <0.05 |
| Pink–Red | 0.46 | 0.07 | Inf | 6.65 | <0.0001 |
| Pink–White | −0.41 | 0.06 | Inf | −7.35 | <0.0001 |
| Pink–Yellow | −1.52 | 0.05 | Inf | −32.13 | <0.0001 |
| Purple–Red | 0.25 | 0.07 | Inf | 3.49 | <0.0001 |
| Purple–White | −0.61 | 0.06 | Inf | −10.40 | <0.0001 |
| Purple–Yellow | −1.73 | 0.05 | Inf | −33.48 | <0.0001 |
| Red–White | −0.86 | 0.06 | Inf | −13.46 | <0.0001 |
| Red–Yellow | −1.98 | 0.06 | Inf | −34.40 | <0.0001 |
| White–Yellow | −1.11 | 0.04 | Inf | −27.63 | <0.0001 |

Table A2.
Post hoc pairwise comparison results from a Poisson regression analysis, using the Holm–Bonferroni method to adjust p-values for multiple testing. Bold p-values indicate statistically significant differences in butterfly visitation frequencies among the compared flower colors by controlling the effect of season on visitation frequencies. In the “Color Contrast” column, each entry lists a color paired with the season in which the data was collected. For example, “Blue Autumn” signifies that the visitation frequency to blue flowers was observed during the autumn season.
Table A2.
Post hoc pairwise comparison results from a Poisson regression analysis, using the Holm–Bonferroni method to adjust p-values for multiple testing. Bold p-values indicate statistically significant differences in butterfly visitation frequencies among the compared flower colors by controlling the effect of season on visitation frequencies. In the “Color Contrast” column, each entry lists a color paired with the season in which the data was collected. For example, “Blue Autumn” signifies that the visitation frequency to blue flowers was observed during the autumn season.
| Color Contrast | Differences in Estimated Marginal Means | Standard Error | df | Z-Statistics | p-Value |
|---|---|---|---|---|---|
| Blue Autumn–Orange Autumn | −2.16 | 0.11 | Inf | −19.41 | <0.0001 |
| Blue Autumn–Pink Autumn | −1.80 | 0.11 | Inf | −15.81 | <0.0001 |
| Blue Autumn–Purple Autumn | −1.59 | 0.12 | Inf | −13.76 | <0.0001 |
| Blue Autumn–Red Autumn | −1.34 | 0.12 | Inf | −11.32 | <0.0001 |
| Blue Autumn–White Autumn | −2.21 | 0.11 | Inf | −19.86 | <0.0001 |
| Blue Autumn–Yellow Autumn | −3.32 | 0.11 | Inf | −30.94 | <0.0001 |
| Blue Autumn–Blue Spring | −1.05 | 0.03 | Inf | −34.15 | <0.0001 |
| Blue Autumn–Orange Spring | −3.21 | 0.12 | Inf | −27.79 | <0.0001 |
| Blue Autumn–Pink Spring | −2.85 | 0.12 | Inf | −24.16 | <0.0001 |
| Blue Autumn–Purple Spring | −2.64 | 0.12 | Inf | −22.06 | <0.0001 |
| Blue Autumn–Red Spring | −2.39 | 0.12 | Inf | −19.53 | <0.0001 |
| Blue Autumn–White Spring | −3.25 | 0.12 | Inf | −28.24 | <0.0001 |
| Blue Autumn–Yellow Spring | −4.37 | 0.11 | Inf | −39.14 | <0.0001 |
| Orange Autumn–Pink Autumn | 0.36 | 0.06 | Inf | 6.48 | <0.0001 |
| Orange Autumn–Purple Autumn | 0.57 | 0.06 | Inf | 9.56 | <0.0001 |
| Orange Autumn–Red Autumn | 0.82 | 0.06 | Inf | 12.67 | <0.0001 |
| Orange Autumn–White Autumn | −0.05 | 0.05 | Inf | −0.90 | >0.05 |
| Orange Autumn–Yellow Autumn | −1.16 | 0.04 | Inf | −28.26 | <0.0001 |
| Orange Autumn–Blue Spring | 1.11 | 0.12 | Inf | 9.63 | <0.0001 |
| Orange Autumn–Orange Spring | −1.05 | 0.03 | Inf | −34.15 | <0.0001 |
| Orange Autumn–Pink Spring | −0.69 | 0.06 | Inf | −10.78 | <0.0001 |
| Orange Autumn–Purple Spring | −0.48 | 0.07 | Inf | −7.15 | <0.0001 |
| Orange Autumn–Red Spring | −0.23 | 0.07 | Inf | −3.19 | <0.05 |
| Orange Autumn–White Spring | −1.09 | 0.06 | Inf | −18.62 | <0.0001 |
| Orange Autumn–Yellow Spring | −2.21 | 0.05 | Inf | −43.09 | <0.0001 |
| Pink Autumn–Purple Autumn | 0.21 | 0.06 | Inf | 3.24 | <0.05 |
| Pink Autumn–Red Autumn | 0.46 | 0.07 | Inf | 6.65 | <0.0001 |
| Pink Autumn–White Autumn | −0.41 | 0.06 | Inf | −7.35 | <0.0001 |
| Pink Autumn–Yellow Autumn | −1.52 | 0.05 | Inf | −32.13 | <0.0001 |
| Pink Autumn–Blue Spring | 0.75 | 0.12 | Inf | 6.37 | <0.0001 |
| Pink Autumn–Orange Spring | −1.41 | 0.06 | Inf | −22.13 | <0.0001 |
| Pink Autumn–Pink Spring | −1.05 | 0.03 | Inf | −34.15 | <0.0001 |
| Pink Autumn–Purple Spring | −0.84 | 0.07 | Inf | −11.84 | <0.0001 |
| Pink Autumn–Red Spring | −0.59 | 0.08 | Inf | −7.82 | <0.0001 |
| Pink Autumn–White Spring | −1.45 | 0.06 | Inf | −22.99 | <0.0001 |
| Pink Autumn–Yellow Spring | −2.57 | 0.06 | Inf | −45.54 | <0.0001 |
| Purple Autumn–Red Autumn | 0.25 | 0.07 | Inf | 3.49 | <0.05 |
| Purple Autumn–White Autumn | −0.61 | 0.06 | Inf | −10.40 | <0.0001 |
| Purple Autumn–Yellow Autumn | −1.73 | 0.05 | Inf | −33.48 | <0.0001 |
| Purple Autumn–Blue Spring | 0.54 | 0.12 | Inf | 4.54 | <0.0001 |
| Purple Autumn–Orange Spring | −1.62 | 0.07 | Inf | −24.15 | <0.0001 |
| Purple Autumn–Pink Spring | −1.26 | 0.07 | Inf | −17.69 | <0.0001 |
| Purple Autumn–Purple Spring | −1.05 | 0.03 | Inf | −34.15 | <0.0001 |
| Purple Autumn–Red Spring | −0.80 | 0.08 | Inf | −10.20 | <0.0001 |
| Purple Autumn–White Spring | −1.66 | 0.07 | Inf | −24.98 | <0.0001 |
| Purple Autumn–Yellow Spring | −2.78 | 0.06 | Inf | −46.24 | <0.0001 |
| Red Autumn–White Autumn | −0.86 | 0.06 | Inf | −13.46 | <0.0001 |
| Red Autumn–Yellow Autumn | −1.98 | 0.06 | Inf | −34.40 | <0.0001 |
| Red Autumn–Blue Spring | 0.29 | 0.12 | Inf | 2.39 | >0.05 |
| Red Autumn–Orange Spring | −1.87 | 0.07 | Inf | −26.08 | <0.0001 |
| Red Autumn–Pink Spring | −1.51 | 0.08 | Inf | −19.98 | <0.0001 |
| Red Autumn–Purple Spring | −1.30 | 0.08 | Inf | −16.62 | <0.0001 |
| Red Autumn–Red Spring | −1.05 | 0.03 | Inf | −34.15 | <0.0001 |
| Red Autumn–White Spring | −1.91 | 0.07 | Inf | −26.86 | <0.0001 |
| Red Autumn–Yellow Spring | −3.03 | 0.07 | Inf | −46.43 | <0.0001 |
| White Autumn–Yellow Autumn | −1.11 | 0.04 | Inf | −27.63 | <0.0001 |
| White Autumn–Blue Spring | 1.16 | 0.12 | Inf | 10.05 | <0.0001 |
| White Autumn–Orange Spring | −1.00 | 0.06 | Inf | −17.09 | <0.0001 |
| White Autumn–Pink Spring | −0.64 | 0.06 | Inf | −10.14 | <0.0001 |
| White Autumn–Purple Spring | −0.43 | 0.07 | Inf | −6.52 | <0.0001 |
| White Autumn–Red Spring | −0.18 | 0.07 | Inf | −2.57 | >0.05 |
| White Autumn–White Spring | −1.05 | 0.03 | Inf | −34.15 | <0.0001 |
| White Autumn–Yellow Spring | −2.16 | 0.05 | Inf | −42.67 | <0.0001 |
| Yellow Autumn–Blue Spring | 2.27 | 0.11 | Inf | 20.35 | <0.0001 |
| Yellow Autumn–Orange Spring | 0.11 | 0.05 | Inf | 2.16 | >0.05 |
| Yellow Autumn–Pink Spring | 0.47 | 0.06 | Inf | 8.38 | <0.0001 |
| Yellow Autumn–Purple Spring | 0.68 | 0.06 | Inf | 11.32 | <0.0001 |
| Yellow Autumn–Red Spring | 0.93 | 0.07 | Inf | 14.28 | <0.0001 |
| Yellow Autumn–White Spring | 0.07 | 0.05 | Inf | 1.30 | >0.05 |
| Yellow Autumn–Yellow Spring | −1.05 | 0.03 | Inf | −34.15 | <0.0001 |
| Blue Spring–Orange Spring | −2.16 | 0.11 | Inf | −19.41 | <0.0001 |
| Blue Spring–Pink Spring | −1.80 | 0.11 | Inf | −15.81 | <0.0001 |
| Blue Spring–Purple Spring | −1.59 | 0.12 | Inf | −13.76 | <0.0001 |
| Blue Spring–Red Spring | −1.34 | 0.12 | Inf | −11.32 | <0.0001 |
| Blue Spring–White Spring | −2.21 | 0.11 | Inf | −19.86 | <0.0001 |
| Blue Spring–Yellow Spring | −3.32 | 0.11 | Inf | −30.94 | <0.0001 |
| Orange Spring–Pink Spring | 0.36 | 0.06 | Inf | 6.48 | <0.0001 |
| Orange Spring–Purple Spring | 0.57 | 0.06 | Inf | 9.56 | <0.0001 |
| Orange Spring–Red Spring | 0.82 | 0.06 | Inf | 12.67 | <0.0001 |
| Orange Spring–White Spring | −0.05 | 0.05 | Inf | −0.90 | >0.05 |
| Orange Spring–Yellow Spring | −1.16 | 0.04 | Inf | −28.26 | <0.0001 |
| Pink Spring–Purple Spring | 0.21 | 0.06 | Inf | 3.24 | <0.05 |
| Pink Spring–Red Spring | 0.46 | 0.07 | Inf | 6.65 | <0.0001 |
| Pink Spring–White Spring | −0.41 | 0.06 | Inf | −7.35 | <0.0001 |
| Pink Spring–Yellow Spring | −1.52 | 0.05 | Inf | −32.13 | <0.0001 |
| Purple Spring–Red Spring | 0.25 | 0.07 | Inf | 3.49 | <0.05 |
| Purple Spring–White Spring | −0.61 | 0.06 | Inf | −10.40 | <0.0001 |
| Purple Spring–Yellow Spring | −1.73 | 0.05 | Inf | −33.48 | <0.0001 |
| Red Spring–White Spring | −0.86 | 0.06 | Inf | −13.46 | <0.0001 |
| Red Spring–Yellow Spring | −1.98 | 0.06 | Inf | −34.40 | <0.0001 |
| White Spring–Yellow Spring | −1.11 | 0.04 | Inf | −27.63 | <0.0001 |
References
- Durairaj, P.; Sinha, B. Review of butterflies (Lepidoptera: Rhopalocera) from Arunachal Pradesh: Conservation status and importance of research in protected areas. Zool. Surv. India. Zool. Future Educ. Res. 2015, 61–77. [Google Scholar]
- Parasharya, B.M.; Jani, J.J. Butterflies of Gujarat; Anand Agricultural University: Anand, India, 2007; p. 138. [Google Scholar]
- Ghazanfar, M.; Malik, M.F.; Hussain, M.; Iqbal, R.; Younas, M. Butterflies and their contribution in ecosystem: A review. J. Entomol. Zool. Stud. 2016, 4, 115–118. [Google Scholar]
- Sharma, M.; Sharma, N. Nectar resource use by butterflies in Gir Wildlife Sanctuary, Sasan, Gujarat. Biol. Forum J. 2013, 5, 56–63. [Google Scholar]
- Tiple, A.D.; Deshmukh, V.P.; Dennis, R.L. Factors influencing nectar plant resource visits by butterflies on a university campus: Implications for conservation. Nota Lepidopterol. 2005, 28, 213–224. [Google Scholar]
- Jain, A.; Kunte, K.; Webb, E.L. Flower specialization of butterflies and impacts of non-native flower use in a transformed tropical landscape. Biol. Conserv. 2016, 201, 184–191. [Google Scholar] [CrossRef]
- Van der Poel, P.; Kc, S. Overview of new butterfly records (Lepidoptera: Papilionoidea) for Nepal since Smith (2010). Bionotes 2022, 24, 272–280. [Google Scholar]
- Khanal, B.; Chalise, M.K.; Solanki, G.S. Diversity of butterflies with respect to altitudinal rise at various pockets of the Langtang National Park, central Nepal. Int. Multidiscip. Res. J. 2012, 2, 41–48. [Google Scholar]
- Sajan, K.C.; Sapkota, A. Additional distribution records of butterflies (Lepidoptera: Rhopalocera) with seven species new to Nepal. Biodiversitas J. Biol. Divers. 2022, 23, 2711–2738. [Google Scholar]
- Smith, C. Butterflies of Nepal. In Wild is Beautiful Publication; Craftsman Press: Bankok, Thailand, 1989. [Google Scholar]
- DPR. Government of Nepal, Ministry of Forests and Environment; Department of Plant Resources: Kathmandu, Nepal, 2021. [Google Scholar]
- Zhang, H.-H.; Wang, W.-L.; Yu, Q.; Xing, D.-H.; Xu, Z.-B.; Duan, K.; Zhu, J.-Q.; Zhang, X.; Li, Y.-P.; Hu, S.-J. Spatial distribution of pollinating butterflies in Yunnan Province, Southwest China with resource conservation implications. Insects 2020, 11, 525. [Google Scholar] [CrossRef]
- Kunze, J.; Gumbert, A. The combined effect of color and odor on flower choice behavior of bumble bees in flower mimicry systems. Behav. Ecol. 2001, 12, 447–456. [Google Scholar] [CrossRef]
- Fenster, C.B.; Armbruster, W.S.; Wilson, P.; Dudash, M.R.; Thomson, J.D. Pollination syndromes and floral specialization. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 375–403. [Google Scholar] [CrossRef]
- Takahashi, K.; Itino, T. Visitation frequencies of bumblebees and swallowtail butterflies to flowers and the nectar sugar concentration of Rhododendron kaempferi and R. japonicum in mountains of central Japan. J. Pollinat. Ecol. 2017, 21, 92–97. [Google Scholar] [CrossRef]
- Subedi, B.; Stewart, A.B.; Neupane, B.; Ghimire, S.; Adhikari, H. Butterfly species diversity and their floral preferences in the Rupa Wetland of Nepal. Ecol. Evol. 2021, 11, 2086–2099. [Google Scholar] [CrossRef]
- Zhang, S.; Han, J.; Qian, Q.; Zhao, J.; Ma, X.; Song, S. Flower color preferences of Aporia bieti (Lepidoptera: Pieridae) in the Xiama Forest Farm, Gansu, China. Acta Ecol. Sin. 2018, 38, 345–350. [Google Scholar] [CrossRef]
- McEwen, J.R.; Vamosi, J.C. Floral color versus phylogeny in structuring subalpine flowering communities. Proc. R. Soc. B Biol. Sci. 2010, 277, 2957–2965. [Google Scholar] [CrossRef]
- Reverté, S.; Retana, J.; Gómez, J.M.; Bosch, J. Pollinators show flower color preferences but flowers with similar colors do not attract similar pollinators. Ann. Bot. 2016, 118, 249–257. [Google Scholar] [CrossRef]
- Blackiston, D.; Briscoe, A.D.; Weiss, M.R. Color vision and learning in the monarch butterfly, Danaus plexippus (Nymphalidae). J. Exp. Biol. 2011, 214, 509–520. [Google Scholar] [CrossRef]
- Santhosh, S.; Basavarajappa, S. Study on nectar plants of few butterfly species at agriculture ecosystems of Chamarajanagar District, Karnataka, India. Int. J. Entomol. Res. 2016, 1, 40–48. [Google Scholar]
- Pohl, N.B.; Van Wyk, J.; Campbell, D.R. Butterflies show flower color preferences but not constancy in foraging at four plant species. Ecol. Entomol. 2011, 36, 290–300. [Google Scholar] [CrossRef]
- Qin, J.D.; Wang, C.Z. The relation of interaction between insects and plants to evolution. Acta Entomol. Sin. 2001, 44, 360–365. [Google Scholar]
- Tudor, O.; Dennis, R.L.H.; Greatorex-Davies, J.N.; Sparks, T.H. Flower preferences of woodland butterflies in the UK: Nectaring specialists are species of conservation concern. Biol. Conserv. 2004, 119, 397–403. [Google Scholar] [CrossRef]
- Arya, M.K.; Verma, A.; Chandra, H. Sex-specific extra floral feeding preferences among subtropical butterfly fauna of terai arc landscape, southern Himalaya, India. Indian J. Ecol. 2020, 47, 196–204. [Google Scholar]
- Nimbalkar, R.K.; Chandekar, S.K.; Khunte, S.P. Butterfly diversity in relation to nectar food plants from Bhor Tahsil, Pune District, Maharashtra, India. J. Threat. Taxa 2011, 3, 1601–1609. [Google Scholar] [CrossRef]
- Shrestha, B.R.; Timsina, B.; Münzbergová, Z.; Dostálek, T.; Gaudel, P.; Basnet, T.B.; Rokaya, M.B. Butterfly-plant interactions and body size patterns along an elevational gradient in the Manang region of central Nepal. J. Mt. Sci. 2020, 17, 1115–1127. [Google Scholar] [CrossRef]
- Krenn, H.W.; Mühlberger, N. Groundplan anatomy of the proboscis of butterflies (Papilionoidea, Lepidoptera). Zool. Anz. J. Comp. Zool. 2002, 241, 369–380. [Google Scholar] [CrossRef]
- Arbulo, N.; Santos, E.; Salvarrey, S.; Invernizzi, C. Proboscis length and resource utilization in two Uruguayan bumblebees: Bombus atratus Franklin and Bombus bellicosus Smith (Hymenoptera: Apidae). Neotrop. Entomol. 2011, 40, 72–77. [Google Scholar] [CrossRef]
- Bergerot, B.; Fontaine, B.; Renard, M.; Cadi, A.; Julliard, R. Preferences for exotic flowers do not promote urban life in butterflies. Landsc. Urban Plan. 2010, 96, 98–107. [Google Scholar] [CrossRef]
- Tiple, A.D.; Khurad, A.M.; Dennis, R.L.H. Adult butterfly feeding–nectar flower associations: Constraints of taxonomic affiliation, butterfly, and nectar flower morphology. J. Nat. Hist. 2009, 43, 855–884. [Google Scholar] [CrossRef]
- Hardy, P.B.; Sparks, T.H.; Isaac, N.J.B.; Dennis, R.L.H. Specialism for larval and adult consumer resources among British butterflies: Implications for conservation. Biol. Conserv. 2007, 138, 440–452. [Google Scholar] [CrossRef]
- Ehrlich, P.R.; Raven, P.H. Butterflies and plants: A study in coevolution. Evolution 1964, 18, 586–608. [Google Scholar] [CrossRef]
- Vilella-Arnizaut, I.B.; Roeder, D.V.; Fenster, C.B. Use of botanical gardens as arks for conserving pollinators and plant-pollinator interactions: A case study from the United States Northern Great Plains. J. Pollinat. Ecol. 2022, 31, 53–69. [Google Scholar] [CrossRef]
- McDermott Long, O.; Warren, R.; Price, J.; Brereton, T.M.; Botham, M.S.; Franco, A.M.A. Sensitivity of UK butterflies to local climatic extremes: Which life stages are most at risk? J. Anim. Ecol. 2017, 86, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Smith, C. A Photographic Pocket Guide to: Butterflies of Nepal; Himalayan Map House (P.) Ltd.: Kathmandu, Nepal, 2011. [Google Scholar]
- Parmar, G.; Lamichhane, D.; Pandey, J. Catalogue of Plants of National Botanical Garden, Nepal; National Botanical Garden: Lalitpur, Nepal, 2022. [Google Scholar]
- Briscoe, A.D.; Bernard, G.D.; Szeto, A.S.; Nagy, L.M.; White, R.H. Not all butterfly eyes are created equal: Rhodopsin absorption spectra, molecular identification, and localization of ultraviolet-, blue-, and green-sensitive rhodopsin-encoding mRNAs in the retina of Vanessa cardui. J. Comp. Neurol. 2003, 458, 334–349. [Google Scholar] [CrossRef]
- Swihart, S.L. The neural basis of color vision in the butterfly, Papilio troilus. J. Insect Physiol. 1970, 16, 1623–1636. [Google Scholar] [CrossRef]
- Scherer, C.; Kolb, G. The influence of color stimuli on visually controlled behavior in Aglais urticae L. and Pararge aegeria L. (Lepidoptera). J. Comp. Physiol. 1987, 161, 891–898. [Google Scholar] [CrossRef]
- Muñoz-Galicia, D.; Castillo-Guevara, C.; Lara, C. Innate and learnt color preferences in the common green-eyed white butterfly (Leptophobia aripa): Experimental evidence. PeerJ 2021, 9, e12567. [Google Scholar] [CrossRef]
- Kandori, I.; Yamaki, T. Reward and non-reward learning of flower colors in the butterfly Byasa alcinous (Lepidoptera: Papilionidae). Naturwissenschaften 2012, 99, 705–713. [Google Scholar] [CrossRef]
- Raju, A.S.; Bhattacharya, A.; Rao, S.P. Nectar host plants of some butterfly species at Visakhapatnam. Sci. Cult. 2004, 70, 187–190. [Google Scholar]
- Corbet, S.A. Butterfly nectaring flowers: Butterfly morphology and flower form. Entomol. Exp. Appl. 2000, 96, 289–298. [Google Scholar] [CrossRef]
- Ranta, E.; Lundberg, H. Resource partitioning in bumblebees: The significance of differences in proboscis length. Oikos 1980, 35, 298–302. [Google Scholar] [CrossRef]
- Szigeti, V.; Vajna, F.; Kőrösi, Á.; Kis, J. Are all butterflies equal? Population-wise proboscis length variation predicts flower choice in a butterfly. Anim. Behav. 2020, 163, 135–143. [Google Scholar] [CrossRef]
- Sultana, S.; Rahman, S.; Akand, S.; Hoque, M.F.; Miah, M.S.; Bashar, M.A. Butterfly probosces and their functional relations with the nectar plants in some selected forests. J. Biodivers. Conserv. Bioresour. Manag. 2017, 3, 93–102. [Google Scholar] [CrossRef]
- Mertens, J.E.J.; Brisson, L.; Janeček, Š.; Klomberg, Y.; Maicher, V.; Sáfián, S.; Delabye, S.; Potocký, P.; Kobe, I.N.; Pyrcz, T. Elevational and seasonal patterns of butterflies and hawkmoths in plant-pollinator networks in tropical rainforests of Mount Cameroon. Sci. Rep. 2021, 11, 9710. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).