The Study of Exotic and Invasive Plant Species in Gullele Botanic Garden, Addis Ababa, Ethiopia
Abstract
1. Introduction
2. Materials and Methods
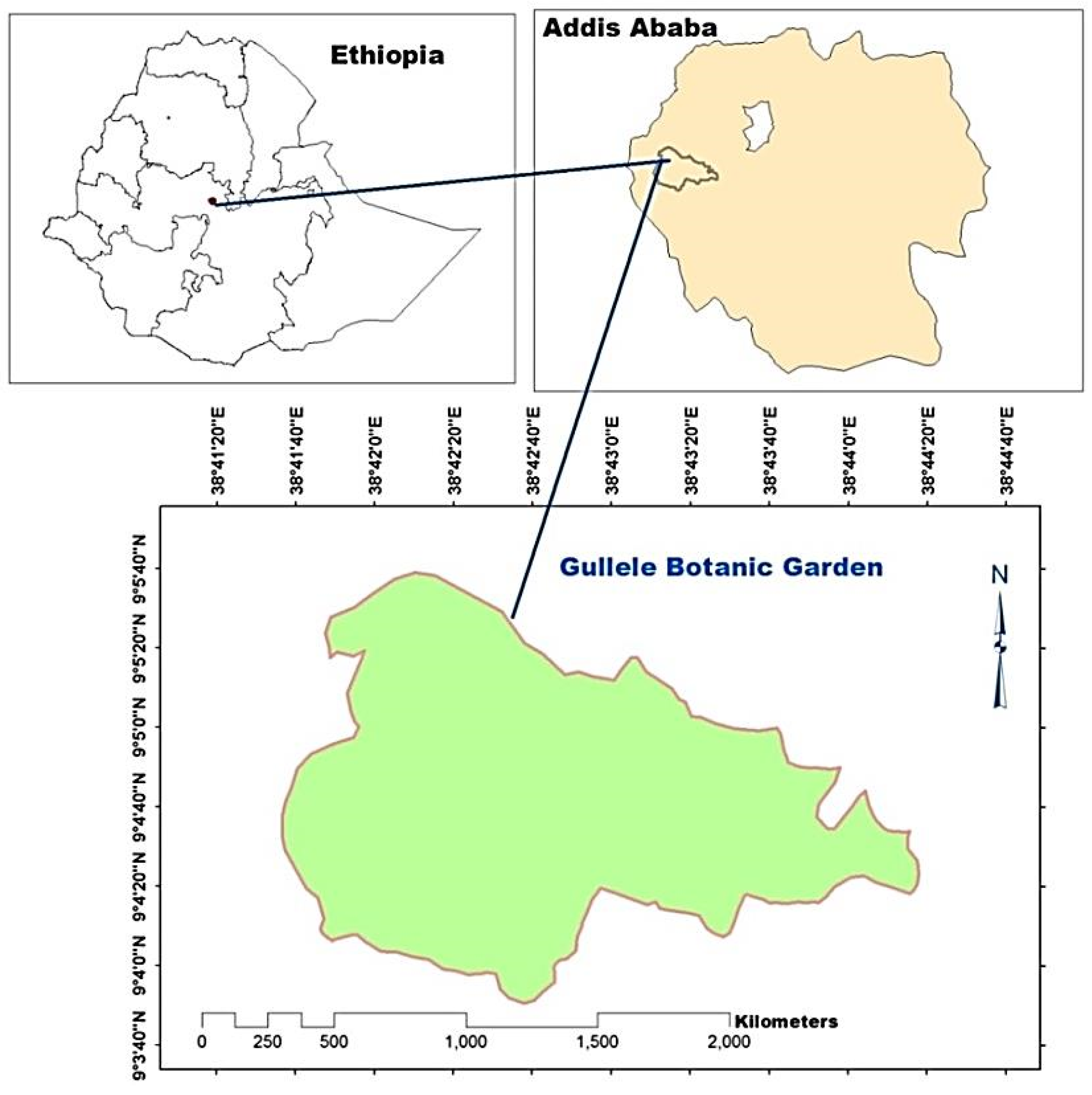
2.1. Study Area Description
2.2. Data Collection
Vegetation Data Collection
2.3. Data Analysis
2.3.1. Vegetation Data Analysis
2.3.2. Floristic Similarity Analysis between Land Use Types
2.3.3. Spatial Data Analysis
3. Result
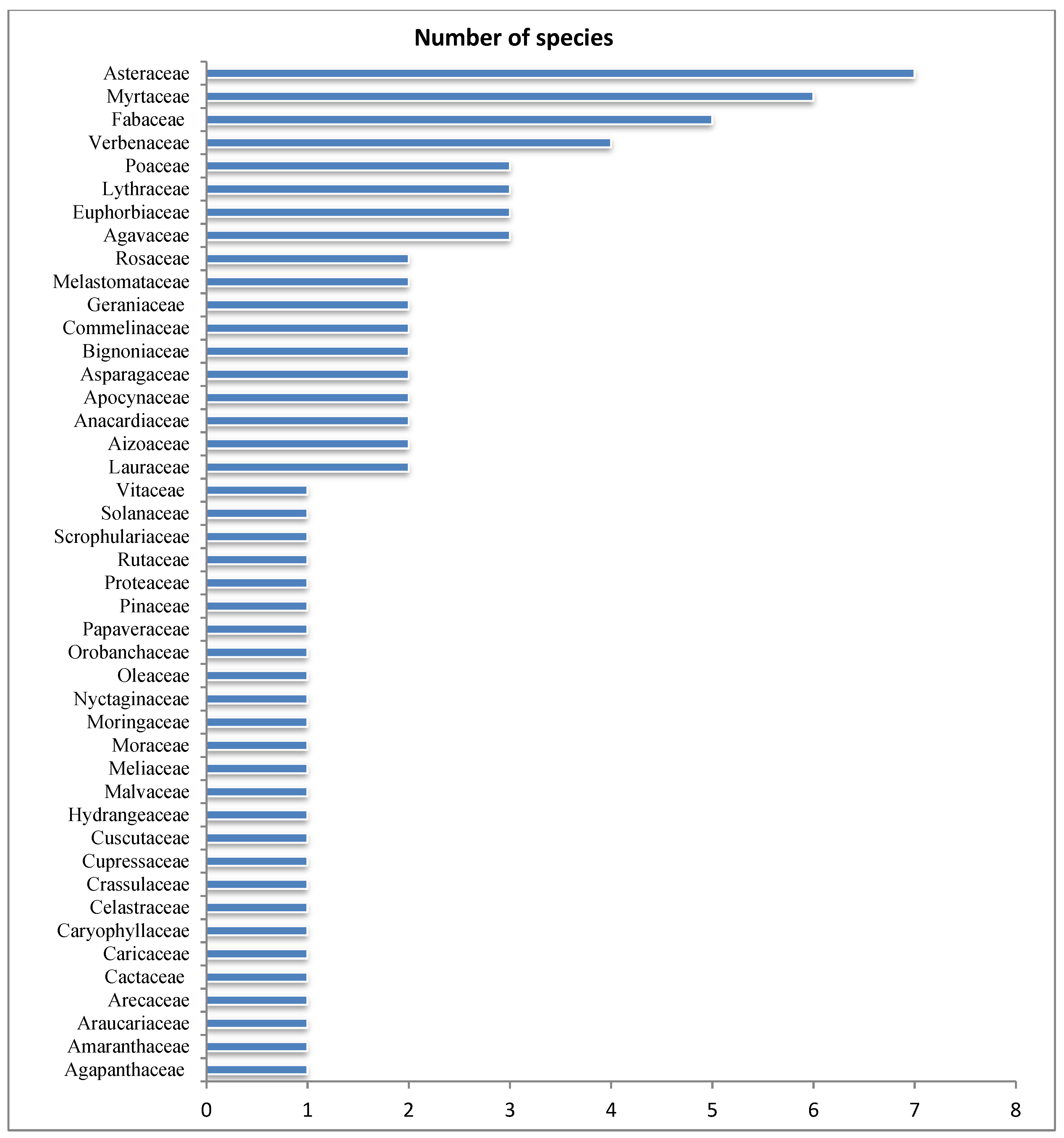
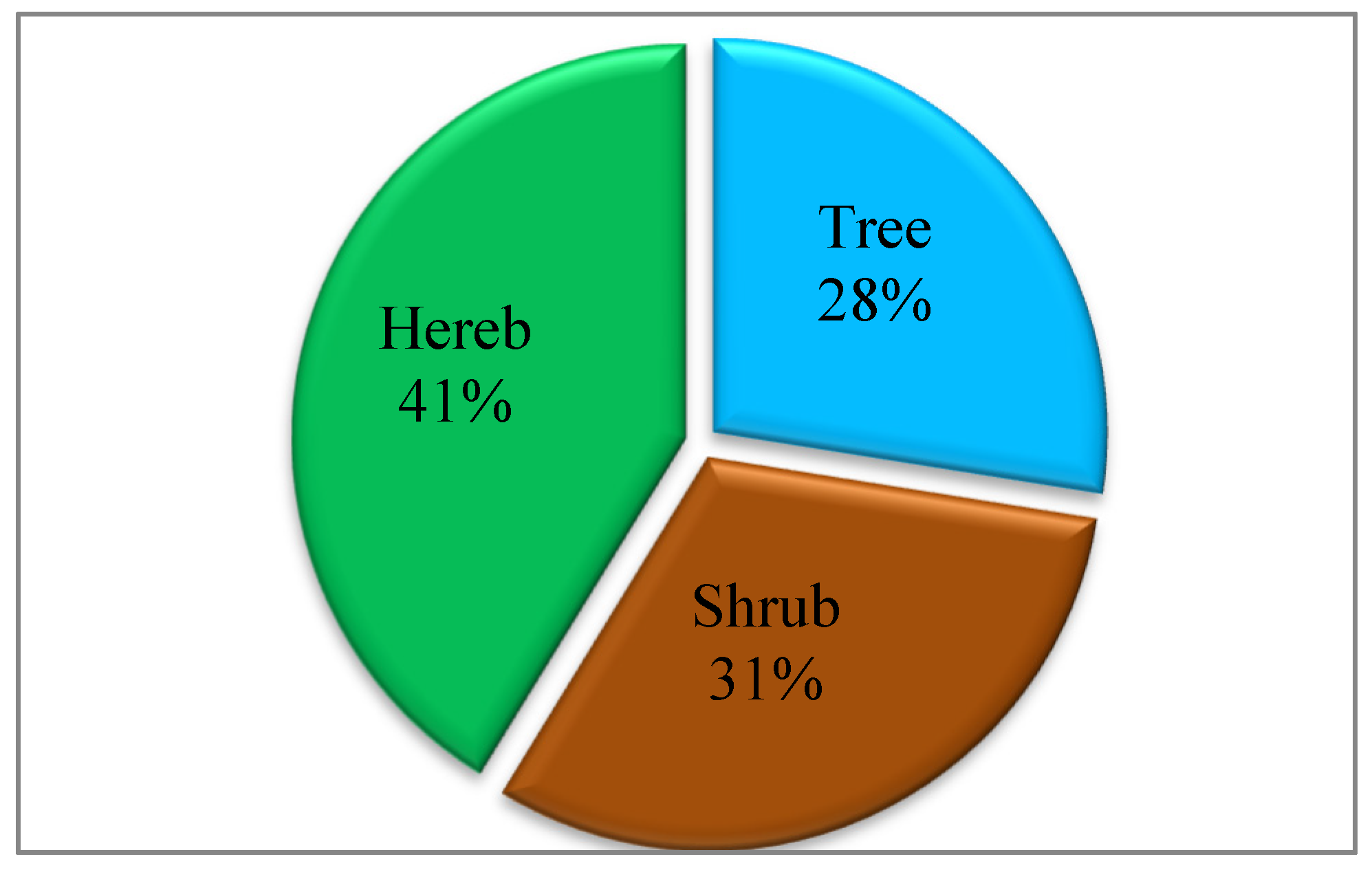
3.1. Exotic, Invasive and Potentially Invasive Plant Species in Gullele Botanic Garden
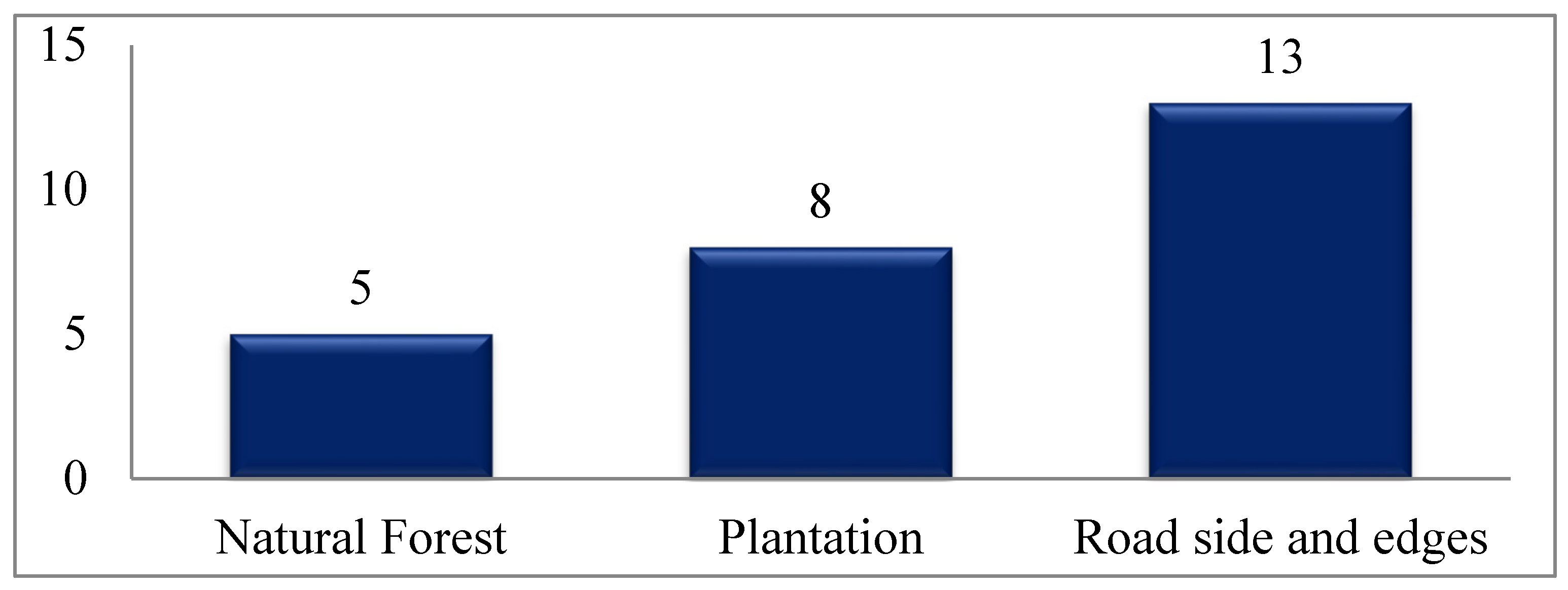
3.2. Exotic, Invasive and Potentially Invasive Species Abundance and Density in Different Land Use Types
3.3. Exotic, Invasive and Potentially Invasive Species Diversity in Different Land Use Types
3.4. Floristic Similarity Analysis between Land Use Types
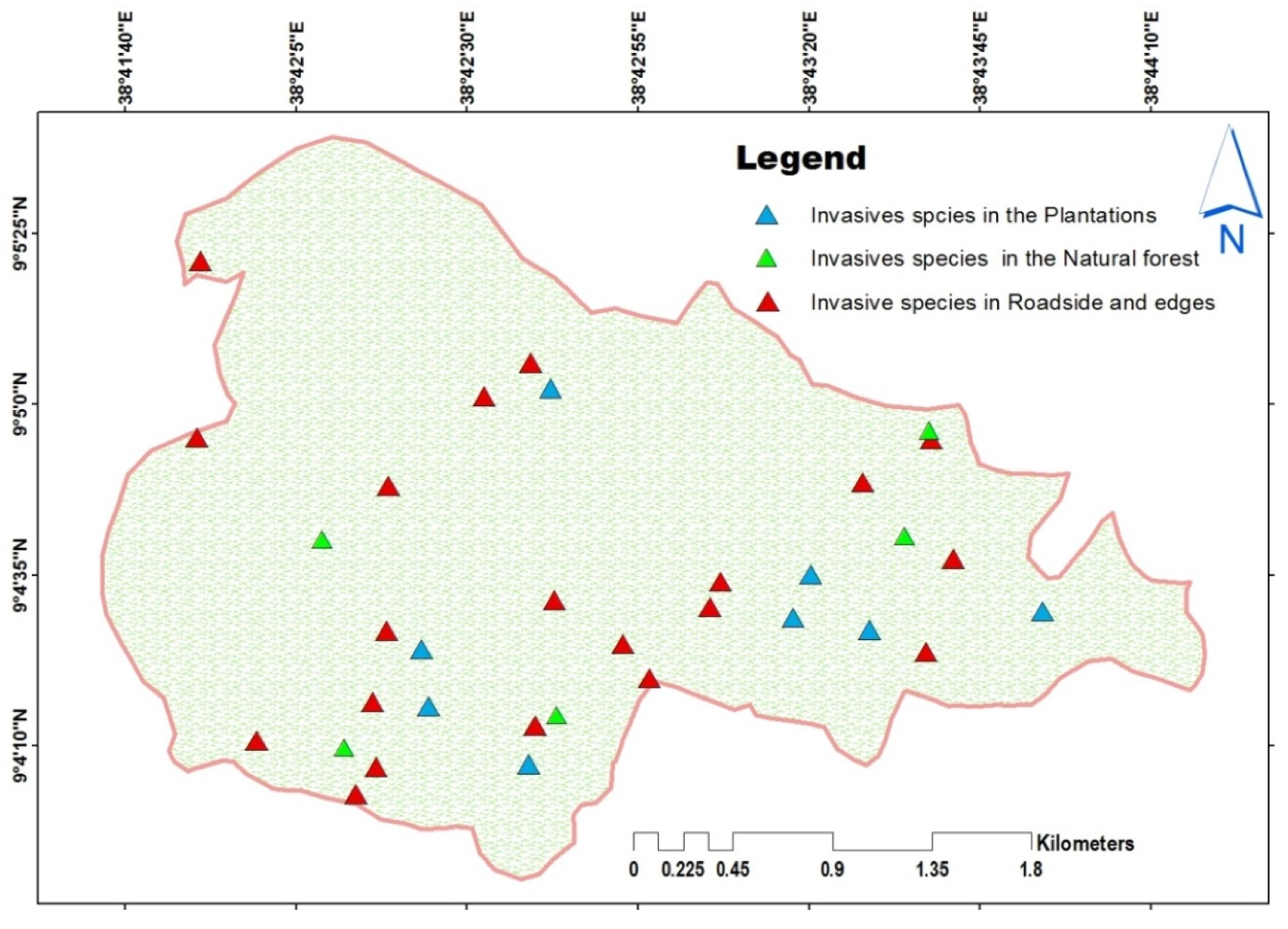
3.5. Invasive species in Gullele Botanic Garden
Invasive Species Distribution and Land Use Types
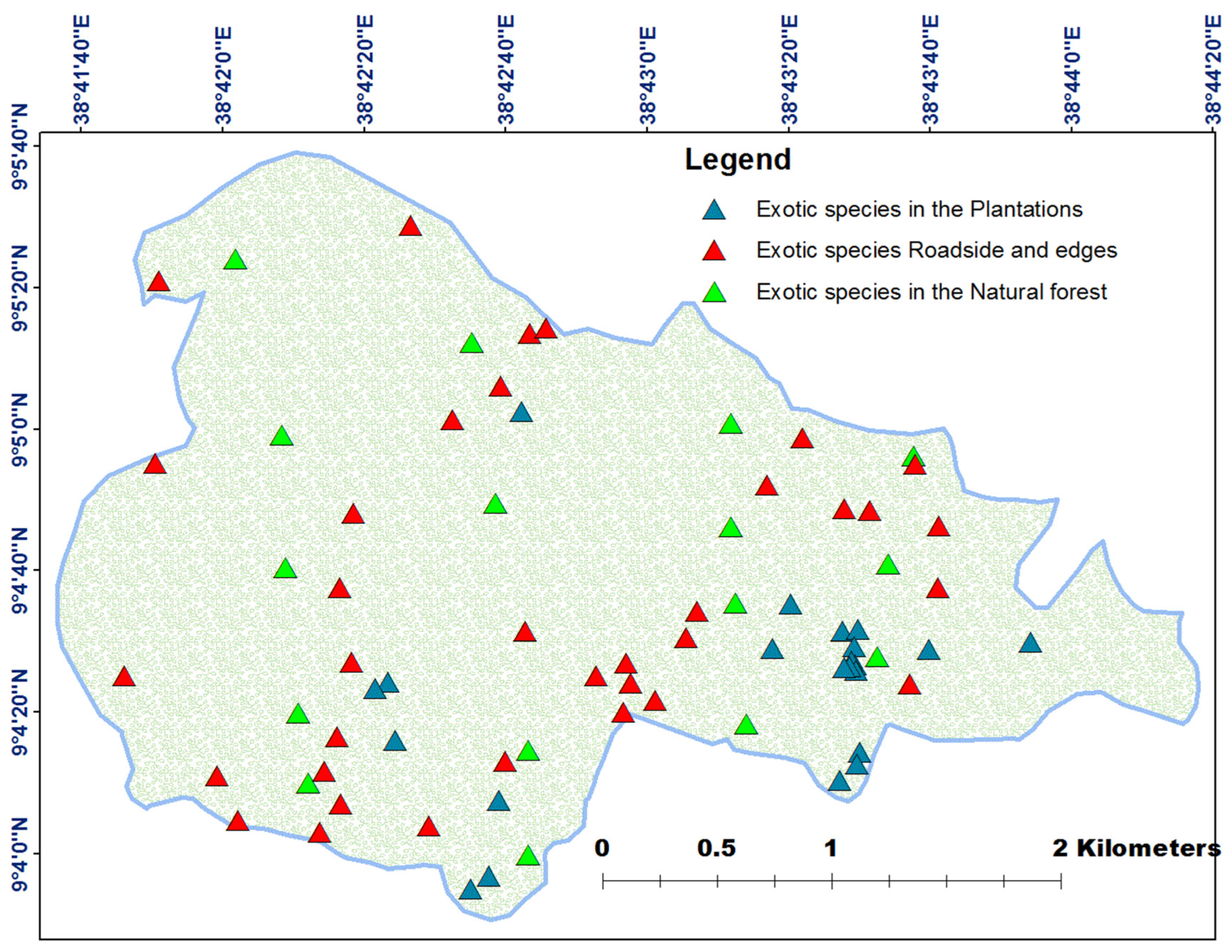
3.6. Spatial Distribution of Exotic Species in the Garden
4. Discussion
4.1. Exotic Plant Species in Gullele Botanic Garden
4.2. Species Abundance, Density and Diversity in the Sampled Land Use Types
4.3. Spatial Distribution of Exotic and Invasive Species in the Garden
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. List of Exotic Species
| No. | Species Name | Family | Habit | Status Invasiveness |
|---|---|---|---|---|
| 1 | Acacia decurrens Willd. | Fabaceae | T | PI |
| 2 | Acacia mearnsii De Wild. | Fabaceae | T | PI |
| 3 | Acacia melanoxylon R. Br. | Fabaceae | T | I |
| 4 | Acacia saligna (Labill.) Wendl. | Fabaceae | S | I |
| 5 | Agapanthus africanus T.Durand and Schinz | Agapanthaceae | H | NI |
| 6 | Agave americana L. | Agavaceae | S | NI |
| 7 | Agave sisalana Perro ex Eng. | Agavaceae | S | NI |
| 8 | Aloysia triphylla (L’Herit.) Britton | Verbenaceae | S | NI |
| 9 | Aptenia cordifolia (Tenore) V. Steenis | Aizoaceae | H | NI |
| 10 | Araucaria heterophylla (Salisb.) Franco | Araucariaceae | T | NI |
| 11 | Argemone mexicana L. | Papaveraceae | H | I |
| 12 | Arundo donax L. | Poaceae | H | NI |
| 13 | Azadirachta indica A. Juss. | Meliaceae | T | NI |
| 14 | Bougainvillea glabra | Nyctaginaceae | S/C | NI |
| 15 | Callistemon citrinus (Curtis) Skeels. | Myrtaceae | T | NI |
| 16 | Callistephus chinensis (L.) Nees | Asteraceae | H | NI |
| 17 | Carica papaya L. (Caricaceae). | Caricaceae | T | NI |
| 18 | Carpobrotus edulis (L.) L. Bolus | Aizoaceae | H | NI |
| 19 | Centradenia floribunda shawl | Melastomataceae | H | NI |
| 20 | Chlorophytum comosum (Thunb.) Jacques | Asparagaceae | H | NI |
| 21 | Chrysanthemum leucanthemum L. | Asteraceae | H | NI |
| 22 | Citrus aurantiifolia (Christm.) Swingle | Rutaceae | S | NI |
| 23 | Cordyline australis (G.Forst.) Endl. | Asparagaceae | T | NI |
| 24 | Cordyline fruticosa (L.) A.Chev. | Asparagaceae | H | NI |
| 25 | Crassula ovata (Miller) Druce | Crassulaceae | H | NI |
| 26 | Cuphea hyssopifolia Kunth | Lythraceae | H | NI |
| 27 | Cuphea ignea A.DC | Lythraceae | H | NI |
| 28 | Cuphea micropetala Kunth | Lythraceae | H | NI |
| 29 | Cupressus lusitanica Mill | Cupressaceae | T | NI |
| 30 | Cuscuta campestris Yuncker | Cuscutaceae | H | I |
| 31 | Cyathula uncinulata (Schrad.) Schinz * | Commelinaceae | H | I |
| 32 | Cymbopogon citratus (DC.) Stapf. | Poaceae | H | NI |
| 33 | Dianthus barbatus L. | Caryophyllaceae | H | NI |
| 34 | Distictis buccinatoria (DC.) A.H. | Bignoniaceae | HC | NI |
| 35 | Duranta erecta L. | Verbenaceae | S | NI |
| 36 | Duranta repens L. | Verbenaceae | S | NI |
| 37 | Eucalyptus camaldulensis Dehnh. | Myrtaceae | T | NI |
| 38 | Eucalyptus citriodora Hook. | Myrtaceae | T | NI |
| 39 | Eucalyptus globulus Labill. | Myrtaceae | T | NI |
| 40 | Euonymus fortunei (Turcz.) Hand.-Mazz. | Celastraceae | S | NI |
| 41 | Euphorbia milii Des Moulins | Euphorbiaceae | S | NI |
| 42 | Ficus benjamina Linn. | Moraceae | S | NI |
| 43 | Galinsoga parviflora Cav. | Asteraceae | H | I |
| 44 | Grevillea robusta R.Br. | Proteaceae | T | NI |
| 45 | Hibiscus rosa-sinensis L. | Malvaceae | S | NI |
| 46 | Hydrangea macrophylla (Thunb.) Ser. | Hydrangeaceae | H | NI |
| 47 | Iresine herbstii Lindl. | Amaranthaceae | H | NI |
| 48 | Jacaranda mimosifolia D.Don | Bignoniaceae | T | NI |
| 49 | Jatropha curcas L. | Euphorbiaceae | T | NI |
| 50 | Lantana camara L. | Verbenaceae | S | I |
| 51 | Lavandula angustifolia Mill. | Lauraceae | S | NI |
| 52 | Ligustrum vulgare L. | Oleaceae | S | NI |
| 53 | Malus domestica Borkh. | Rosaceae | S | NI |
| 54 | Mangifera indica L. | Anacardiaceae | T | NI |
| 55 | Melaleuca alternifolia Cheel. | Myrtaceae | T | NI |
| 56 | Moringa oleifera Lam. | Moringaceae | T | NI |
| 57 | Nerium oleander L. | Apocynaceae | S | I |
| 58 | Nicotiana glauca Graham | Solanaceae | S | I |
| 59 | Opuntia ficus-indica (L.) Miller. | Cactaceae | S | NI |
| 60 | Orobanche minor Smith | Orobanchaceae | H | NI |
| 61 | Osteospermum fruticosum (L.) Norl. | Asteraceae | H | NI |
| 62 | Pelargonium asperum Willd. | Geraniaceae | H | NI |
| 63 | Pelargonium zonale (L.) L’Hér. | Geraniaceae | H | NI |
| 64 | Persea americana Mill. | Lauraceae | T | NI |
| 65 | Phalaris arundinacea L. | Poaceae | H | NI |
| 66 | Pinus patula Schiede ex Schltdl. & Cham. | Pinaceae | T | NI |
| 67 | Psidium guajava L. | Myrtaceae | S | I |
| 68 | Ricinus communis L. * | Euphorbiaceae | H | I |
| 69 | Rosa pendulina L. | Rosaceae | S | NI |
| 70 | Schinus molle L. | Anacardiaceae | T | NI |
| 71 | Senecio cineraria | Asteraceae | H | NI |
| 72 | Senna didymobotrya (Fresen.) Irwin & Barneby * | Fabaceae | S | I |
| 73 | Silybum marianum (L.) Gaertn. | Asteraceae | H | NI |
| 74 | Striga gesnerioides (Willd.) Vatke * | Scrophulariaceae | H | I |
| 75 | Tagetes minuta L. | Asteraceae | H | NI |
| 76 | Tibouchina urvilleana (DC.) Cogn. | Melastomataceae | T | NI |
| 77 | Tradescantia pallida (Rose) Hunt | Commelinaceae | H | NI |
| 78 | Vinca major L. | Apocynaceae | HC | NI |
| 79 | Vitis vinifera L. | Vitaceae | WC | NI |
| 80 | Washingtonia filifera (Linden ex Andre) H. Wendl. | Arecaceae | S | NI |
References
- Carwardine, M. Alien Species: What They Are and Why They Are Such a Threat. Discov. From the Team at BBC Wildlife Magazine. 2017. Available online: https://www.discoverwildlife.com/animal-facts/alien-species-facts (accessed on 10 May 2023).
- Kumar, A.; Prasad, S. Threats of invasive alien plant species. Int. Res. J. Manag. Sci. Tech. 2014, 4, 605–624. [Google Scholar]
- Richardson, D.M.; Binggeli, P.; Schroth, G. Invasive agroforestry trees: Problems and solutions. Agrof. Biod. Conserv. Trop. Lands. 2004, 15, 371–396. [Google Scholar]
- Pyšek, P.; Richardson, D.M.; Rejmánek, M.; Webster, G.L.; Williamson, M.; Kirschner, J. Alien plants in checklists and floras: Towards better communication between taxonomists and ecologists. Taxon 2004, 53, 131–143. [Google Scholar] [CrossRef]
- Krishnan, S.; Novy, A. The role of botanic gardens in the twenty-first century. CABI Rev. 2017, 1–10. [Google Scholar] [CrossRef]
- Pagad, S.; Genovesi, P.; Carnevali, L.; Scalera, R.; Clout, M. IUCN SSC Invasive Species Specialist Group: Invasive Alien Species Information Management Supporting Practitioners, Policy Makers and Decision Takers. 2015. Available online: https://researchspace.auckland.ac.nz/handle/2292/33532 (accessed on 3 September 2023).
- Kolar, C.S.; Lodge, D.M. Progress in invasion biology: Predicting invaders. Trend. Ecol. Evol. 2001, 16, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Ascensão, F.; Capinha, C. Aliens on the Move: Transportation Networks and Non-Native Species. In Railway Ecology; Borda-de-Água, L., Barrientos, R., Beja, P., Pereira, H., Eds.; Springer: Cham, Switzerland, 2017; pp. 65–80. [Google Scholar] [CrossRef]
- Castri, D. History of Biological Invasions with Special Emphasis on the Old World. Biological Invasions: A Global Perspective. 1989, pp. 1–30. Available online: https://cir.nii.ac.jp/crid/1572543025638845952 (accessed on 14 September 2023).
- Hobbs, H.A. Invasive Species in a Changing World; Island Press: Washington, DC, USA, 2000. [Google Scholar]
- Haber, E.; Network, A. Guide to Monitoring Exotic and Invasive Plants; Environment Canada: Ottawa, ON, Canada, 1997. [Google Scholar]
- D’Antonio, C.; Levine, J.; Thomsen, M. Ecosystem resistance to invasion and the role of propagule supply: A California perspective. J. Med. Econ. 2001, 2, 233–246. [Google Scholar]
- Thinley, U.; Gurung, D.B.; Sonam, T.; Uden, K. A Study and Survey on Key Invasive Plant Species in Southwestern Bhutan, India, 2022; pp. 1–67.
- Engels, J.M.; Hawkes, J.G.; Worede, M. Plant Genetic Resources of Ethiopia; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Fessehaie, R.; Tessema, T. Alien Plant Species Invasions in Ethiopia: Challenges and Responses. In Proceedings of the International Workshop on Parthenium Weed in Ethiopia, Ethiopia. Available online: https://ipmil.cired.vt.edu/wp-content/uploads/2014/07/10-Fessehaie.pdf (accessed on 23 April 2023).
- Shiferaw, W.; Demissew, S.; Bekele, T. Invasive alien plant species in Ethiopia: Ecological impacts on biodiversity a review paper. Int. J. Mol. Biol. 2018, 3, 171–178. [Google Scholar] [CrossRef]
- Wassie, S.B. Natural resource degradation tendencies in Ethiopia: A review. Environ. Syst. Res. 2020, 9, 33. [Google Scholar] [CrossRef]
- Gullele Botanic Garden. Organization’s Website. 2022. Available online: https://gullelebotanicgarden.yolasite.com/more-info.php#! (accessed on 18 October 2023).
- Argaw, T. Opportunities of Botanical Garden in Environmental and Development Education to Support School Based Instruction in Ethiopia. J. Biol. Agr. Heal. 2015, 5, 92–110. [Google Scholar]
- Seta, T.; Belay, B. BOTANIC GARDEN PROFILE Gullele Botanic Garden, Addis Ababa (Ethiopia): Current status, Challenges and Opportunities. Sibbaldia Inter. J. Bot. Gard. Horti. 2022, 21, 13–34. [Google Scholar] [CrossRef]
- Morton, W.H. Geological Map of Addis Ababa; Addis Ababa University, Geology Department: Addis Ababa, Ethiopia, 1974. [Google Scholar]
- Ellenberg, D.; Mueller-Dombois, D. Aims and Methods of Vegetation Ecology; Wiley: New York, NY, USA, 1974; pp. 45–66. [Google Scholar]
- Kent, M. Vegetation Description and Data Analysis: A Practical Approach, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2012; pp. 1–8. [Google Scholar]
- Thomas, G.; Sucher, R.; Wyatt, A.; Jiménez, I. Ex situ species conservation: Predicting plant survival in botanic gardens based on climatic provenance. Biol. Conser. 2022, 265, 109410. [Google Scholar] [CrossRef]
- Mokotjomela, T.M.; Rahlao, S.J.; Vukeya, L.R.; Baltzinger, C.; Mangane, L.V.; Willis, C.K.; Mutshinyalo, T.M. The Diversity of Alien Plant Species in South Africa’s National Botanical and Zoological Gardens. Diversity 2023, 15, 407. [Google Scholar]
- Wondafrash, M.; Wingfield, M.J.; Wilson, J.R.; Hurley, B.P.; Slippers, B.; Paap, T. Botanical gardens as key resources and hazards for biosecurity. Biodiv. Conser. 2021, 30, 1929–1946. [Google Scholar] [CrossRef]
- Tadesse, M. Flora of Ethiopia and Eritrea, The National Herbarium, Addis Ababa, Ethiopia: Uppsala, Sweden, 2004; 1–408.
- Castro-Díez, P.; Vaz, A.S.; Silva, J.S.; Van Loo, M.; Alonso, Á.; Aponte, C.; Bayón, Á.; Bellingham, P.J.; Chiuffo, M.C.; DiManno, N.; et al. Global effects of non-native tree species on multiple ecosystem services. Biol. Rev. 2019, 94, 1477–1501. [Google Scholar] [CrossRef]
- Burgiel, S.W.; Muir, A.A. Invasive Species, Climate Change and Ecosystem-Based Adaptation: Addressing Multiple Drivers of Global Change; IUCN: Washington, DC, USA, 2010; pp. 1–55. [Google Scholar]
- Witt, A.; Beale, T.; Van Wilgen, B.W. An assessment of the distribution and potential ecological impacts of invasive alien plant species in eastern Africa. Trans. Royal. Soc. S. Afr. 2018, 73, 217–236. [Google Scholar] [CrossRef]
- Van Kleunen, M.; Essl, F.; Pergl, J.; Brundu, G.; Carboni, M.; Dullinger, S.; Early, R.; González-Moreno, P.; Groom, Q.J.; Hulme, P.E.; et al. The changing role of ornamental horticulture in alien plant invasions. Biol. Rev. 2018, 93, 1421–1437. [Google Scholar] [CrossRef] [PubMed]
- Son, D.; Waldman, B.; Song, U. Effects of land-use types and the exotic species, Hypochaeris radicata L., on plant diversity in human-transformed landscapes of the biosphere reserve, Jeju Island, Korea. Plant Diver. 2023, 45, 685–693. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Peng, P.; Wang, G.; Zhao, G.; Zhou, Y.; Tang, Z. Mapping the distribution and dispersal risks of the alien invasive plant Ageratina adenophora in China. Diversity 2022, 14, 915. [Google Scholar] [CrossRef]
- Richardson, D.M.; Pyšek, P.; Rejmanek, M.; Barbour, M.G.; Panetta, F.D.; West, C.J. Naturalization and invasion of alien plants: Concepts and definitions. Diver. Distrib. 2000, 6, 93–107. [Google Scholar] [CrossRef]
- Oh, M.; Heo, Y.; Lee, E.J.; Lee, H. Major environmental factors and traits of invasive alien plants determining their spatial distribution. J. Ecol. Environ. 2021, 45, 29. [Google Scholar] [CrossRef]
- IUCN/PACO. Invasive Plants Affecting Protected Areas of West Africa. Management for Reduction of Risk for Biodiversity; Gland, IUCN: Ouagadougou, Burkino Faso, 2013. [Google Scholar]
- Moyo, H.P.; Fatunbi, A.O. Utilitarian perspective of the invasion of some South African biomes by Acacia mearnsii. Glob. J. Environ. Res. 2010, 4, 6–17. [Google Scholar]
| Land Use Types | Abundance | Sampled Area (m2) | Density (Species/m2) |
|---|---|---|---|
| Natural forest | 128 | 400 | 0.32 |
| Roadside and edges | 1045 | 725 | 1.44 |
| Plantation | 285 | 475 | 0.6 |
| Total | 1458 | 1600 | 2.36 |
| Community | Shannon–Wiener Diversity Index (H′) | Shannon Evenness (J′) |
|---|---|---|
| Natural forest | 0.06 | 0.05 |
| Roadside and edges of the garden | 3.09 | 0.82 |
| Plantation | 0.63 | 0.29 |
| Land Use Type | Natural Forest | Roadside and Edges of the Garden | Plantation |
|---|---|---|---|
| Natural forest | 1 | 0.22 | 0.15 |
| Roadside and edges of the garden | - | 1 | 0.40 |
| Plantations | - | - | 1 |
| No. | Species Name | Family | Habit | LUT Found |
|---|---|---|---|---|
| 1 | Acacia decurrens Willd. | Fabaceae | T | NF, RE, PL |
| 2 | Acacia mearnsii De Wild. | Fabaceae | T | RE |
| 3 | Acacia melanoxylon R. Br. | Fabaceae | T | NF, RE, PL |
| 4 | Acacia saligna (Labill.) Wendl. | Fabaceae | S | NF, RE |
| 5 | Argemone mexicana L. | Papaveraceae | H | RE |
| 6 | Cuscuta campestris Yuncker | Cuscutaceae | H | NF, RE |
| 7 | Cyathula uncinulata (Schrad.) Schinz | Commelinaceae | H | NF, RE |
| 8 | Galinsoga parviflora Cav. | Asteraceae | H | PL |
| 9 | Lantana camara L. | Verbanaceae | S | NF, RE |
| 10 | Nerium oleander L. | Apocynaceae | S | RE, PL |
| 11 | Nicotiana glauca Graham | Solanaceae | S | RE |
| 12 | Psidium guajava L. | Myrtaceae | S | PL |
| 13 | Ricinus communis L. | Euphorbiaceae | H | RE, PL |
| 14 | Senna didymobotrya (Fresen.) Irwin & Barneby | Fabaceae | S | RE |
| 15 | Striga gesnerioides (Willd.) Vatke | Scrophulariaceae | H | RE, PL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girmay, M.; Gebrehiwot, K.; Atinafe, E.; Tareke, Y.; Belay, B. The Study of Exotic and Invasive Plant Species in Gullele Botanic Garden, Addis Ababa, Ethiopia. J. Zool. Bot. Gard. 2024, 5, 36-50. https://doi.org/10.3390/jzbg5010003
Girmay M, Gebrehiwot K, Atinafe E, Tareke Y, Belay B. The Study of Exotic and Invasive Plant Species in Gullele Botanic Garden, Addis Ababa, Ethiopia. Journal of Zoological and Botanical Gardens. 2024; 5(1):36-50. https://doi.org/10.3390/jzbg5010003
Chicago/Turabian StyleGirmay, Mehari, Kflay Gebrehiwot, Ergua Atinafe, Yared Tareke, and Birhanu Belay. 2024. "The Study of Exotic and Invasive Plant Species in Gullele Botanic Garden, Addis Ababa, Ethiopia" Journal of Zoological and Botanical Gardens 5, no. 1: 36-50. https://doi.org/10.3390/jzbg5010003
APA StyleGirmay, M., Gebrehiwot, K., Atinafe, E., Tareke, Y., & Belay, B. (2024). The Study of Exotic and Invasive Plant Species in Gullele Botanic Garden, Addis Ababa, Ethiopia. Journal of Zoological and Botanical Gardens, 5(1), 36-50. https://doi.org/10.3390/jzbg5010003






