From Incision to Immunity: Integrating Surgery and Immunotherapy in Non-Small Cell Lung Cancer
Abstract
1. Introduction
2. Literature Search Strategy
3. Immunotherapy
3.1. Approved Immunotherapeutic Agents
3.2. PD1/PD-L1 Inhibitors/Antibodies
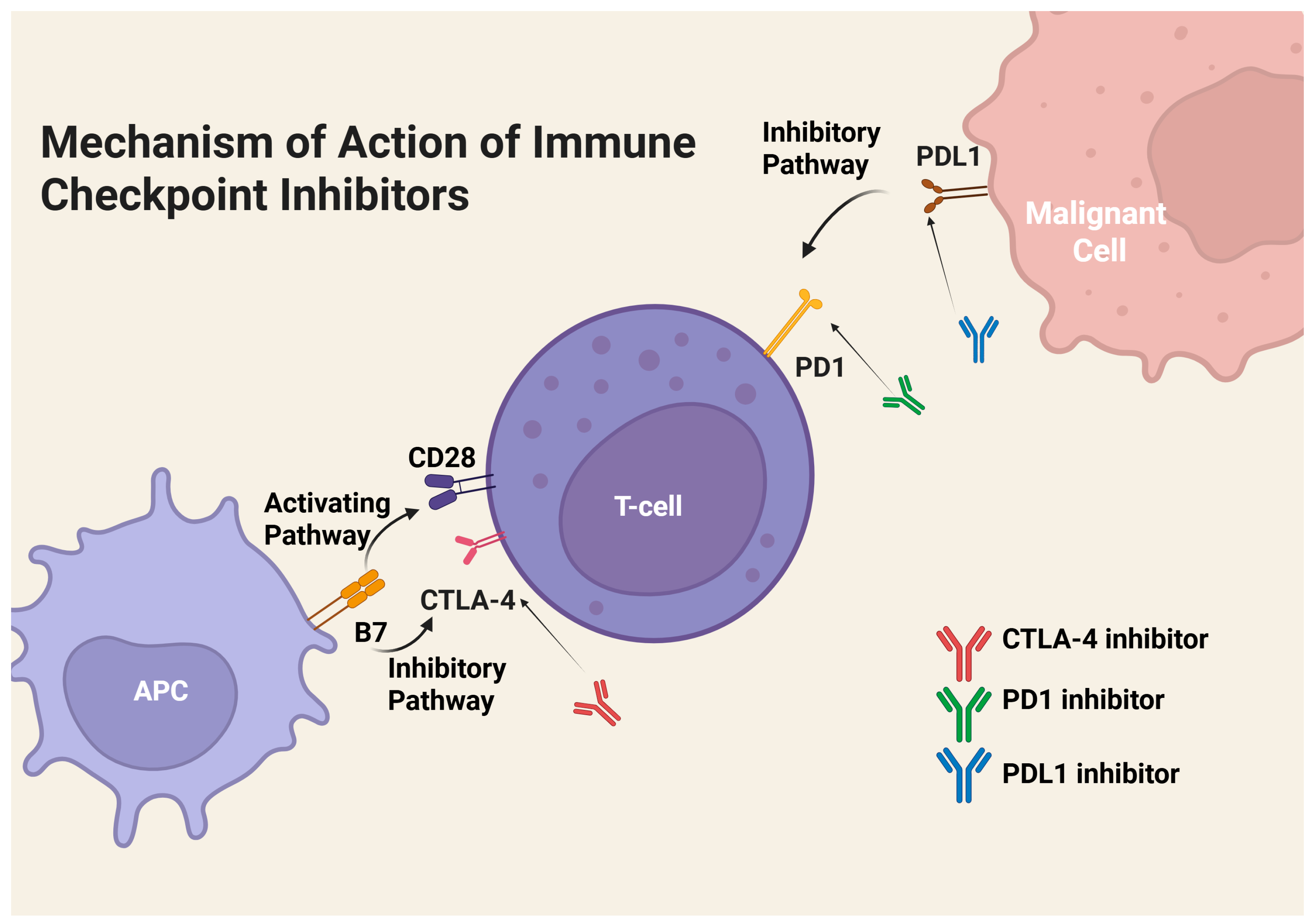
3.2.1. PD-1/PD-L1 Mechanism of Action
3.2.2. PD-1/PD-L1 Outcomes
3.3. CTLA-4 Inhibitors/Antibodies
3.3.1. CTLA-4 Mechanism of Action
3.3.2. CTLA-4 Outcomes
3.4. Immunotherapy Resistance
3.5. Overcoming Immunotherapy Resistance
4. Surgical Management
4.1. Role of Surgery in NSCLC
4.2. Surgical Procedures Options
4.2.1. Sublobar Resection
Wedge Resection
Segmentectomy
4.2.2. Lobectomies
4.2.3. Pneumonectomy
5. Emerging Surgical Approaches
5.1. Video-Assisted Thoracic Surgery
5.2. Robotic-Assisted Thoracic Surgery
6. Multimodal Approaches and Personalized Medicine
6.1. Integration of Surgery and Immunotherapy
6.2. Role of Clinical Team
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NSCLC | Non-small cell lung cancer |
| PD-1 | Programed cell death receptor 1 |
| PD-1L | Programed cell death receptor ligand 1 |
| CTLA-4 | Cytotoxic T-Lymphocyte Antigen 4 |
| OS | Overall Survival |
| PFS | Progression-free survival |
| EFS | Event-free survival |
| DFS | Disease-free survival |
| HR | Hazard ratios |
| RR | Risk ratios |
| TCR | T-cell receptor |
| ICI | Immune checkpoint inhibitors |
| bsABs | Bispecific Antibodies |
| EGFR | Epidermal Growth Factor Receptor |
| ctDNA | Circulating Tumor DNA |
| VATS | Video-assisted thoracic surgery |
| RATS | Robotic-assisted surgery |
| FVC | Forced vital capacity |
| FEV1 | Forced expiratory volume in 1 s |
| RCT | Randomized control trial |
| NK | Natural Killer |
| MDT | Multidisciplinary team |
References
- Gridelli, C.; Rossi, A.; Carbone, D.P.; Guarize, J.; Karachaliou, N.; Mok, T.; Petrella, F.; Spaggiari, L.; Rosell, R. Non-small-cell lung cancer. Nat. Rev. Dis. Primer 2015, 1, 15009. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes. Dis. 2022, 10, 1367–1401. [Google Scholar] [CrossRef]
- Salvà, F.; Felip, E. Neoadjuvant chemotherapy in early-stage non-small cell lung cancer. Transl. Lung Cancer Res. 2013, 2, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Araghi, M.; Mannani, R.; Heidarnejad maleki, A.; Hamidi, A.; Rostami, S.; Safa, S.H.; Faramarzi, F.; Khorasani, S.; Alimohammadi, M.; Tahmasebi, S.; et al. Recent advances in non-small cell lung cancer targeted therapy; an update review. Cancer Cell Int. 2023, 23, 162. [Google Scholar] [CrossRef]
- Uramoto, H.; Tanaka, F. Recurrence after surgery in patients with NSCLC. Transl. Lung Cancer Res. 2014, 3, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Hardavella, G.; Magouliotis, D.E.; Chalela, R.; Januszewski, A.; Dennstaedt, F.; Putora, P.M.; So, A.; Bhowmik, A. Stage I and II nonsmall cell lung cancer treatment options. Breathe 2024, 20, 230219. [Google Scholar] [CrossRef]
- Wang, G.; Liu, L.; Zhang, J.; Li, S. The analysis of prognosis factor in patients with non-small cell lung cancer receiving pneumonectomy. J. Thorac. Dis. 2020, 12, 1366. [Google Scholar] [CrossRef]
- Yu, K.R.; Julliard, W.A. Sublobar Resection of Non-Small-Cell Lung Cancer: Wedge Resection vs. Segmentectomy. Curr. Oncol. 2024, 31, 2497–2507. [Google Scholar] [CrossRef]
- Deng, X.F.; Jiang, L.; Liu, Q.X.; Zhou, D.; Hou, B.; Cui, K.; Min, J.X.; Dai, J.G. Lymph node micrometastases are associated with disease recurrence and poor survival for early-stage non-small cell lung cancer patients: A meta-analysis. J. Cardiothorac. Surg. 2016, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, A.; Canavan, M.E.; Zhan, P.L.; Udelsman, B.V.; Ely, S.; Wigle, D.A.; Martin, L.; Jeffrey Yang, C.F.; Boffa, D.J.; Dhanasopon, A.P. Salvage lung resection after immunotherapy is feasible and safe. JTCVS Open 2024, 20, 141–150. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef]
- O’Brien, M.; Paz-Ares, L.; Marreaud, S.; Dafni, U.; Oselin, K.; Havel, L.; Esteban, E.; Isla, D.; Martinez-Marti, A.; Faehling, M.; et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): An interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022, 23, 1274–1286. [Google Scholar] [CrossRef] [PubMed]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Felip, E.; Altorki, N.; Zhou, C.; Csőszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase 3 trial. Lancet Lond. Engl. 2021, 398, 1344–1357. [Google Scholar] [CrossRef] [PubMed]
- Cascone, T.; William, W.N.; Weissferdt, A.; Leung, C.H.; Lin, H.Y.; Pataer, A.; Godoy, M.C.B.; Carter, B.W.; Federico, L.; Reuben, A.; et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: The phase 2 randomized NEOSTAR trial. Nat. Med. 2021, 27, 504–514. [Google Scholar] [CrossRef]
- Sandbank, E.; Eckerling, A.; Margalit, A.; Sorski, L.; Ben-Eliyahu, S. Immunotherapy during the Immediate Perioperative Period: A Promising Approach against Metastatic Disease. Curr. Oncol. 2023, 30, 7450–7477. [Google Scholar] [CrossRef]
- Liu, L.L.; Skribek, M.; Harmenberg, U.; Gerling, M. Systemic inflammatory syndromes as life-threatening side effects of immune checkpoint inhibitors: Case report and systematic review of the literature. J. Immunother. Cancer 2023, 11, e005841. [Google Scholar] [CrossRef]
- Deboever, N.; McGrail, D.J.; Lee, Y.; Tran, H.T.; Mitchell, K.G.; Antonoff, M.B.; Hofstetter, W.L.; Mehran, R.J.; Rice, D.C.; Roth, J.A.; et al. Surgical approach does not influence changes in circulating immune cell populations following lung cancer resection. Lung Cancer 2022, 164, 69–75. [Google Scholar] [CrossRef]
- Fukuda, S.; Suda, K.; Hamada, A.; Tsutani, Y. Recent Advances in Perioperative Immunotherapies in Lung Cancer. Biomolecules 2023, 13, 1377. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Jassem, J. Multidisciplinary team care in advanced lung cancer. Transl. Lung Cancer Res. 2020, 9, 1690–1698. [Google Scholar] [CrossRef]
- Nurwidya, F.; Murakami, A.; Takahashi, F.; Takahashi, K. Molecular Mechanisms Contributing to Resistance to Tyrosine Kinase-Targeted Therapy for Non-Small Cell Lung Cancer. Cancer Biol. Med. 2012, 9, 18–22. [Google Scholar] [CrossRef]
- Mithoowani, H.; Febbraro, M. Non-Small-Cell Lung Cancer in 2022: A Review for General Practitioners in Oncology. Curr. Oncol. 2022, 29, 1828–1839. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Remon, J.; Hellmann, M.D. First-Line Immunotherapy for Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 586–597. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Five-Year Outcomes With Pembrolizumab Versus Chemotherapy for Metastatic Non–Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50%. J. Clin. Oncol. 2021, 39, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Passiglia, F.; Galvano, A.; Gristina, V.; Barraco, N.; Castiglia, M.; Perez, A.; La Mantia, M.; Russo, A.; Bazan, V. Is there any place for PD-1/CTLA-4 inhibitors combination in the first-line treatment of advanced NSCLC?—A trial-level meta-analysis in PD-L1 selected subgroups. Transl. Lung Cancer Res. 2021, 10, 3106–3119. [Google Scholar] [CrossRef]
- Kerrigan, K.; Puri, S. Role of CTLA Inhibition in Management of Non-Small Cell Lung Cancer. Curr. Oncol. Rep. 2022, 24, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Chehrazi-Raffle, A.; Reddi, S.; Salgia, R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: A comprehensive review of registration trials and future considerations. J. Immunother. Cancer 2018, 6, 8. [Google Scholar] [CrossRef]
- Neumann, M.; Murphy, N.; Seetharamu, N. The Evolving Role of PD-L1 Inhibition in Non-Small Cell Lung Cancer: A Review of Durvalumab and Avelumab. Cancer Med. J. 2022, 5, 31–45. [Google Scholar]
- Pai-Scherf, L.; Blumenthal, G.M.; Li, H.; Subramaniam, S.; Mishra-Kalyani, P.S.; He, K.; Zhao, H.; Yu, J.; Paciga, M.; Goldberg, K.B.; et al. FDA Approval Summary: Pembrolizumab for Treatment of Metastatic Non-Small Cell Lung Cancer: First-Line Therapy and Beyond. Oncologist 2017, 22, 1392–1399. [Google Scholar] [CrossRef]
- Johnson, M.L.; Cho, B.C.; Luft, A.; Alatorre-Alexander, J.; Geater, S.L.; Laktionov, K.; Kim, S.W.; Ursol, G.; Hussein, M.; Lim, F.L.; et al. Durvalumab With or Without Tremelimumab in Combination With Chemotherapy as First-Line Therapy for Metastatic Non–Small-Cell Lung Cancer: The Phase III POSEIDON Study. J. Clin. Oncol. 2023, 41, 1213–1227. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, S.; Du, Y.; Sun, L.; Jiang, H.; Zhang, B.; Sun, G.; Wang, R. Efficacy and safety of programmed death 1 inhibitors in patients with advanced non-small cell lung cancer: A meta-analysis. Cancer Manag. Res. 2019, 11, 4619–4630. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.N.; Wang, T.; Ma, J.Z.; Sui, H.; Deng, W.L. The efficacy and safety of PD-1/PD-L1 inhibitors versus chemotherapy in patients with previously treated advanced non-small-cell lung cancer. Medicine 2021, 100, e25145. [Google Scholar] [CrossRef]
- Roussos, P.; Migkou, M. Impact of PD-1/PD-L1 inhibitors on survival in stage III non-small-cell lung cancer: A systematic review. Cancer Pathog. Ther. 2024, 2, 155–163. [Google Scholar] [CrossRef]
- Vellanki, P.J.; Mulkey, F.; Jaigirdar, A.A.; Rodriguez, L.; Wang, Y.; Xu, Y.; Zhao, H.; Liu, J.; Howe, G.; Wang, J.; et al. FDA Approval Summary: Nivolumab with Ipilimumab and Chemotherapy for Metastatic Non-small Cell Lung Cancer, A Collaborative Project Orbis Review. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 3522–3527. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Caro, R.B.; Zurawski, B.; Kim, S.W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Zieliński, P.; Stępień, M.; Chowaniec, H.; Kalyta, K.; Czerniak, J.; Borowczyk, M.; Dwojak, E.; Mroczek, M.; Dworacki, G.; Ślubowska, A.; et al. Resistance in Lung Cancer Immunotherapy and How to Overcome It: Insights from the Genetics Perspective and Combination Therapies Approach. Cells 2025, 14, 587. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, H. Immunotherapy resistance in non-small-cell lung cancer: From mechanism to clinical strategies. Front. Immunol. 2023, 14, 1129465. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Hellmann, M.D. Acquired Resistance to Immune Checkpoint Inhibitors. Cancer Cell 2020, 37, 443–455. [Google Scholar] [CrossRef]
- Shao, J.; Jin, Y.; Jin, C. A new approach to overcoming resistance to immunotherapy: Nanotechnology. Front. Oncol. 2023, 13, 1210245. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, Q.; Shen, J.; Wei, T.; Shen, W.; Zhang, N.; Luo, P.; Zhang, J. The Effect of Smoking on the Immune Microenvironment and Immunogenicity and Its Relationship With the Prognosis of Immune Checkpoint Inhibitors in Non-small Cell Lung Cancer. Front. Cell Dev. Biol. 2021, 9, 745859. [Google Scholar] [CrossRef]
- McQuade, J.L.; Daniel, C.R.; Hess, K.R.; Mak, C.; Wang, D.Y.; Rai, R.R.; Park, J.J.; Haydu, L.E.; Spencer, C.; Wongchenko, M.; et al. The association of BMI and outcomes in metastatic melanoma: A retrospective, multicohort analysis of patients treated with targeted therapy, immunotherapy, or chemotherapy. Lancet Oncol. 2018, 19, 310–322. [Google Scholar] [CrossRef]
- Martín-Ruiz, A.; Fiuza-Luces, C.; Rincón-Castanedo, C.; Fernández-Moreno, D.; Gálvez, B.G.; Martínez-Martínez, E.; Martín-Acosta, P.; Coronado, M.J.; Franco-Luzón, L.; González-Murillo, Á.; et al. Benefits of exercise and immunotherapy in a murine model of human non-small-cell lung carcinoma. Exerc. Immunol. Rev. 2020, 26, 100–115. [Google Scholar]
- Cheng, W.; Kang, K.; Zhao, A.; Wu, Y. Dual blockade immunotherapy targeting PD-1/PD-L1 and CTLA-4 in lung cancer. J. Hematol. Oncol. 2024, 17, 54. [Google Scholar] [CrossRef]
- Reck, M.; Ciuleanu, T.E.; Schenker, M.; Bordenave, S.; Cobo, M.; Juan-Vidal, O.; Reinmuth, N.; Richardet, E.; Felip, E.; Menezes, J.; et al. Five-year outcomes with first-line nivolumab plus ipilimumab with 2 cycles of chemotherapy versus 4 cycles of chemotherapy alone in patients with metastatic non-small cell lung cancer in the randomized CheckMate 9LA trial. Eur. J. Cancer 2024, 211, 114296. [Google Scholar] [CrossRef]
- Gao, X.; Xu, N.; Li, Z.; Shen, L.; Ji, K.; Zheng, Z.; Liu, D.; Lou, H.; Bai, L.; Liu, T.; et al. Safety and antitumour activity of cadonilimab, an anti-PD-1/CTLA-4 bispecific antibody, for patients with advanced solid tumours (COMPASSION-03): A multicentre, open-label, phase 1b/2 trial. Lancet Oncol. 2023, 24, 1134–1146. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ma, Y.; Fan, Y.; Zhou, J.; Yang, N.; Yu, Q.; Zhuang, W.; Song, W.; Wang, Z.M.; Li, B.; et al. A multicenter, open-label phase Ib/II study of cadonilimab (anti PD-1 and CTLA-4 bispecific antibody) monotherapy in previously treated advanced non-small-cell lung cancer (AK104-202 study). Lung Cancer 2023, 184, 107355. [Google Scholar] [CrossRef]
- Carlisle, J.W.; Wolner, Z.; Pannu, S.; Mitchell, C.; Hsu, M.; Aijaz, A.; Johnson, M.; Naqash, A.R. Bispecific Antibodies in Non-Small Cell Lung Cancer: From Targeted Innovation to Real-World Integration. Am. Soc. Clin. Oncol. Educ. Book 2025, 45, e472792. [Google Scholar] [CrossRef] [PubMed]
- Schram, A.M.; Goto, K.; Kim, D.W.; Macarulla, T.; Hollebecque, A.; O’Reilly, E.M.; Ou, S.H.I.; Rodon, J.; Rha, S.Y.; Nishino, K.; et al. Efficacy of Zenocutuzumab in NRG1 Fusion-Positive Cancer. N. Engl. J. Med. 2025, 392, 566–576. [Google Scholar] [CrossRef] [PubMed]
- HARMONi-A Study Investigators; Fang, W.; Zhao, Y.; Luo, Y.; Yang, R.; Huang, Y.; He, Z.; Zhao, H.; Li, M.; Li, K.; et al. Ivonescimab Plus Chemotherapy in Non-Small Cell Lung Cancer with EGFR Variant: A Randomized Clinical Trial. JAMA 2024, 332, 561–570. [Google Scholar] [CrossRef]
- Marinello, A.; Tagliamento, M.; Pagliaro, A.; Conci, N.; Cella, E.; Vasseur, D.; Remon, J.; Levy, A.; Dall’Olio, F.G.; Besse, B. Circulating tumor DNA to guide diagnosis and treatment of localized and locally advanced non-small cell lung cancer. Cancer Treat. Rev. 2024, 129, 102791. [Google Scholar] [CrossRef]
- Duffy, M.J. Circulating tumor DNA (ctDNA) as a biomarker for lung cancer: Early detection, monitoring and therapy prediction. Tumour Biol. 2024, 46, S283–S295. [Google Scholar] [CrossRef]
- Borg, M.; Wen, S.W.C.; Andersen, R.F.; Timm, S.; Hansen, T.F.; Hilberg, O. Methylated Circulating Tumor DNA in Blood as a Tool for Diagnosing Lung Cancer: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 3959. [Google Scholar] [CrossRef]
- Guo, K.; Lu, J.; Lou, Y.; Zheng, S. Prognostic value of postoperative ctDNA detection in patients with early non-small cell lung cancer: A systematic review and meta-analysis. Ther. Adv. Med. Oncol. 2023, 15, 17588359231177008. [Google Scholar] [CrossRef]
- Steele, J.; Taylor, E.; Khuri, S.; Houldsworth, A. Therapy gone viral: Exploring the use of oncolytic viruses in non-small cell lung cancer immunotherapy. Cancer Treat. Res. Commun. 2025, 44, 100971. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Z.; Sun, J.X.; Liu, C.Q.; Pan, R.Y.; Zeng, N.; Zhang, S.H.; An, Y.; Xu, M.Y.; Zhong, X.Y.; Ma, S.Y.; et al. Efficacy of oncolytic virus in the treatment of intermediate-to-advanced solid tumors: A systematic review and meta-analysis. J. Virol. 2025, 99, e00640-25. [Google Scholar] [CrossRef] [PubMed]
- Wolf, E.; Sacchi de Camargo Correia, G.; Li, S.; Zhao, Y.; Manochakian, R.; Lou, Y. Emerging Immunotherapies for Advanced Non-Small-Cell Lung Cancer. Vaccines 2025, 13, 128. [Google Scholar] [CrossRef]
- O’Reilly, D.; Botticella, A.; Barry, S.; Cotter, S.; Donington, J.S.; Le Pechoux, C.; Naidoo, J. Treatment Decisions for Resectable Non–Small-Cell Lung Cancer: Balancing Less With More? Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e389950. [Google Scholar] [CrossRef]
- Salvicchi, A.; Tombelli, S.; Mugnaini, G.; Gonfiotti, A. Lung Segmentectomy in NSCLC Surgery. Life 2023, 13, 1284. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef]
- Sublobar Resections—ClinicalKey. Available online: https://www.clinicalkey.com/#!/content/playContent/1-s2.0-S1547412723000117?returnurl=https:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS1547412723000117%3Fshowall%3Dtrue&referrer=https:%2F%2Fpubmed.ncbi.nlm.nih.gov%2F (accessed on 31 August 2025).
- Moon, Y.; Lee, K.Y.; Moon, S.W.; Park, J.K. Sublobar Resection Margin Width Does Not Affect Recurrence of Clinical N0 Non-small Cell Lung Cancer Presenting as GGO-Predominant Nodule of 3 cm or Less. World J. Surg. 2017, 41, 472–479. [Google Scholar] [CrossRef]
- Magouliotis, D.E.; Zotos, P.A.; Karamolegkou, A.P.; Tatsios, E.; Spiliopoulos, K.; Athanasiou, T. Long-Term Survival after Extended Sleeve Lobectomy (ESL) for Central Non-Small Cell Lung Cancer (NSCLC): A Meta-Analysis with Reconstructed Time-to-Event Data. J. Clin. Med. 2022, 12, 204. [Google Scholar] [CrossRef]
- Zalepugas, D.; Koryllos, A.; Stoelben, E.; Ludwig, C. Sleeve lobectomy versus lobectomy for primary treatment of non-small–cell lung cancer: A single-center retrospective analysis. J. Surg. Oncol. 2021, 123, 553–559. [Google Scholar] [CrossRef]
- Waseda, R.; Iwasaki, A. Extended sleeve lobectomy: Its place in surgical therapy for centrally located non-small cell lung cancer and a review of technical aspects. J. Thorac. Dis. 2018, 10 (Suppl. S26), S3103–S3108. [Google Scholar] [CrossRef]
- Balderson, S.S.; D’Amico, T.A. Thoracoscopic lobectomy for the management of non-small cell lung cancer. Curr. Oncol. Rep. 2008, 10, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Pneumonectomy for Non–Small Cell Lung Cancer—ClinicalKey. Available online: https://www.clinicalkey.com/#!/content/playContent/1-s2.0-S1055320716000144?returnurl=https:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS1055320716000144%3Fshowall%3Dtrue&referrer=https:%2F%2Fpubmed.ncbi.nlm.nih.gov%2F (accessed on 2 September 2025).
- Agostini, P.J.; Lugg, S.T.; Adams, K.; Smith, T.; Kalkat, M.S.; Rajesh, P.B.; Steyn, R.S.; Naidu, B.; Rushton, A.; Bishay, E. Risk factors and short-term outcomes of postoperative pulmonary complications after VATS lobectomy. J. Cardiothorac. Surg. 2018, 13, 28. [Google Scholar] [CrossRef]
- Howington, J.A.; Blum, M.G.; Chang, A.C.; Balekian, A.A.; Murthy, S.C. Treatment of Stage I and II Non-small Cell Lung Cancer: Diagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2013, 143 (Suppl. S5), e278S–e313S. [Google Scholar] [CrossRef]
- Deng, H.Y.; Qiu, X.M.; Zhu, D.X.; Tang, X.; Zhou, Q. Video-Assisted Thoracoscopic Sleeve Lobectomy for Centrally Located Non-small Cell Lung Cancer: A Meta-analysis. World J. Surg. 2021, 45, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.L.; Mack, S.J.; Rshaidat, H.; Collins, M.L.; Whitehorn, G.L.; Grenda, T.R.; Evans, N.R., 3rd; Okusanya, O.T. Wedge Resection Outcomes: A Comparison of Video-Assisted and Robot-Assisted Wedge Resections. Ann. Thorac. Surg. 2024, 118, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Zheng, Y.; Yuan, Y.; Han, D.; Cao, Y.; Zhang, Y.; Li, C.; Xiang, J.; Zhang, Z.; Niu, Z.; et al. Robotic-assisted Versus Video-assisted Thoracoscopic Lobectomy: Short-term Results of a Randomized Clinical Trial (RVlob Trial). Ann. Surg. 2022, 275, 295. [Google Scholar] [CrossRef]
- Syrigos, N.; Fyta, E.; Goumas, G.; Trontzas, I.P.; Vathiotis, I.; Panagiotou, E.; Nikiteas, N.I.; Kotteas, E.; Dimitroulis, D. Robotic-Assisted Thoracoscopic Surgery Versus Video-Assisted Thoracoscopic Surgery: Which Is the Preferred Approach for Early-Stage NSCLC? J. Clin. Med. 2025, 14, 3032. [Google Scholar] [CrossRef]
- Tsutani, Y.; Handa, Y.; Shimada, Y.; Ito, H.; Ikeda, N.; Nakayama, H.; Yoshimura, K.; Okada, M. Comparison of cancer control between segmentectomy and wedge resection in patients with clinical stage IA non–small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2021, 162, 1244–1252.e1. [Google Scholar] [CrossRef]
- Altorki, N.; Wang, X.; Kozono, D.; Watt, C.; Landrenau, R.; Wigle, D.; Port, J.; Jones, D.R.; Conti, M.; Ashrafi, A.S.; et al. Lobar or Sublobar Resection for Peripheral Stage IA Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 388, 489–498. [Google Scholar] [CrossRef]
- Saji, H.; Okada, M.; Tsuboi, M.; Nakajima, R.; Suzuki, K.; Aokage, K.; Aoki, T.; Okami, J.; Yoshino, I.; Ito, H.; et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): A multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022, 399, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Soldath, P.; Ryom, P.; Petersen, R.H. Long-term survival after sleeve lobectomy versus pneumonectomy for non-small cell lung cancer. Surg. Oncol. 2025, 58, 102168. [Google Scholar] [CrossRef]
- Akutay, S.; Ceyhan, Ö. The relationship between fear of surgery and affecting factors in surgical patients. Perioper. Med. 2023, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Reza, T.; Grezenko, H.; Barker, C.; Bakht, D.; Faran, N.; Abdullah Yahya, N.; Affaf, M.; Mohamed, H.; Gasim, R.; IKH Almadhoun, M.K.; et al. Emotional Stress and Immune Response in Surgery: A Psychoneuroimmunological Perspective. Cureus 2023, 15, e48727. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Tie, Y.; Tu, C.; Wei, X. Surgical trauma-induced immunosuppression in cancer: Recent advances and the potential therapies. Clin. Transl. Med. 2020, 10, 199–223. [Google Scholar] [CrossRef]
- Kim, E.Y.; Hong, T.H. Changes in total lymphocyte count and neutrophil-to-lymphocyte ratio after curative pancreatectomy in patients with pancreas adenocarcinoma and their prognostic role. J. Surg. Oncol. 2019, 120, 1102–1111. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, P.; Sun, Z.; Wang, Y.; Chen, J.; Miao, C. Surgical trauma induces postoperative T-cell dysfunction in lung cancer patients through the programmed death-1 pathway. Cancer Immunol. Immunother. 2015, 64, 1383–1392. [Google Scholar] [CrossRef]
- Elias, A.W.; Kasi, P.M.; Stauffer, J.A.; Thiel, D.D.; Colibaseanu, D.T.; Mody, K.; Joseph, R.W.; Bagaria, S.P. The Feasibility and Safety of Surgery in Patients Receiving Immune Checkpoint Inhibitors: A Retrospective Study. Front. Oncol. 2017, 7, 121. [Google Scholar] [CrossRef]
- Wang, J.; Su, X.; Yang, L.; Qiao, F.; Fang, Y.; Yu, L.; Yang, Q.; Wang, Y.; Yin, Y.; Chen, R.; et al. The influence of myeloid-derived suppressor cells on angiogenesis and tumor growth after cancer surgery. Int. J. Cancer 2016, 138, 2688–2699. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, S.; Geng, P.; Zhang, H.; Zhang, H.; Tang, A.; Xie, X. Minimally invasive video-assisted versus conventional open thyroidectomy on immune response: A meta analysis. Int. J. Clin. Exp. Med. 2015, 8, 2593–2599. [Google Scholar]
- Liu, J.; Blake, S.J.; Yong, M.C.R.; Harjunpää, H.; Ngiow, S.F.; Takeda, K.; Young, A.; O’Donnell, J.S.; Allen, S.; Smyth, M.J.; et al. Improved Efficacy of Neoadjuvant Compared to Adjuvant Immunotherapy to Eradicate Metastatic Disease. Cancer Discov. 2016, 6, 1382–1399. [Google Scholar] [CrossRef]
- Liu, J.; O’Donnell, J.S.; Yan, J.; Madore, J.; Allen, S.; Smyth, M.J.; Teng, M.W.L. Timing of neoadjuvant immunotherapy in relation to surgery is crucial for outcome. Oncoimmunology 2019, 8, e1581530. [Google Scholar] [CrossRef]
- De Lucia, A.; Mazzotti, L.; Gaimari, A.; Zurlo, M.; Maltoni, R.; Cerchione, C.; Bravaccini, S.; Delmonte, A.; Crinò, L.; Borges de Souza, P.; et al. Non-small cell lung cancer and the tumor microenvironment: Making headway from targeted therapies to advanced immunotherapy. Front. Immunol. 2025, 16, 1515748. [Google Scholar] [CrossRef]
- Galetta, D.; De Marinis, F.; Spaggiari, L. Rescue Surgery after Immunotherapy/Tyrosine Kinase Inhibitors for Initially Unresectable Lung Cancer. Cancers 2022, 14, 2661. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Li, R.; Sun, J.; Lou, Y.; Zhang, W.; Bai, H.; Wang, H.; Shen, J.; Jing, B.; Shi, C.; et al. Erlotinib as Neoadjuvant Therapy in Stage IIIA (N2) EGFR Mutation-Positive Non-Small Cell Lung Cancer: A Prospective, Single-Arm, Phase II Study. Oncologist 2019, 24, 157-e64. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.Z.; Chen, K.N.; Chen, C.; Gu, C.D.; Wang, J.; Yang, X.N.; Mao, W.M.; Wang, Q.; Qiao, G.B.; Cheng, Y.; et al. Erlotinib Versus Gemcitabine Plus Cisplatin as Neoadjuvant Treatment of Stage IIIA-N2 EGFR-Mutant Non–Small-Cell Lung Cancer (EMERGING-CTONG 1103): A Randomized Phase II Study. J. Clin. Oncol. 2019, 37, 2235–2245. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, F.; Hu, H.; Wang, S.; Li, Y.; Hu, H.; Chen, H. Gefitinib as neoadjuvant therapy for resectable stage II-IIIA non–small cell lung cancer: A phase II study. J. Thorac. Cardiovasc. Surg. 2021, 161, 434–442.e2. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.; Nagasaka, M. Neoadjuvant EGFR-TKI therapy in Non-Small cell lung cancer. Cancer Treat. Rev. 2024, 126, 102724. [Google Scholar] [CrossRef] [PubMed]
- Saeteng, S.; Chewaskulyong, B.; Charoentum, C.; Lertprasertsuke, N.; Euathrongchit, J.; Tajarernmuang, P.; Klunklin, P.; Siwachat, S.; Kongkarnka, S.; Wannasopha, Y.; et al. The Impact of a Multidisciplinary Team Conference on Non-Small Cell Lung Cancer Care: Time Barriers and Long-Term Outcomes. J. Clin. Med. 2024, 13, 5276. [Google Scholar] [CrossRef] [PubMed]
- Bilfinger, T.V.; Albano, D.; Perwaiz, M.; Keresztes, R.; Nemesure, B. Survival Outcomes Among Lung Cancer Patients Treated Using a Multidisciplinary Team Approach. Clin. Lung Cancer 2018, 19, 346–351. [Google Scholar] [CrossRef] [PubMed]


| Immunotherapeutic Class | Mechanism of Action | Survival | Limitations | Ref. |
|---|---|---|---|---|
| PD-1 Inhibitors | Immunoglobins directly inhibit PD-1 receptors on T-cells, which generates an immune response | OS saw a 13-month improvement when compared to chemotherapy | Cannot be used when PD-L1 expression is low | [4,28,29,30] |
| PD-L1 inhibitors | Immunoglobins directly inhibit PD-L1 on malignant cells, which generates an immune response | OS HR of 0.85 when compared to chemotherapy | Cannot be used when PD-L1 expression is low | [4,28,29,30] |
| CTLA-4 inhibitors | Immunoglobulins directly inhibit CTLA-4 receptors on T-cells, allowing T-cell activation and the initiation of an immune response against malignant cells | PFS HR of 0.75 compared to chemotherapy | Can cause system-wide reactions | [4,24,26,27,31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janes, M.J.; Schmidt, A.A.; Krieg, G.A.; Chouhan, A.S.; Wakefield, M.R.; Fang, Y. From Incision to Immunity: Integrating Surgery and Immunotherapy in Non-Small Cell Lung Cancer. Immuno 2025, 5, 48. https://doi.org/10.3390/immuno5040048
Janes MJ, Schmidt AA, Krieg GA, Chouhan AS, Wakefield MR, Fang Y. From Incision to Immunity: Integrating Surgery and Immunotherapy in Non-Small Cell Lung Cancer. Immuno. 2025; 5(4):48. https://doi.org/10.3390/immuno5040048
Chicago/Turabian StyleJanes, Michael J., Aidan A. Schmidt, Garret A. Krieg, Amitoj S. Chouhan, Mark R. Wakefield, and Yujiang Fang. 2025. "From Incision to Immunity: Integrating Surgery and Immunotherapy in Non-Small Cell Lung Cancer" Immuno 5, no. 4: 48. https://doi.org/10.3390/immuno5040048
APA StyleJanes, M. J., Schmidt, A. A., Krieg, G. A., Chouhan, A. S., Wakefield, M. R., & Fang, Y. (2025). From Incision to Immunity: Integrating Surgery and Immunotherapy in Non-Small Cell Lung Cancer. Immuno, 5(4), 48. https://doi.org/10.3390/immuno5040048






