Salt Stress Effects on the Growth, Photosynthesis and Antioxidant Enzyme Activities in Maize (Zea mays L.) Cultivars †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Cultivation and Processing
2.2. Measurements and Sampling
2.3. Statistical Analyses
3. Results
3.1. Effect of Salt Stress on Physiological and Morphological Traits
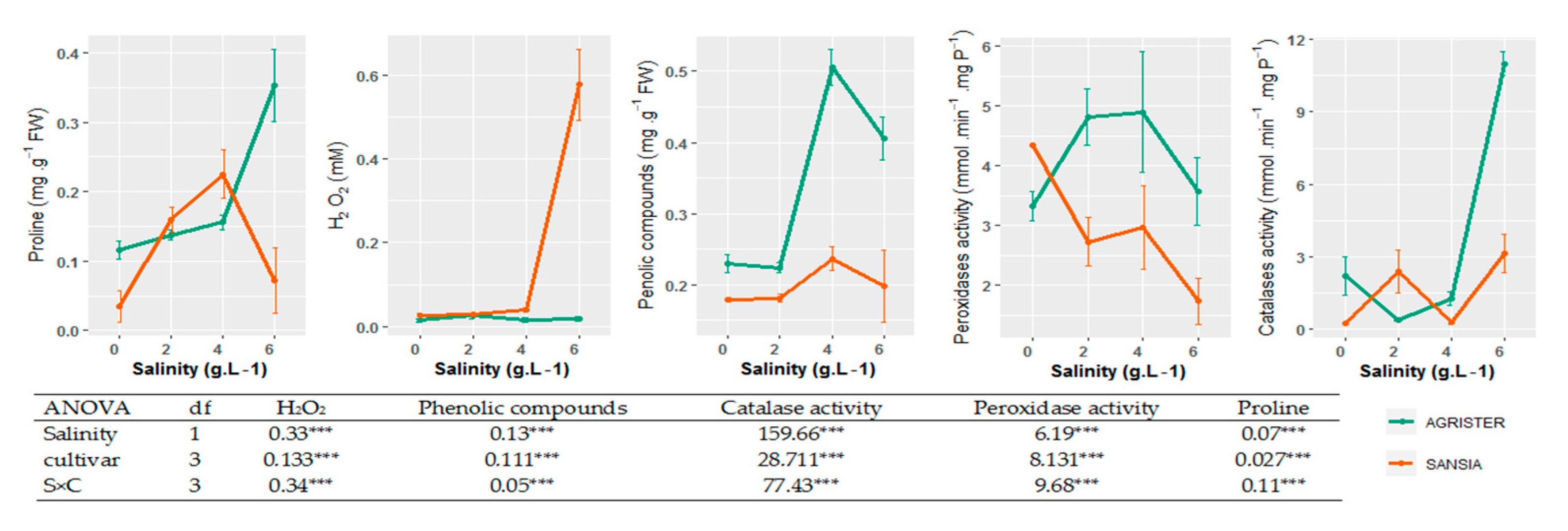
3.2. Effect of Salt Stress on the Antioxidants Enzymes, H2O2, Proline, and Phenolic Compounds
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ben Khaled, A.; Hayek, T.; Mansour, E.; Hannachi, H.; Lachiheb, B.; Ferchichi, A. Evaluating salt tolerance of 14 barley accessions from southern Tunisia using multiple parameters. J. Agric. Sci. 2012, 4, 27–38. [Google Scholar] [CrossRef] [Green Version]
- AbdElgawad, H.; Zinta, G.; Hegab, M.M.; Pandey, R.; Asard, H.; Abuelsoud, W. High salinity induces different oxidative stress and antioxidant responses in maize seedlings organs. Front. Plant Sci. 2016, 7, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Lu, Y.; Wu, M.; Liang, E.; Li, Y.; Zhang, D.; Yin, Z.; Ren, X.; Dai, Y.; Deng, D.; et al. Ability to Remove Na+ and Retain K+ Correlates with Salt Tolerance in Two Maize Inbred Lines Seedlings. Front. Plant Sci. 2016, 7, 1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ditta, A. Salt tolerance in cereals: Molecular mechanisms and applications. In Molecular Stress Phsyiology of Plants; Rout, G., Das, A., Eds.; Springer: New Delhi, India, 2013; pp. 133–154. [Google Scholar]
- Huqe, M.A.S.; Haque, M.S.; Sagar, A.; Uddin, M.N.; Hossain, M.A.; Hossain, A.Z.; Rahman, M.M.; Wang, X.; Al-Ashkar, I.; Ueda, A.; et al. Characterization of Maize Hybrids (Zea mays L.) for Detecting Salt Tolerance Based on Morpho-Physiological Characteristics, Ion Accumulation and Genetic Variability at Early Vegetative Stage. Plants 2011, 10, 2549. [Google Scholar] [CrossRef] [PubMed]
- Carpici, E.B.; Celik, N.; Bayram, G. The effects of salt stress on the growth, biochemical parameter and mineral element content of some maize (Zea mays L.) cultivars. Afr. J. Biotechnol. 2010, 9, 6937–6942. [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Filek, M.; Walas, S.; Mrowiec, H.; Rudolphy-Skórska, E.; Sieprawska, A.; Biesaga-Kościelniak, J. Membrane permeability and micro-and macroelement accumulation in spring wheat cultivars during the short-term effect of salinity-and PEG-induced water stress. Acta Physiol. Plant. 2012, 34, 985–995. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Noreen, Z.; Ashraf, M. Assessment of variation in antioxidative defense system in salt treated pea (Pisum sativum L.) cultivars and its putative use as salinity tolerance markers. J. Plant Physiol. 2009, 166, 1764–1774. [Google Scholar] [CrossRef]
- Egley, G.H.; Paul, R.N.; Vaughn, K.C.; Duke, S.O. Role of peroxidase in the development of water-impermeable seed coats in Sida spinosa L. Planta 1983, 157, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Hichem, H.; Mounir, D. Differential responses of two maize (Zea mays L.) varieties to salt stress: Changes on polyphenols composition of foliage and oxidative damages. Ind. Crops Prod. 2009, 30, 144–151. [Google Scholar] [CrossRef]
| Traits | Shoot Length (cm) | Shoot Dry Weight (g) | Chlorophyll Content (g/mL) | RMP (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cultivar | Agrister | Sancia | Agrister | Sancia | Agrister | Sancia | Agrister | Sancia | |
| Salinity | 0 g·L−1 | 34.66 ± 0.24 a | 33.58 ± 0.42 a | 13.56 ± 0.98 a | 10.96 ± 0.61 a | 39.68 ± 0.45 b | 39.44 ± 0.75 a | 86.82 ± 0.89 d | 83.84 ± 0.32 d |
| 2 g·L−1 | 30.83 ± 0.47 b | 28.67 ± 0.62 b | 10.34 ± 0.13 b | 9.13 ± 0.08 b | 41.45 ± 0.03 ab | 40.33 ± 0.58 a | 88.51 ± 0.19 c | 92.74 ± 1.18 c | |
| 4 g·L−1 | 29.83 ± 0.94 b | 27.10 ± 0.29 c | 10.00 ± 0.33 b | 8.77 ± 0.26 b | 41.67 ± 1.55 a | 37.46 ± 0.38 b | 94.38 ± 0.73 b | 96.56 ± 0.65 b | |
| 6 g·L−1 | 28.08 ± 0.51 c | 26.00 ± 0.49 d | 9.18 ± 0.20 b | 7.69 ± 0.13 c | 42.29 ± 0.17 a | 36.46 ± 0.74 b | 97.14 ± 0.18 a | 98.90 ± 0.55 a | |
| ANOVA | df | ||||||||
| Salinity | 1 | 168,515 *** | 47,962 *** | 8817 * | 564,379 *** | ||||
| Cultivar | 3 | 24,402 *** | 15,999 *** | 48,628 *** | 10,055 ** | ||||
| S × C | 3 | 2118 n.s. | 1951 n.s. | 30,741 *** | 41,812 *** | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kthiri, Z.; Hammami, M.D.E.; Marzougui, O.; Jabeur, M.B.; Aouadi, A.; Karmous, C.; Hamada, W. Salt Stress Effects on the Growth, Photosynthesis and Antioxidant Enzyme Activities in Maize (Zea mays L.) Cultivars. Environ. Sci. Proc. 2022, 16, 73. https://doi.org/10.3390/environsciproc2022016073
Kthiri Z, Hammami MDE, Marzougui O, Jabeur MB, Aouadi A, Karmous C, Hamada W. Salt Stress Effects on the Growth, Photosynthesis and Antioxidant Enzyme Activities in Maize (Zea mays L.) Cultivars. Environmental Sciences Proceedings. 2022; 16(1):73. https://doi.org/10.3390/environsciproc2022016073
Chicago/Turabian StyleKthiri, Zayneb, Mohamed Dhia Eddine Hammami, Oumaima Marzougui, Maissa Ben Jabeur, Amal Aouadi, Chahine Karmous, and Walid Hamada. 2022. "Salt Stress Effects on the Growth, Photosynthesis and Antioxidant Enzyme Activities in Maize (Zea mays L.) Cultivars" Environmental Sciences Proceedings 16, no. 1: 73. https://doi.org/10.3390/environsciproc2022016073
APA StyleKthiri, Z., Hammami, M. D. E., Marzougui, O., Jabeur, M. B., Aouadi, A., Karmous, C., & Hamada, W. (2022). Salt Stress Effects on the Growth, Photosynthesis and Antioxidant Enzyme Activities in Maize (Zea mays L.) Cultivars. Environmental Sciences Proceedings, 16(1), 73. https://doi.org/10.3390/environsciproc2022016073






