Abstract
The results of calculations by the DFT method with the exchange–correlation functional B3LYP 6–31 G** of the electronic and spatial structure of the 3,4-ethylenedioxythiophene oligomer containing 12 units (E12) in the charge states 0, +1, +2, +3 and +4 were obtained. Increasing the charge reproduces the increase in the degree of doping. The received results allow us to conclude that in the oxidized oligomer of 3,4-ethylenedioxythiophene containing 12 monomer units, conductivity is provided by the formation of two polarons at the ends of the chain at a high degree of doping. For oligomers with a different number of units in poly 3,4-ethylenedioxythiophene, more complex polaron structures can be realized by charge carriers.
1. Introduction
Conductive polymers (CP) belonging to the so-called “synthetic metal” are polymers with a conjugated carbon chain. They have electrical, electronic, magnetic and optical properties of metals, but retain the plastic properties of conventional polymers, facilitating their processing and further use. Their conductivity, when small amounts of dopants are introduced into the matrix of the original polyconjugated polymers with a typical conductivity of 10−10 to 10−5 Sm·sm−1, significantly increases, reaching the conductivity of semiconductors or even metals from 1 to 10−5 Sm·sm−1.
Doping is performed by chemical or electrochemical oxidation (p-doping) or reduction (n-doping) of the polymer. In this case, the polymer chains acquire, respectively, positive or negative charges, which are compensated by the formation of a polymer matrix of intermolecular complexes in the electrolyte solution with polyions of opposite sign. By adjusting the level of doping, it is possible to change the conductivity of the CP in a wide range.
The main task in the study of CP is to establish the nature and characteristics of charge carriers. As follows from the literature [1,2,3], polarons and/or bipolarons with or without spin can be the charge carriers, ensuring the conductivity of CP. The unambiguous resolution of this task can significantly facilitate the development of new materials with improved properties based on CP for their use in molecular electronics.
Derivatives of polythiophene, including poly 3,4-ethylenedioxythiophene, are the most studied among CP. However, even for this polymer, there are conflicting opinions about the nature of charge carriers and its dependence on the length of the polymer chain.
2. Calculation Methods and Models
The proposed communication presents the results of a study of the electronic and spatial structure of a 3,4-ethylenedioxythiophene oligomer containing 12 units (E12) in charge states 0, +1, +2, +3 and +4, obtained by the density functional theory method with exchange–correlation functional B3LYP 6–31 G**. The increase in charge simulates an increase in the degree of doping. An unrestricted approach was used (UB3LYP functional) for calculations in charge states +1 and +2.
3. Results and Discussion
For the electroneutral state of the E12 oligomer, the calculated C–C bond lengths between adjacent monomer units are 1.433 Å, which is typical of the benzenoid phase (see Figure 1).
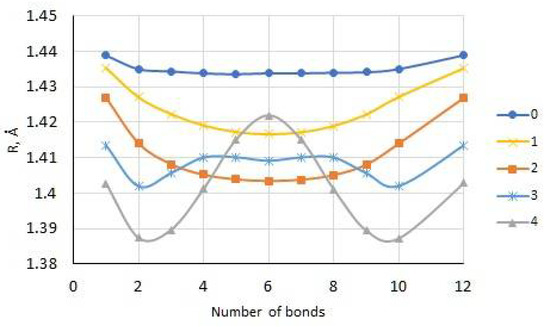
Figure 1.
Dependence of the length of C–C bonds between monomeric units on the charge state of the E12 oligomer. Bond numbering begins with the bond between the first and second monomer units.
However, already at a low degree of doping, i.e., for the E121+ cation, the lengths of these bonds decrease monotonically from the ends of the chain to its center, reaching a minimum value of 1.417 Å at bond 6 (central), which is typical for the quinoid phase of the systems under study. For the E122+ bication, a similar dependence of the bond lengths between monomeric units is also observed. However, these bond lengths are still significantly smaller compared to similar bond lengths in the E121+ cation, which determines the increase in the contribution of the quinoid phase. For higher oxidation states (+3 and +4), on the corresponding curves of the dependence of the charge on the bond number between the intermonomer units, two minima appear in the bond regions 2 and 3, as well as 9 and 10, which indicates a further increase in the contribution of the quinoid phase and the formation at the ends of the oligomer chain of two separated polarons. Similar dependences were obtained for charged pyrrole oligomers [4].
When a positive charge appears in the E12 oligomer, a polaron “hole” level appears in the gap between the energies of the upper occupied molecular orbital (HOMO) and the lower unoccupied molecular orbital (LUMO) of the E121+ cation, which is 0.46 eV away from the top of the valence band (Figure 2).
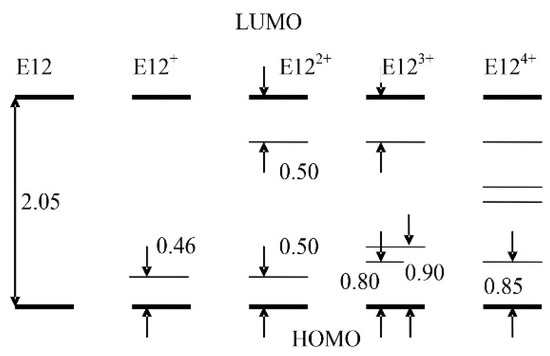
Figure 2.
Formation of the polaron band (eV) depending on the charge state of the E12 oligomer.
Since, during the formation of the E121+ cation, an electron is removed from the HOMO level of the E12 oligomer, the level corresponding to the formation of a “hole” will lie above the HOMO level, as occurs when one electron is removed from a doubly occupied molecular orbital. For the neutral oligomer E12, the width of this gap is 2.06 eV (Figure 3), which shows the dependence of E(LUMO)-E(HOMO) on the number of monomer units. Furthermore, in the text, the HOMO level will be identified by the top of the valence band, and the LUMO level by the bottom of the conduction band.
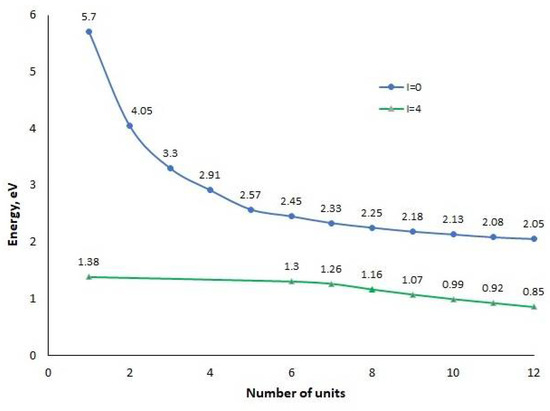
Figure 3.
Energy gap E(LUMO)-E(HOMO), eV.
With a further increase in the degree of doping, two polaron hole levels appear in the E122+ bication, spaced from the top of the valence band and the bottom of the conduction band by 0.50 eV. There are three such levels in the E123+ cation, two of which lie at a distance of 0.8 eV from the top of the valence band, and one is 0.90 eV away from the bottom of the conduction band. For the E124+ cation, there are already four such hole levels that arise during the fourfold ionization of the E12 oligomer, which in pairs give rise to the formation of a polaron band in the energy gap between the HOMO and LUMO levels. The structure of the molecular orbital corresponding to the lowest level, which lies at a distance of 0.85 eV from the HOMO level (Figure 4), clearly demonstrates the formation of two polarons at the ends of the E12 oligomer. With an appropriate interpretation, confirmation of the fact of the formation of two polarons can be obtained from the corresponding experimental data [5,6].
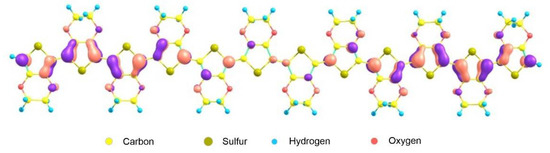
Figure 4.
Structure of the molecular orbital localized at the lowest polaron level in the gap between the HOMO and LUMO energies of the E124+ oligomer.
Figure 4 shows that the quinoid phase of the considered molecular orbital of the E124+ cation is localized at the ends of the chain in two regions, each of which captures four monomeric units. This indicates that the resulting vacancies, “holes”, are not delocalized over the entire oligomeric chain, which should be expected within the framework of the band theory of solids. Holes are localized in two four-link regions of the oligomeric chain of the E124+ cation; so, a bipolaron forms. The appearance of a bipolaron in E124+, as well as a polaron in the E121+ cation, causes structural deformation of the carbon chain of the oligomer (transition of the benzenoid phase to the quinoid phase). Two positive charges of a bipolaron, each with a charge of +2e, influence each other and behave like a pair. Both polarons and bipolarons, under the action of an electric field, are able to move along the polymer chain, leading to the reorganization of double and single bonds in the conjugated system (see Figure 5).
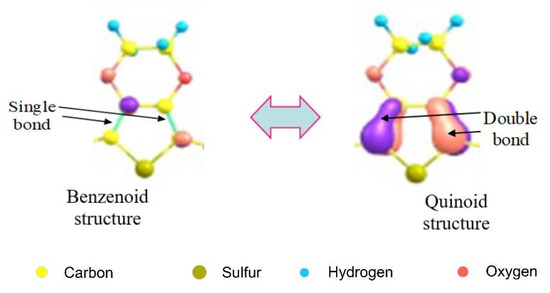
Figure 5.
Benzoic and quinoid structures of the monomer units of the E124+ cation.
4. Conclusions
Thus, the obtained results allow us to conclude that in the oxidized 3,4-ethylenedioxythiophene oligomer containing 12 monomer units, at a high degree of doping, the conductivity is provided by the formation of two polarons at the ends of the chain. For oligomers with a different number of units and 3,4-ethylenedioxythiophene, more complex polaron structures can be charge carriers.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/materproc2022009020/s1.
Author Contributions
Conceptualization, V.L. and M.T.; methodology, V.L., A.D. and O.T.; software, A.D. and O.T.; validation, M.T. and O.F.; formal analysis, O.T. and O.F.; investigation, V.L. and O.F.; writing—original draft preparation, M.T., V.L. and A.D.; writing—review and editing, A.D. and M.T.; visualization, O.T. and O.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- e Silva, G.M. Electric-field effects on the competition between polarons and bipolarons in conjugated polymers. Phys. Rev. B 2000, 61, 10777–10781. [Google Scholar] [CrossRef]
- Zade, S.S.; Bendikov, M. Theoretical study of long oligothiophene dications: Bipolaron vs. polaron pair vs. triplet state. J. Phys. Chem. B 2006, 110, 15839–15846. [Google Scholar] [CrossRef] [PubMed]
- Zamoshchik, N.; Salzner, U.; Bendikov, M. Nature of charge carriers in long doped oligothiophenes: The effect of counterions. J. Phys. Chem. C 2008, 112, 8408–8418. [Google Scholar] [CrossRef]
- Dai, Y.; Wei, C.; Blaisten-Barojas, E. Bipolarons and polaron pairs in oligopyrrole dications. Comput. Theor. Chem. 2012, 993, 7–12. [Google Scholar] [CrossRef]
- Fichou, D.; Horowitz, G.; Xu, B.; Gamier, F. Stoichiometric control of the successive generation of the radical cation and dication of extended aconjugated oligothiophenes: A quantitative model for doped polythiophene. Synth. Met. 1990, 39, 243–259. [Google Scholar] [CrossRef]
- Van Haare, J.A.E.H.; Havinga, E.E.; Van Dongen, J.L.J.; Janssen, R.A.J.; Cornil, J.; Brédas, J.L. Redox states of long oligothiophenes: Two polarons on a single chain. Chem. A Eur. J. 1998, 4, 1509–1522. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).