Abstract
This study examines some relevant limnological and hydrogeochemical characteristics of the most emblematic pit lake of the Iberian Pyrite Belt (IPB). Corta Atalaya (CA) open pit mine is known for its large size and for being one of the most important exploitations of copper in Europe. Despite its historical importance, little is known about the lake formed in this open pit. During this study, the pit lake presented a surface area of 16 ha, a maximum depth of 106 m, and a 5.8 hm3 volume of acid and metal-enriched water. CA pit lake shows permanent chemical stratification (meromictic lake), where three layers with different density and chemical composition can be differentiated: (i) a superficial layer of 5 ± 2 m water depth, with electric conductivity (EC) between 5.4–6.64 mS/cm, and oxygenated and Fe(III)-rich mixolimnion; (ii) an intermediate layer (between 5–30 m, chemocline), exhibiting strong vertical changes in parameters such as temperature (T) and EC, which show an increase with depth; and (iii) a thick bottom layer from 30 m to 106 m depth, with anoxic, elevated EC (47 mS/cm) and T (32 °C) values, and a concentration of Fe as Fe(II) (monimolimnion).The characterization of the water column is essential to know the potential sources of strategic and critical raw materials, and to evaluate their possible recuperation, thereby activating a circular economy.
1. Introduction
The intensive mining carried out in the Iberian Pyrite Belt (IPB) has left a legacy of mine pits with considerable size (e.g., Las Cruces open pit, which is up to 1.6 km long, 0.90 km wide, and 250 m height). Among them, Corta Atalay1a pit lake (CA) boasts significant dimensions, measuring 1.2 Km in length, 0.9 Km in width, and 360 m in height [1] (Figure 1). CA is situated in the northwest of the urban nucleus of the Riotinto Mines in the province of Huelva, Spain. CA was once a site for extracting massive sulphides like zinc (Zn), copper (Cu), and lead (Pb) from the San Dionisio polymetallic deposit. The extraction operations began in 1885 and concluded in 1992.

Figure 1.
Panoramic view of Corta Atalaya pit lake. Yellow star indicates the geographical location of the Riotinto mines (Huelva, Spain).
In the IPB, most pit lakes exhibit acidic characteristics with a pH value of 3 [1]. They also contain high concentrations of sulphate and various metals such as iron (Fe), aluminium (Al), Cu, Zn, and manganese (Mn). Within this context, meromictic lakes, characterized by permanent chemical stratification, are more common than holomictic lakes, which experience winter mixing that homogenizes the water column. Throughout the spring to the end of autumn, a thermal stratification process occurs in all these pit lakes [1,2].
During this study, the lake presented 106 m depth, and the mine open pit was partly flooded; thus, column water could present hydrological and geochemical evolution.
The objectives of the present paper are (i) to establish a first approach to the physical limnology of the mine pit lake, investigating its possible vertical stratification (thermal and/or chemical), and (ii) to characterize the chemical composition of the pit lake waters.
2. Geology
The IPB is a world-class metallogenic province situated in the southwestern corner of the Iberian Peninsula, extending from the north of Seville to the south of Lisbon [3]. The IPB had more than 2000 million tons of ore and has a long history (since pre-Roman times) of extensive mining, comprising more than 80 mines, including historical ore deposits such as Riotinto, Tharsis, Aznalcóllar [4], and Las Cruces. The lithostratigraphic sequence of the IPB consists of Upper Devonian to Middle Carboniferous volcanic and sedimentary rocks, including the PQ group (composed of phyllites and quartzites), the volcanic–sedimentary complex (VSC; formed by shales, greywakes, and mostly acid volcanic rocks), and the Culm group (a flysch-like sequence of shales and greywackes). The mineralization is dominated by pyrite, with lesser amounts of sphalerite, chalcopyrite, galena, and cassiterite [3]. The pit lakes of the IPB share a common geological framework defined by the ubiquitous presence of pyrite, abundant aluminosilicates, and a marked scarcity of carbonate minerals. These mineralogical features have favoured the oxidative dissolution of pyrite, and the subsequent formation of acid mine drainage (AMD) [1].
3. Materials and Methods
The field measurements and water sampling were carried out every two months, from May 2013 to May 2014. Environmental conditions for the water column of the pit lake were measured with a Hydrolab Datasonde S5 probe (Hach, Loveland CO, USA). Water samples were collected from different depths using an opaque, 2.2-L PVC bottle (Beta Plus Wildlife Supply). All samples were filtered on site with 0.45 μm membrane filters from Millipore, stored in 125 mL polyethylene bottles, acidified with HNO3 and and kept at 4 °C during transport.
Water samples were analysed by atomic absorption spectrometry (AAS, Varian SpectrAA 220FS), for Na, K, Mg, Ca, Fe, Cu, Mn and Zn. The determination of elements (Al, Ag, As, Ba, Cd, Co, Cr, Mo, Pb, Sb, Tl, Th, V and U), depending on their content, was performed by ICP-AES (Varian Vista MPX) or ICP-MS (Agilent 7500 ce). Depending on the pH and electrical conductivity values, some determinations of S or SiO2 were analysed by ICP-AES (Varian Vista MPX equipment). The accuracy of the analytical methods was verified against certified reference waters (TM-27.3 and TMDA-51.3 of National Water Research Institute), and close agreement with certified values was achieved for all metals. 115In, 89Y, 159Tb and 209Bi were used as an internal standard for the calibration and measurement of ICP-MS determinations. Pt was used as an internal standard for the calibration and measurement of ICP-MS determinations. Fe(II) concentration was measured by reflectance photometry with a Merck RQflex10 reflectometer and Reflectoquant analytical strips.
Solid bulk samples mineralogy was characterized by powder XRD using a PANanalytical X’Pert Pro diffractometer with Cu Kα radiation (40 kV, 40 mA) with a graphite monochromator, High Score software, and the ICDD database. For routine XRD inspections, 2–70° 2θ scans were used with a 0.5 s counting time per step.
4. Result and Discussion
The CA pit lake presents a permanent chemical stratification, known as a meromictic lake, characterized by the presence of three distinct layers with varying densities and chemical compositions (Figure 2). These layers can be differentiated as follows: (1) A superficial layer (mixolimnion) that presents a depth of approximately 5 ± 2 m. It is characterized by the presence of oxygen and an enrichment in Fe(III) (Figure 3). Its electric conductivity (EC) ranges between 5.4–6.64 mS/cm. (2) An intermediate layer, situated between 5 and 30 m depth, known as the chemocline, that shows strong vertical variations in parameters such as temperature (T) and EC. As depth increases, both T and EC show a progressive increase. (3) A bottom layer, extending from 30 to 106 m of depth; it is anoxic and presents high values of EC (47 mS/cm) and T (32 °C) (Figure 2). Additionally, it is characterized by elevated concentrations of Fe in the form of Fe(II), and is referred to as the monimolimnion (Figure 3).
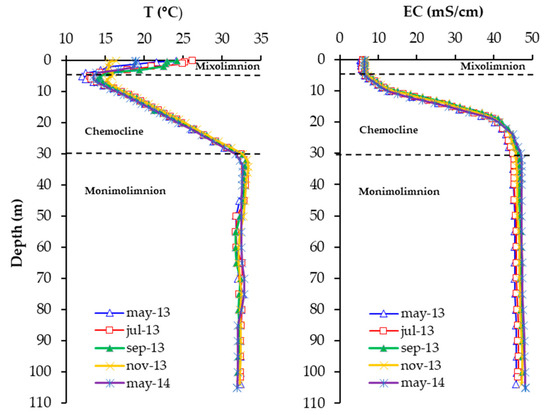
Figure 2.
Seasonal depth profiles of T and EC in the Corta Atalaya pit lake obtained in 2013 and 2014.
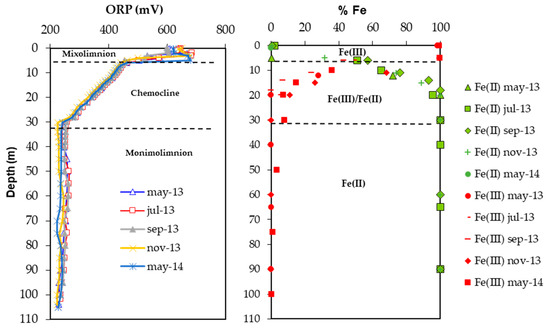
Figure 3.
Percentage distribution of Fe(II) and Fe(III) in the Corta Atalaya water column and its relationship with the ORP values. A thin mixolimnion presents Fe(III), and a transition zone (chemocline) where Fe(III) decreases and Fe(II) increases up to 30 m depth; from here, the dominant species is Fe(II).
The water column’s pH (2.2–2.8) is quite homogenous, with minor seasonal variations. Dissolved oxygen (DO) values are only measured in the mixolimnion, presenting zero values from 5 m depth. The monimolimnion waters show a much lower redox potential (ORP) than the mixolimnion (220 versus 650 mV, respectively) (Figure 3) and a much higher concentration of dissolved solids, as indicated by the EC. The vertical trends in these parameters show slight seasonal variations, but their general features remain relatively constant throughout the year. The increase in density (conditioned by T and EC values) from the surface to 30 m depth is significant, providing the water column with considerable stability.
The pit lake Fe concentrations follow the pattern of an EC profile. In general, when the lake is stratified chemically, the mixolimnion displays high redox values (ORP ~650 mV) that correspond to oxygen-saturated conditions where iron is present mainly as Fe(III) (Figure 3). The total Fe concentration in this layer is less than that of the monimolimnion, and can be attributed to both the effects of dilution associated with runoff and to actual hydrolysis/precipitation of Fe(III), the latter process being helped by the alkalinity contribution supplied by the entry of freshwater. Down to 5 m depth, the Fe concentration is between 650–930 mg/L, increasing in the chemocline down to the monimolimnion (30 m), where it reached the highest values (up to 41 g/L), predominantly in the form of Fe(II). Since the superficial area is in contact with the atmosphere and its waters present DO, the majority of the dissolved Fe is present as Fe(III). In the anoxic layer (monimolimnion), the dissolved Fe is predominantly in the form of Fe(II) (Figure 3). Fe concentrations in mixolimnion are elevated when compared to other pit lakes of IPB [1], and in comparison to monimolimnion are extremely high [3,5].
The majority of acidic pit lakes have a pH < 3 due to iron buffering [1,3]. The hydrolysis of Fe(III) and its subsequent precipitation is a significant reaction that occurs in AMD systems [6], and promotes the formation of different Fe(III) minerals depending on the Eh–pH conditions of the aquatic system (e.g., schwertmannite, goethite, ferrihydrite). In the CA pit lake, the concentration of Fe(III) in mixolimnion is primarily controlled by the solubility of schwertmannite (Figure 4). This oxyhydroxysulphate mineral is usually formed in acid–sulphate waters [7,8], eliminating Fe and SO4 from the system, but also affects some metal adsorption and co-precipitation processes as well as Cu, arsenic (As), Pb, Zn, cobalt (Co), vanadium (V), cadmium (Cd), nickel (Ni) [7], thorium (Th), uranium (U), and rare earth elements [9].
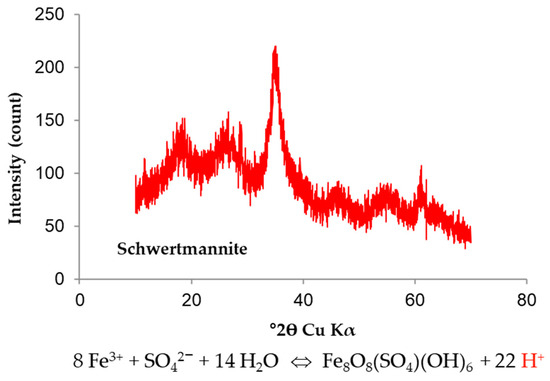
Figure 4.
Powder X-ray diffraction pattern of natural schwertmannite sampled in the mixolimnion of Corta Atalaya pit lake.
Concentrations of elements such as SO4, Al, Zn Cu, Mn, Co, Cd, and Ni follow the pattern of an EC profile, except for Cu and As. Monimolimnion presents very high concentrations, reaching values of up to 112 g/L sulphate, 2.8 g/L Al, 5.5 g/L Zn, 405 mg/L Cu, 437 mg/L Mn, 52 mg/L As, 27 mg/L Co, 14 mg/L Cd and 6 mg/L Ni, hence water presents acidity values of more than 80,000 mg/L eq. CaCO3.
Currently, this water volume, particularly the monimolimnion, represents a reservoir of elements of high economic interest, many of them included in the EU Critical Raw Material list 2023 [10]. Implementing their recovery would result in (i) the recycling of these elements and (ii) improvement in water quality. Improvements in the water quality of the pit lake have been achieved by Cu recovery and sludge disposal. As a consequence, the lake has shifted from a meromictic to a holomictic state, over 99% of the dissolved Fe has precipitated as schwertmannite, the total acidity of the water column has dropped by 35%, and the pH has risen from 2.5 to 4.1 [11].
5. Conclusions
This work documents for the first time the hydrochemical and limnological characterization of the Corta Atalaya pit lake, showing the data of its water column, which is permanently stratified. CA has been classified as meromictic lake showing a well-defined chemocline separating an anoxic, Fe(II)- and metal-rich monimolimnion, and an oxygenated and Fe(III)-rich mixolimnion. This last layer presents slight seasonal variations in parameters as pH, EC, T, and Eh, influenced by climatic factors, whereas the chemocline and monimolimnion are more compositionally and thermally stable.
As far as we know, there is no other mining lake in the world in which monimolimnion presents similar concentrations of elements such as SO4, Fe, Al, Zn, Cu, Mn, As, Co, Cd and Ni, representing a unique laboratory for experimentation.
Understanding the limnological and hydrochemical characteristics of pit lakes enables the estimation of water volumes in each layer, along with the identification of elements that may be of interest. This knowledge promotes the development of economically viable and environmentally sustainable recovery processes, while fostering a circular economy approach that transforms AMD into valuable resources for the industry.
Author Contributions
Conceptualization, E.S., E.L.-P. and R.A., supervision, E.S., B.R.-T. and R.A., investigation, E.S., R.A., B.R.-T. and E.L.-P., formal analysis, E.S., B.R.-T. and F.J.G., validation, E.S. and B.R.-T., writing—original draft preparation, E.S., B.R.-T., E.L.-P., F.J.G. and R.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been financially supported with funds from EMED-Tartessus Company, in collaboration with Autónoma University Foundation and Instituto Geológico y Minero de España.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are not publicly available due to privacy restrictions.
Acknowledgments
We acknowledge the support provided by Nuria Rodriguez González (Astrobiology Center), Moustafa Malki (Centro de Biología Molecular Severo Ochoa), and mine staff during the field works. This study is a contribution the Horizon Europe project GSEU (HORIZON-CL5-2021-D3-02-14, Project 101075609).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sánchez España, J.; López-Pamo, E.; Santofimia, E.; Diez Ercilla, M. The acidic mine pit lakes of Iberian Pyrite Belt: An approach to their physical limnology and hydrogeochemistry. Appl. Geochem. 2008, 23, 1260–1287. [Google Scholar] [CrossRef]
- Santofimia, E.; López-Pamo, E.; Reyes, J. Changes in stratification and iron redox cycle of an acidic pit lake in relation with climatic factors and physical processes. J. Geochem. Explor. 2012, 116–117, 40–50. [Google Scholar] [CrossRef]
- Geller, W.; Schultze, M.; Kleinmann, R.; Wolkersdorfer, C. Acid Pit Lake. The Legacy of Coal and Metal Surface Mines; Environmental Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2013; pp. 315–342. [Google Scholar] [CrossRef]
- Leistel, J.M.; Marcoux, E.; Thiéblemont, D.; Quesada, C.; Sánchez, A.; Almodóvar, G.R.; Pascual, E.; Sáez, R. The volcanic-hosted massive sulphide deposits of the Iberian Pyrite Belt. Review and preface to the Thematic Issue. Min. Depos. 1998, 33, 2–30. [Google Scholar] [CrossRef]
- López-Pamo, E.; Sánchez-España, J.; Diez, M.; Santofimia, E.; Reyes, J. Cortas Mineras de la Faja Pirítica: Inventario e Hidroquímica; Publicaciones del Instituto Geológico y Minero de España: Madrid, Spain, 2008; pp. 49–61. [Google Scholar]
- Nordstrom, D.K.; Alpers, C.N. Geochemistry of acid mine water. In The Environmental Geochemistry of Mineral Deposits; Plumlee, G.S., Logsdon, M.K., Eds.; The Society of Economic Geologists, Inc.: Littleton, CO, USA, 1999; Volume 6A, pp. 133–160. [Google Scholar]
- Santofimia, E.; López-Pamo, E.; Montero, E. Selective precipitation of schwertmannite in a stratified acidic pit lake of Iberian Pyrite Belt. Mineral. Mag. 2015, 79, 497–513. [Google Scholar] [CrossRef]
- Bigham, J.M.; Nordstrom, D.K. Iron and aluminum hydroxysulfates from acid sulfate waters. In Sulfate Minerals: Crystallography, Geochemistry, and Environmental Significance; Alpers, C.N., Jambor, J.L., Nordstrom, D.K., Eds.; Reviews in Mineralogy and Geochemistry; United States Geological Survey: Reston, VA, USA, 2000; Volume 40, pp. 351–403. [Google Scholar] [CrossRef]
- Santofimia, E.; González, F.J.; Rincón-Tomás, B.; López-Pamo, E.; Marino, E.; Reyes, J.; Bellido, E. The mobility of thorium, uranium and rare earth elements from Mid Ordovician black shales to acid waters and its removal by goethite and schwertmannite. Chemosphere 2022, 307, 135907. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Study on the Critical Raw Materials for the EU. Final Report; European Commission: Brussels, Belgium, 2023. [CrossRef]
- Gammons, C.; Icopini, G.A. Improvements to the Water Quality of the Acidic Berkeley Pit Lake due to Copper Recovery and Sludge Disposal. Mine Water Environ. 2020, 39, 427–439. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).