Abstract
Cerium dioxide nanoparticles (CeO2NP) show antioxidant enzyme-like properties and reactive oxygen species (ROS) scavenging activity, making them a promising material for potential therapeutic applications in neurodegenerative diseases. The objective of this work was to assess the biological behavior of CeO2NP in human SH-SY5Y neuronal and A172 glial cells by means of the MTT assay and the γH2AX assay. Despite the significant dose- and time-dependent NP internalization by both cell lines, nanoceria generally presented scarce cytotoxicity or genotoxicity, essentially restricted to the highest NP doses and longest exposure times. In conclusion, a high biocompatibility of CeO2NP was observed under the conditions tested.
1. Introduction
Cerium dioxide nanoparticles (CeO2NP) show antioxidant enzyme mimetic properties and oxygen free radical scavenging activity in biological systems. These unique structure-dependent features make them a promising material for potential biomedical applications, namely as an antitumor agent for regenerative therapy, gene therapy, or targeted drug delivery. However, their cellular uptake, action mechanism, and potential adverse effects are not totally understood yet [1].
Specifically, many central nervous system (CNS) diseases are characterized by the accumulation of reactive oxygen species (ROS), which induce severe damage to the brain tissues and irreversible neurodegeneration. CeO2NP has been raised as a novel potential agent in the treatment of neurodegenerative diseases, due mostly to their remarkable property to reduce oxidative stress in damaged cells through their ROS scavenging ability, the wide range of free radicals they can scavenge, and their self-regenerating redox cycle [2]. Moreover, recent studies in animal models showed that CeO2NP can cross the blood-brain barrier (BBB) due to their nanoscale diameters [3].
On this basis, the main objective of this work was to assess whether CeO2NP could induce adverse effects at the cellular and/or genetic level to verify their suitability for their application in the diagnosis and treatment of nervous system diseases. To achieve this aim, the possible alterations in SH-SY5Y neuronal and A172 glial cell viability and induction of DNA double-strand breaks were determined in the presence of a wide dose range of CeO2NP (1–100 µg/mL) by means of the MTT assay and the γH2AX assay, respectively. In the first stage, the physicochemical characterization of the CeO2NP and their ability to be taken up by the cells were assessed.
2. Materials and Methods
Cerium (IV) oxide nanopowder (CAS No. 1306-38-3) was obtained from Sigma-Aldrich Co.; according to the supplier, their primary particle size was <25 nm. The average hydrodynamic diameter and zeta potential in both cell culture media were analyzed by dynamic light scattering (DLS) and mixed mode measurement phase analysis light scattering (M3-PALS), respectively, using a Zetasizer Nano-ZS (Model ZEN 3600; Malvern Instruments Ltd., Malvern, UK).
CeO2NP internalization by both cell types was evaluated by flow cytometry, using a FACSCalibur cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) [4]. The potential antiproliferative effect of CeO2NP was evaluated by MTT assay, following Mosmann (1983) [5] with some methodological modifications to avoid interference of the NP with the standard protocol [6,7], using a SPECTROstar Nano microplate reader (BMG Labtech, Ortenberg, Germany). To evaluate the potential CeO2NP genotoxicity, the γH2AX assay was carried out by flow cytometry in a FACSCalibur cytometer (Becton Dickinson) [8]. For all these experiments, both cell types were incubated with a dose range of nanoceria (1–100 µg/mL) for 3, 24, and 48 h. Negative controls used were cell culture media, and positive controls were 200 µg/mL TiO2NP for internalization, 1% Triton X-100 for the MTT assay, and 1 μg/mL bleomycin for the γH2AX assay.
Statistical analyses were performed using the IBM SPSS Statistics package for Windows (version 27.0). Differences among groups were analyzed by Kruskal–Wallis test and the Mann–Whitney U-test for two-by-two comparisons, and associations between two variables were assessed by Pearson’s correlation. All experiments were run at least in triplicate. Experimental data were expressed as mean ± standard error, and a p-value lower than 0.05 was considered significant.
3. Results and Discussion
Table 1 shows the main physicochemical properties of CeO2NP dispersed in both cell culture media at the highest dose used for toxicity tests. Results revealed that CeO2NP were stable and did not agglomerate in any culture medium since their hydrodynamic size remained with minimal variations, and the zeta potential values showed a stable negative surface charge at all time points tested.

Table 1.
Physicochemical description of cerium dioxide nanoparticles (100 µg/mL).
Flow cytometric analysis of CeO2NP internalization by both cell types showed efficient and similar dose- and time-dependent NP uptake, although intensity was slightly higher in neuronal cells than in glial cells (Figure 1). The results obtained agree with other previous studies, employing different methodologies, that showed dose-dependent CeO2NP uptake in other human cell types [9,10,11].
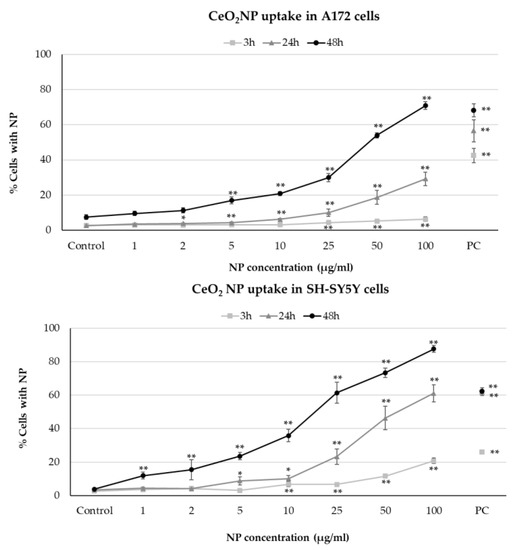
Figure 1.
Flow cytometry analysis of CeO2NP cellular uptake in A172 glial cells (top) and SH-SY5Y neuronal cells (bottom), exposed for 3, 24, and 48 h. * p < 0.05; ** p < 0.01, significant differences regarding the corresponding control. PC, positive control (200 µg/mL TiO2NP).
After cell exposure to CeO2NP, viability was assessed by employing a modified version of the MTT assay (Figure 2). In general, no cytotoxicity was observed at the shortest exposure times (3 and 24 h) for glial cells, since cell viability was not reduced by more than 20%. At 48 h, a dose-dependent relationship was obtained, with significantly decreasing viability at concentrations over 25 µg/mL. In contrast, CeO2NP revealed low cytotoxicity at all times and doses tested in SH-SY5Y cells, maintaining viability values above 80% in general; hence, these NPs did not induce cytotoxicity in this cell line. Our results agree with those obtained in some previous studies that revealed cytotoxicity or drastic decreases in cell viability only at high CeO2NP doses (>50 μg/mL) and/or large exposure periods (>24 h) [9,10,11].

Figure 2.
Cell viability evaluation of A172 glioblastoma cells (top) and SH-SY5Y neuroblastoma cells (bottom) exposed to CeO2NP for 3, 24, and 48 h. * p < 0.01, significant differences with respect to the corresponding control. PC, positive control (1% Triton X-100).
Results obtained from H2AX phosphorylation analysis showed dose- and time-dependent increases in the percentage of glioblastoma cells with γH2AX, significant only at the highest concentration after 3 h of exposure and at all doses tested after 24 h (Figure 3). A similar effect, but slightly more intense (with significant increases at all tested conditions), was observed in neuroblastoma cells.

Figure 3.
H2AX histone phosphorylation analysis after treatment of A172 cells (left) and SH-SY5Y cells (right) with CeO2NP for 3 and 24 h. * p < 0.05; ** p < 0.01, significantly different regarding the corresponding control. PC, positive control (1 μg/mL bleomycin).
Despite the significant increases in phosphorylated H2AX observed in our study, the values obtained were always lower than 6%, indicating a scarce genotoxic potential of CeO2NP in both cell types tested. The genotoxic effects found in different human cell types exposed to these NPs have been controversial. Franchi et al. [12] reported no significant increases in phosphorylated H2AX in fibroblasts exposed to low CeO2NP concentrations (10 µg/mL) for 24 h. Some previous works showed that exposure of human cells to nanoceria doses from 10 to 200 µg/mL did not induce significant primary DNA damage (evaluated by the comet assay) [10,11]. On the contrary, other studies employing the γH2AX test or comet assay revealed that NP doses as low as 6 µg/mL had a higher genotoxic potential even after 3 h of exposure [13,14].
4. Conclusions
The results obtained showed significant dose- and time-dependent NP internalization by both cell lines. Low CeO2NP-induced cytotoxicity was observed in neuronal cells at all times and doses tested. Notable cytotoxicity in glial cells was restricted to 48 h of treatment at concentrations over 25 µg/mL. Genotoxicity obtained in glial and neuronal cells treated with CeO2NP was limited since levels of γH2AX were always lower than 6%.
In general, it is possible to consider a high biocompatibility of CeO2NP under the conditions tested, except for glioblastoma cells exposed for 48 h to medium concentrations. These results provide a better understanding of the interaction of CeO2NP with cellular systems and their possible adverse effects, specifically at the level of the nervous system.
Author Contributions
Conceptualization, N.F.-B., V.V. and B.L.; methodology, A.T., L.M., A.T.R. and N.F.-B.; formal analysis, C.C., S.F. and B.L.; writing—original draft preparation, N.F.-B. and J.M.; writing—review and editing, J.P.T., V.V. and B.L.; supervision, N.F.-B., V.V. and B.L.; funding acquisition, E.P. and V.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Spanish Ministry of Science and Innovation MCIN/AEI/10.13039/501100011033, grant PID2020-114908GA-I00; Xunta de Galicia (ED431B 2022/16); CICA-Disrupting Project (2021SEM-B2); the Spanish Ministry of Education, Culture and Sport (BEAGAL18/00142 to V.V.); and the Portuguese Fundação para a Ciência e a Tecnologia (FCT) (SFRH/BPD/122112/2016 to A.T.R).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This conference proceeding is based on work from COST Action CA 17140 “Cancer Nanomedicine from the Bench to the Bedside”, supported by COST (the European Cooperation in Science and Technology).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thakur, N.; Manna, P.; Das, J. Synthesis and Biomedical Applications of Nanoceria, a Redox Active Nanoparticle. J. Nanobiotechnol. 2019, 17, 84. [Google Scholar] [CrossRef] [PubMed]
- Rzigalinski, B.A.; Carfagna, C.S.; Ehrich, M. Cerium Oxide Nanoparticles in Neuroprotection and Considerations for Efficacy and Safety. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1444. [Google Scholar] [CrossRef] [PubMed]
- Pandit, R.; Chen, L.; Götz, J. The Blood-Brain Barrier: Physiology and Strategies for Drug Delivery. Adv. Drug Deliv. Rev. 2020, 165–166, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bertólez, N.; Costa, C.; Brandão, F.; Kiliç, G.; Teixeira, J.P.; Pásaro, E.; Laffon, B.; Valdiglesias, V. Neurotoxicity Assessment of Oleic Acid-Coated Iron Oxide Nanoparticles in SH-SY5Y Cells. Toxicology 2018, 406–407, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Brandão, F.; Bessa, M.J.; Costa, S.; Valdiglesias, V.; Kiliç, G.; Fernández-Bertólez, N.; Quaresma, P.; Pereira, E.; Pásaro, E.; et al. In Vitro Cytotoxicity of Superparamagnetic Iron Oxide Nanoparticles on Neuronal and Glial Cells. Evaluation of Nanoparticle Interference with Viability Tests. J. Appl. Toxicol. 2016, 36, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Bessa, M.J.; Costa, C.; Reinosa, J.; Pereira, C.; Fraga, S.; Fernández, J.; Bañares, M.A.; Teixeira, J.P. Moving into Advanced Nanomaterials. Toxicity of Rutile TiO2 Nanoparticles Immobilized in Nanokaolin Nanocomposites on HepG2 Cell Line. Toxicol. Appl. Pharmacol. 2017, 316, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bertólez, N.; Costa, C.; Brandão, F.; Kiliç, G.; Duarte, J.A.J.A.; Teixeira, J.P.J.P.; Pásaro, E.; Valdiglesias, V.; Laffon, B. Toxicological Assessment of Silica-Coated Iron Oxide Nanoparticles in Human Astrocytes. Food Chem. Toxicol. 2018, 118, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Rubio, L.; Annangi, B.; Vila, L.; Hernández, A.; Marcos, R. Antioxidant and Anti-Genotoxic Properties of Cerium Oxide Nanoparticles in a Pulmonary-like Cell System. Arch. Toxicol. 2016, 90, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Vila, L.; García-Rodríguez, A.; Cortés, C.; Velázquez, A.; Xamena, N.; Sampayo-Reyes, A.; Marcos, R.; Hernández, A. Effects of Cerium Oxide Nanoparticles on Differentiated/Undifferentiated Human Intestinal Caco-2 cells. Chem. Biol. Interact. 2018, 283, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Singh, S.P.; Chinde, S.; Rahman, M.F.; Mahboob, M.; Grover, P. Toxicity Study of Cerium Oxide Nanoparticles in Human Neuroblastoma Cells. Int. J. Toxicol. 2014, 33, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.P.; Manshian, B.B.; de Souza, T.A.J.; Soenen, S.J.; Matsubara, E.Y.; Rosolen, J.M.; Takahashi, C.S. Cyto- and Genotoxic Effects of Metallic Nanoparticles in Untransformed Human Fibroblast. Toxicol. In Vitro 2015, 29, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Könen-Adıgüzel, S.; Ergene, S. In Vitro Evaluation of the Genotoxicity of CeO2 Nanoparticles in Human Peripheral Blood Lymphocytes Using Cytokinesis-Block Micronucleus Test, Comet Assay, and Gamma H2AX. Toxicol. Ind. Health 2018, 34, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Préaubert, L.; Tassistro, V.; Auffan, M.; Sari-Minodier, I.; Rose, J.; Courbiere, B.; Perrin, J. Very Low Concentration of Cerium Dioxide Nanoparticles Induce DNA Damage, but No Loss of Vitality, in Human Spermatozoa. Toxicol. In Vitro 2018, 50, 236–241. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).