Abstract
An efficient protocol has been developed for the synthesis of a new bioactive sulfonylphthalimide under environmentally friendly conditions. Ultrasonic energy was used to achieve the desired products with excellent yields and high purity, all in solvent-free conditions. The synthesis of this sulfonylphthalimide was carried out using sulfamide and phthalic anhydride. The structure of the synthesized compound was confirmed by 1H, 13C NMR and MS spectral data as well as IR spectroscopy.
1. Introduction
The use of ultrasonic irradiation as an energy source to catalyze the formation of new biomolecules represents an innovative approach in the field of organic synthesis and green chemistry. This method harnesses ultrasonic waves, high-frequency sound waves, to induce chemical reactions efficiently and rapidly [1].
When ultrasonic waves are applied to a chemical reaction, they generate pressure waves and local turbulence in the reaction medium. These extreme conditions promote the breaking of chemical bonds and the formation of reaction products at accelerated rates compared to conventional methods. Consequently, ultrasonic irradiation accelerates the synthesis process and enhances reaction yields [2,3,4,5].
In the specific case of forming a bioactive sulfonylphthalimide, using ultrasonic irradiation offers several advantages. Firstly, it reduces the reaction times required to obtain the desired product, contributing to an overall increase in synthesis efficiency. Additionally, the intensity of ultrasonic waves can be adjusted to selectively control the formation of specific products and minimize the generation of undesirable by-products. Furthermore, this technique can often be performed at room temperature or slightly elevated temperatures, avoiding the need for more energy-intensive reaction conditions.
2. Materials and Methods
2.1. General Procedure for the Synthesis of N-Sulfonylphthalimides
In a 20 mL glass tube, a mixture of phthalic anhydride 1 (1 mmol) and sulfamide 2 (1 mmol) was introduced at room temperature in the presence of triethylamine. The reaction mixture was then sonicated in an ultrasonic bath at a frequency of 40 kHz for 15 min. Once the reaction was complete, the mixture was purified by column chromatography on silica gel, using dichloromethane and ethanol (99/1) as the eluent.
2.2. Spectral Data
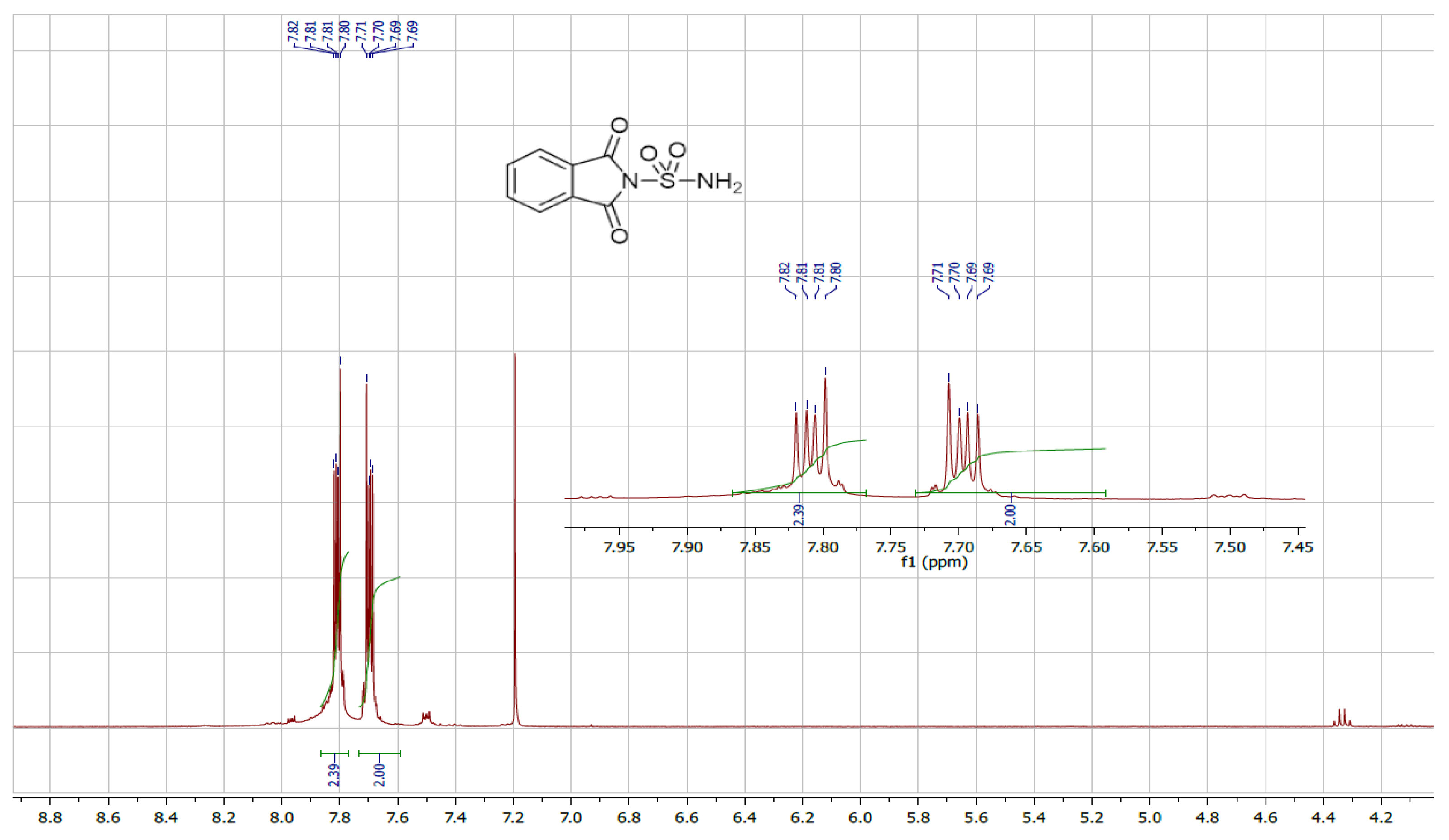
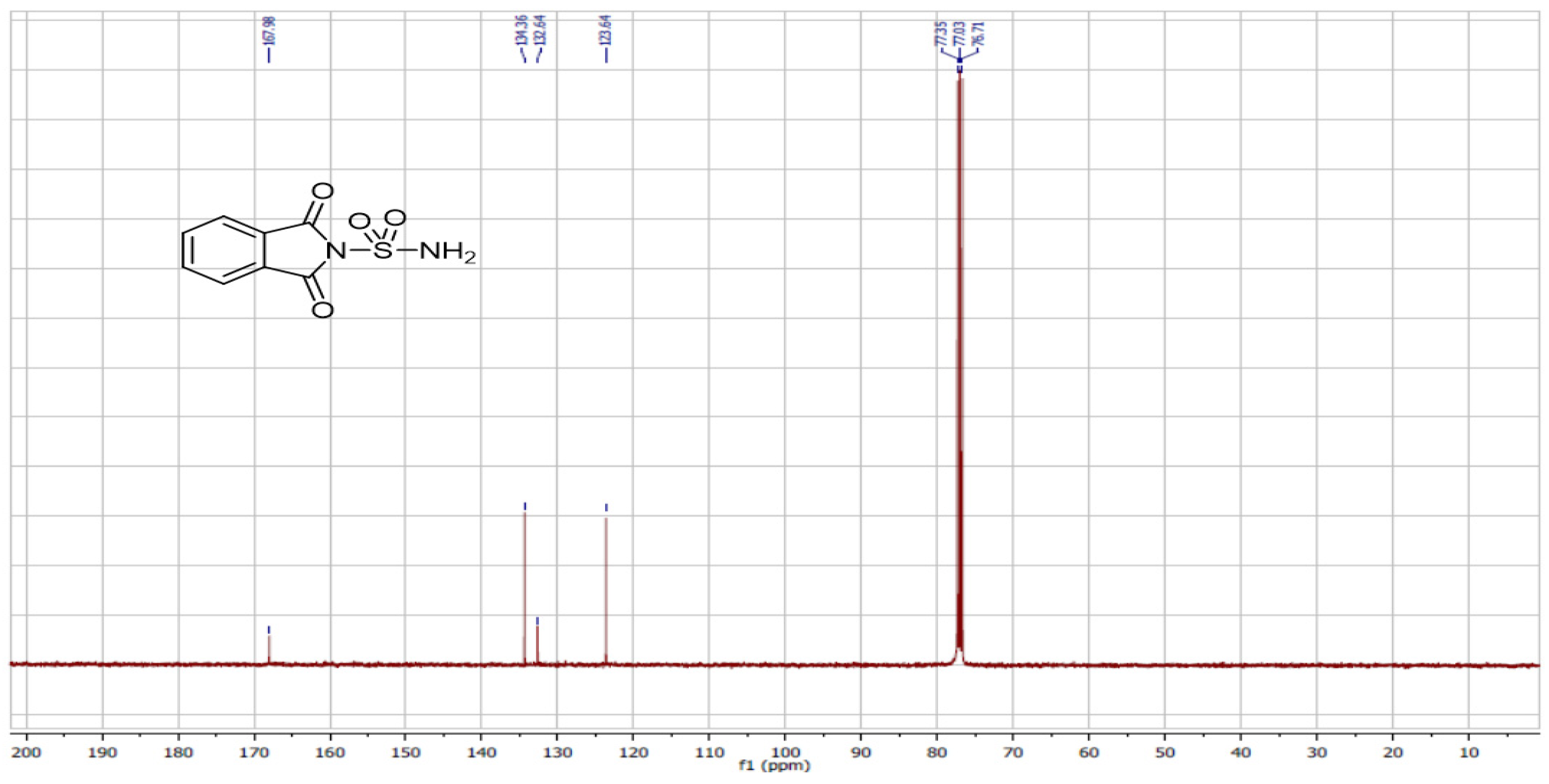
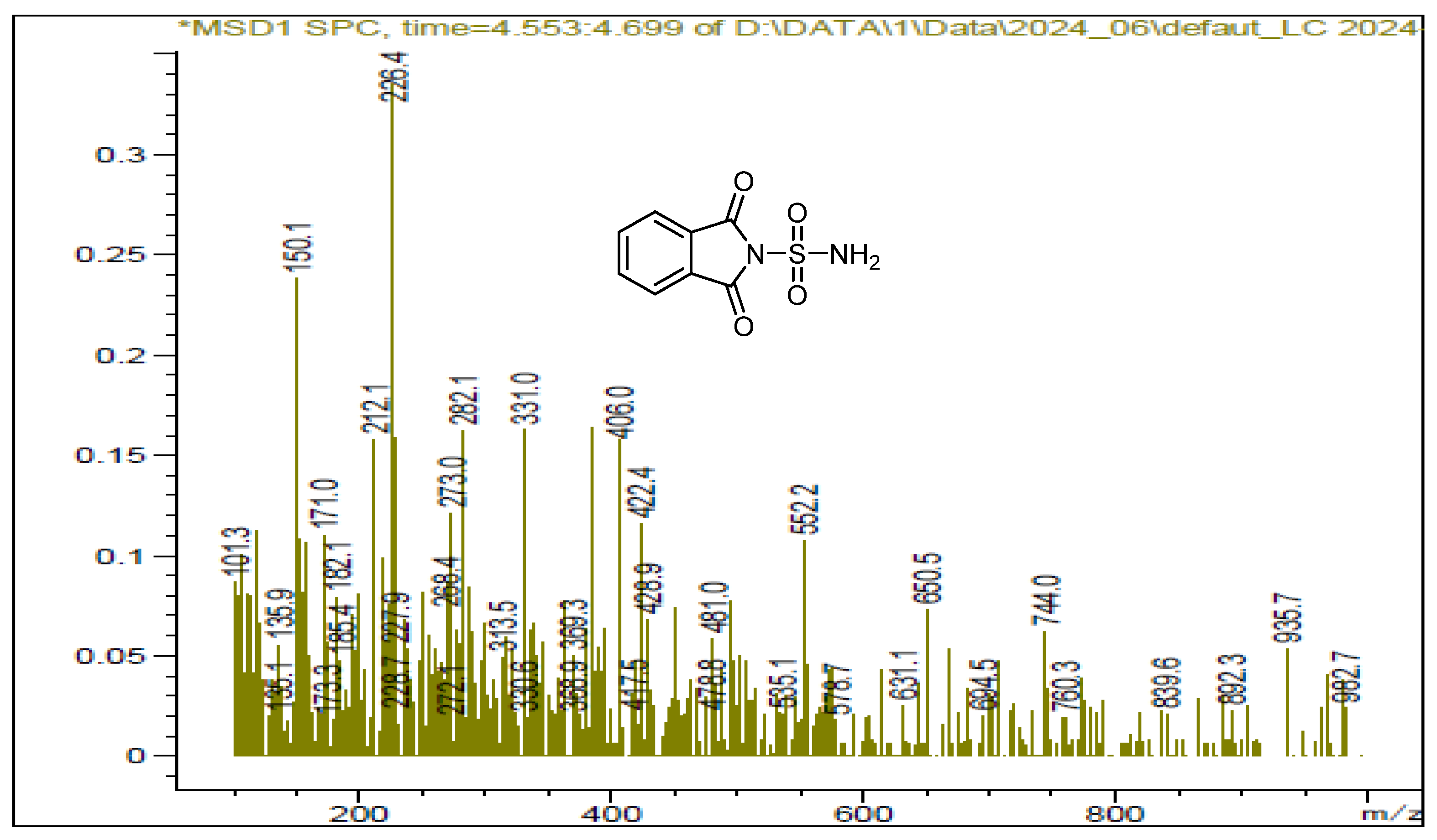
C8H6N2O4S; MW: 226; White solid; m.p. 190–192 °C; yield 87%; 1H NMR (400 MHz, CDCl3): 7.77 and 7.00(m, 4H, C6H4); s(m, 2H, NH2) ppm. 13C NMR (100 MHz, CDCl3): 123.64, 132.64, 134.36, 167.89. IR (KBr): 3388.72 and 3290.79 (NH2); 1372.86, 1179.28 (SO2) cm−1. Ms (m/z): 227.9 [M + 1]+.
3. Results and Discussion
Synthesis
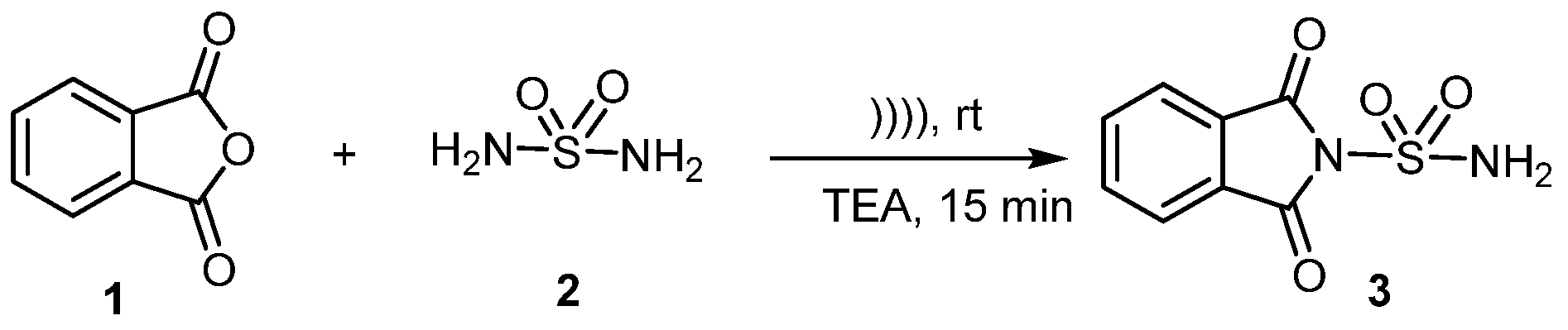
The advancement of novel, environmentally friendly processes for synthesizing new compounds is presently a pivotal focus within the realm of organic chemistry. Hence, ultrasonic irradiation has emerged as a pivotal technique in organic synthesis [6,7]. We herein describe the ultrasound promoted procedure for the synthesis of sulfonylphthalimide 3 by the sulfamide 2 with phthalic anhydride 1, in the presence of triethylamine (Scheme 1). The swift kinetics under mild conditions, coupled with a simple work-up and easy purification process, further enhance the advantages of this protocol.
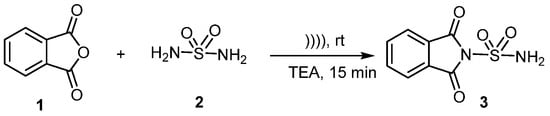
Scheme 1.
Synthesis of new sulfonylphthalimide.
We also utilized ultrasound to carry out the same reaction with solvent removal and reduced temperature. Under these conditions, ultrasound irradiation significantly accelerated the process, reducing the reaction time and yielding the sulfonylphthalimide in 87% yield within 15 min. The structure of the final product was confirmed by NMR 1H, 13C and SM (Figure 1, Figure 2 and Figure 3).
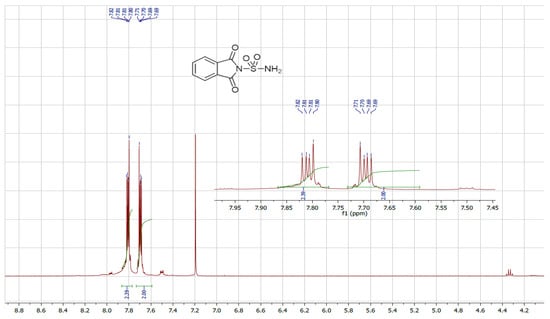
Figure 1.
NMR 1H spectrum of sulfonylphthalimide.
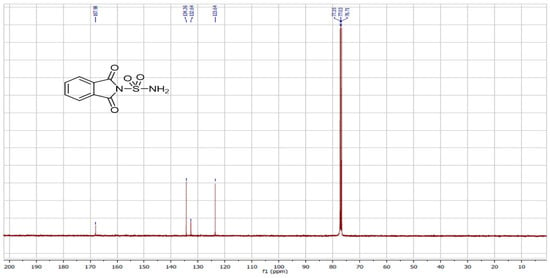
Figure 2.
NMR 13C spectrum of sulfonylphthalimide.
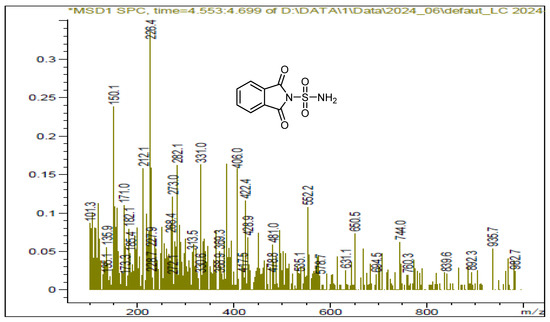
Figure 3.
LCMS spectrum of sulfonylphthalimide.
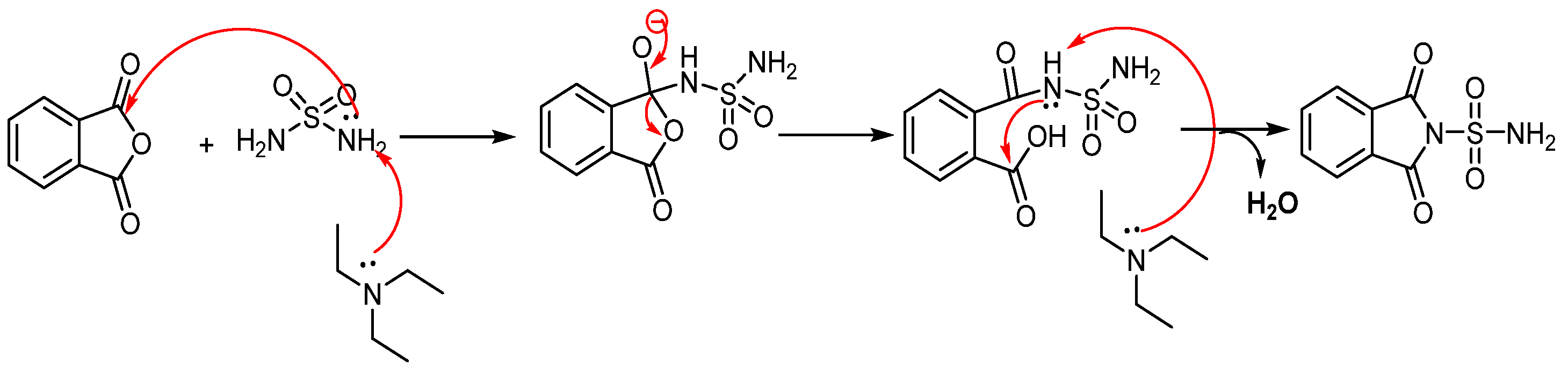
The possible reaction mechanism for the synthesis of sulfonylphthalimide is presented in (Scheme 2).
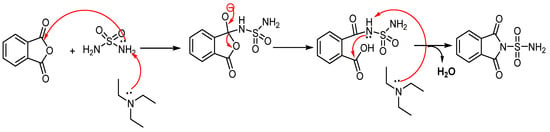
Scheme 2.
Mechanistic proposal for the synthesis of new sulfonylphthalimide.
4. Conclusions
In summary, ultrasonic irradiation provides an effective and sustainable approach for catalyzing the formation of bioactive sulfonylphthalimides, paving the way for new advancements in the synthesis of pharmaceutical and biologically relevant chemical compounds.
Author Contributions
I.G.: methodology; M.B.: writing, review and editing; A.R.: formal analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable.
Acknowledgments
The authors wish to extend their gratitude to the Algerian General Directorate of Scientific Research and Technological Development (DGRSDT) and the Applied Organic Chemistry Laboratory for their support and assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Amaniampong, P.N.; Jérôme, F. Catalysis under ultrasonic irradiation: A sound synergy. Curr. Opin. Green Sutain. Chem. 2020, 22, 7–12. [Google Scholar] [CrossRef]
- Grib, I.; Berredjem, M.; Otmane Rachedi, K.; Djouad, S.E.; Bouacida, S.; Bahadi, R.; Ouk, T.S.; Kadri, M.; Ben Hadda, T.; Belhani, B. Novel N-sulfonylphthalimides: Efficient synthesis, X-ray characterization, spectral investigations, POM analyses, DFT computations and antibacterial activity. J. Mol. Struct. 2020, 1217, 128423. [Google Scholar] [CrossRef]
- Bahadi, R.; Boughoula, R.; Berredjem, M.; Bachari, K.; Bouzina, A.; Bouacida, S.; Sbartai, H.; Benalliouche, F.; Redjemia, R. A convenient synthesis, biological activity and Xray crystallography of novel α-aminophosphonate derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2022, 197, 1150–1156. [Google Scholar] [CrossRef]
- Bouchareb, F.; Berredjem, M.; Bouzina, A.; Guerfi, M. Ultrasound-Promoted, Rapid and Green Synthesis of Phosphonamide Derivatives under Catalyst and Solvent-Free Conditions. Phosphorus Sulfur Silicon Relat. Elem. 2021, 196, 422–430. [Google Scholar] [CrossRef]
- Belhani, B.; Berredjem, M.; Le Borgne, M.; Bouaziz, Z.; Lebreton, J.; Aouf, N.E. A One-Pot, Three-Component, Synthesis of Novel α-Sulfamidophosphonates under Ultrasound Irradiation and Catalyst-Free Conditions. RSC Adv. 2015, 5, 39324–39329. [Google Scholar] [CrossRef]
- Rajagopal, R.; Jarikote, D.V.; Srinivasan, K.V. Ultrasound promoted Suzuki cross-coupling reactions in ionic liquid at ambient conditions. Chem. Commun. 2002, 6, 616–617. [Google Scholar] [CrossRef] [PubMed]
- Giancarlo, C.; Pedro, C. Power ultrasound in organic synthesis: Moving cavitational chemistry from academia to innovative and large-scale applications. Chem. Soc. Rev. 2006, 35, 180–196. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).