Abstract
In recent years, photocatalysis using semiconductor materials has gained significant attention as an effective strategy for dye degradation under mild conditions. Among various metal oxide photocatalysts, zinc ferrite (ZnFe2O4) has gained attention due to its narrow band gap, good stability, low cost, and activation under visible light. ZnFe2O4 nanoparticles (NPs) were synthesized using a co-precipitation process and tested for their photocatalytic effectiveness in degrading synthetic dyes Fast Green FCF and Orange II Sodium Salt under visible light. This study emphasizes the benefits of utilizing ZnFe2O4 as a visible light-activated, cost-effective, and environmentally friendly photocatalyst. These findings add to the growing research on wastewater treatment options.
1. Introduction
Rapid industrialization, urbanization, and insufficient waste management systems continue to be major causes of environmental pollution on Earth [1]. The depletion of natural resources and environmental degradation brought on by industrial growth and environmental crises are currently the main causes of ecosystem damage. Water pollution is a serious environmental problem that puts water, the main component of life on Earth, at serious risk, highlighting the vital role that water plays in sustaining life [2]. Industrial wastewater has numerous negative impacts on ecology and is extremely detrimental to our environment. The textile, cosmetics, printing, paper, and rubber industry sectors produce the majority of wastewater. Massive amounts of extremely hazardous chemicals are produced by the textile industry and discharged at different processing stages [3]. Dyes are the most common and challenging to handle of all the hazardous substances and contaminants. Textiles, food, rubber, cosmetics, paint, pharmaceuticals, paper and pulp, tannery materials, hair, and other materials can all be colored with dye. Dyes are intended to bond with the fibers or surfaces of the materials they are applied to, producing a durable and vivid color. They are usually soluble in water or other solvents. Aquatic life is impacted by these dyes because they color the water and prevent sunlight from reaching the bottom layers [4]. Furthermore, dyes are known to be carcinogenic, mutagenic, and teratogenic, impacting a variety of bacteria and fish species. Human exposure to dyes can cause serious health problems, such as liver, renal, reproductive, brain, and central nervous system disorders [5]. For dye removal, a variety of physicochemical and biological treatment methods have been widely used, including coagulation–flocculation, adsorption, membrane filtering, and microbial degradation. However, these traditional techniques frequently have drawbacks such as a poor performance for stable or non-biodegradable dyes, high operating costs, partial breakdown, and the production of secondary waste [6]. Photocatalytic degradation has drawn a lot of interest lately as an effective and sustainable substitute [7]. This method uses light-irradiated semiconductor catalysts to produce reactive radicals that can mineralize dyes into safe byproducts like CO2 and H2O. Photocatalysis is now regarded as one of the most promising techniques for wastewater purification because of its high efficiency, catalyst reusability, and operation under ambient settings [8]. For example, a recent study described the unique green synthesis of CuFe2O4 nanoparticles (NPs) using plant extracts from Cissus rotundifolia. The photocatalytic dye degradation efficiency of the nanomaterial was evaluated against methylene blue (MB). The results demonstrated the photocatalyst’s effectiveness in water cleanup by showing 82% breakdown under UV–visible light [9]. Sb2O3 NPs were created via the solvothermal procedure in a related investigation carried out by the same research team. The MB dye was used to test the photocatalytic potential. In 60 min, the NPs demonstrated 60% dye degradation. It was discovered that the dye was adsorbed onto the surface of the Sb2O3 nanoball, and degradation was linked to the production of reactive oxygen species (ROS). According to the study’s findings, photocatalysts can be applied practically in industry to remediate wastewater [10]. By adding different transition metals to create composites based on binary and ternary metal oxides and heterojunctions, the photocatalytic efficacy of the catalysts for the destruction of pollutants under visible light is increased [11]. Among these are zinc ferrite nanoparticles, which are regarded as one of the most promising photocatalysts because of their intriguing physiochemical characteristics, superior charge carrier separation features, and high degrading efficiency in visible light. Because ZnFe2O4 NPs have a small energy band gap and Fe3+ ions, they are a powerful photocatalyst. They also possess remarkable optical and magnetic characteristics. Their strong reactivity is a result of both their small particle size and large surface area. As a result, a number of recent studies have concentrated on the synthesis of zinc ferrite [12]. In our present work, the dye degradation efficiency of ZnFe2O4 NPs was tested against the Fast Green FCF and Orange II dyes.
2. Materials and Methods
This study used only analytical-grade chemicals; therefore, no further purification was necessary before usage. Zinc nitrate hexahydrate (Zn(NO3)2·6H2O), ferric nitrate nonahydrate (Fe(NO3)3·9H2O), Sodium hydroxide (NaOH), ethanol, Fast Green FCF and Orange II dyes were procured from Sisco Research Laboratories Pvt. Ltd., Maharashtra, India. A NEUATION iFUGE D08 centrifuge machine was used for centrifuging the reaction mixtures and recovering the precipitates. UV–visible absorption spectra were recorded between 200 and 800 nm using the Agilent Cary UV–Vis Compact Peltier R3.15AL250V spectrophotometer, California, USA. The crystallinity and phase identification purity of the synthesized precursors and the binary nanocomposite were detected using a diffractometer (model No.: D8 Advance Eco, make: Bruker, Germany). FT-IR spectra were obtained using a spectrophotometer (model no.: Nicole 6700, make: Thermo-Scientific, Waltham, MA, USA). The morphology of the nanostructures was analyzed using scanning electron microscope (model: JSM 6490 LV, make: JEOL, Tokyo, Japan).
3. Experiments
3.1. Synthesis of ZnFe2O4 NPs
ZnFe2O4 NPs were synthesized by co-precipitation. Under constant stirring, a precursor solution comprising 0.1 mol Zn(NO3)2·6H2O and 0.2 mol Fe(NO3)3·9H2O was made in 100 mL of distilled water. To cause precipitation, a 10% NaOH solution was added dropwise until pH 9 was reached. After centrifuging and washing with ethanol and water, the precipitate was dried for 5–6 h at 110 °C. To create crystalline ZnFe2O4 NPs, the dried powder was crushed and calcined for four hours at 500 °C.
3.2. Photocatalytic Activity Evaluation
Using visible light, the photocatalytic activity of the NPs was assessed against the Fast Green FCF and Orange II dyes. For this, 50 mL of dye solution (10 ppm) was mixed with 25 mg of NPs. To achieve desorption/absorption equilibrium, the mixture was magnetically stirred for 30 min in the dark before the solution was exposed to light. Every 15 min, 3 mL of the sample was taken out, and the concentration of the degraded dye solution was measured using a UV–visible spectrophotometer (200 nm to 800 nm). Equation (1) was used to determine the dye’s % degradation.
where Co represents the dye absorbance at the beginning, and Ct represents the dye absorbance at time t. Additionally, the kinetics of Fast Green FCF degradation were examined. Plotting ln (Ct) versus time allowed for the evaluation of the degradation kinetics. The kinetic study was performed using a pseudo-first-order kinetic equation.
where Co represents the initial concentration, Ct denotes the concentration at time t, and k is the rate constant. The slope of the plot of ln(Co/Ct) against time gives a rate constant, k.
%Degradation = (Co − Ct)/Co
ln(Co/Ct) = kt
4. Results and Discussion
4.1. Characterization
Two strong peaks were visible in the ZnFe2O4 NPs UV–vis absorption spectra (Figure 1a) at around 221 and 342 nm. The sharp absorption at 221 nm is assigned to O2− → Fe3+ charge transfer transitions within the spinel lattice, while the absorption at 342 nm corresponds to band gap excitation. The measured band gap was 4.6 eV which was calculated through the Tauc plot (Figure 1b) [13]. The FT-IR spectra (Figure 2a) of synthesized ZnFe2O4 NPs, observed between 4000 and 400 cm−1, indicates the presence of distinctive functional groups and the spinel ferrite structure. A wide absorption band at 3386 cm−1 and 3230 cm−1 indicates the presence of adsorbed water or surface hydroxyl groups. The bands at 1695 cm−1 and 1378 cm−1 correspond to H-O-H bending and C-H deformation vibrations, respectively. Sharp bands at 769 cm−1, 543 cm−1, and 455 cm−1 indicate metal–oxygen stretching vibrations in the spinel lattice [14]. The XRD pattern of synthesized ZnFe2O4 NPs revealed (Figure 2b) distinct and sharp peaks at 2θ values of approximately 29.77°, 35.04°, 42.63°, 56.44°, and 62.07°, which correspond to the (220), (311), (400), (511), and (440) crystal planes of the cubic spinel structure, respectively, as per JCPDS card no. 22-1012. The peak at 35.04° shows the presence of the typical (311) plane of ZnFe2O4, indicating the successful development of the spinel ferrite phase. The average crystallite size came out to be 10.206 nm [15]. The SEM image (Figure 3) of the ZnFe2O4 NPs showed a needle-like morphology with a network-like arrangement. The particles appeared elongated and intertwined, forming clusters rather than discrete spherical grains. The surface texture appeared rough and porous, which is characteristic of ferrite nanomaterials synthesized through chemical routes. Similar agglomerated morphologies have been reported for ZnFe2O4 NPs synthesized via green and hydrothermal methods [16].
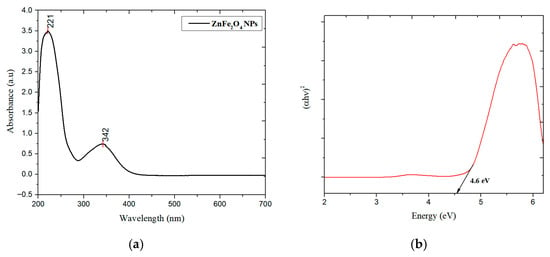
Figure 1.
(a) UV–vis spectra and (b) Tauc plot of ZnFe2O4 NPs.
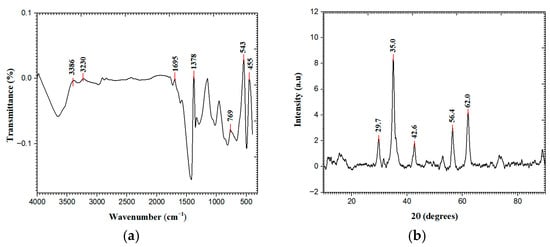
Figure 2.
(a) FT-IR and (b) XRD spectra of ZnFe2O4 NPs.
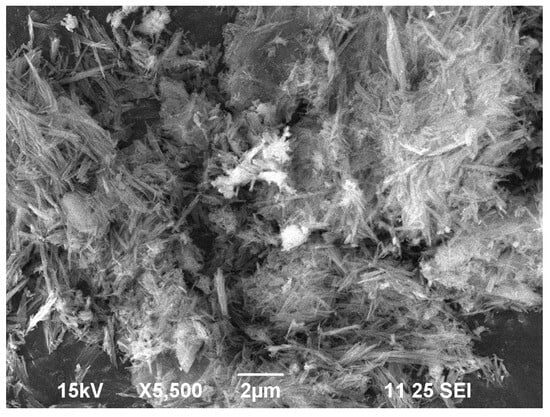
Figure 3.
SEM image of ZnFe2O4 NPs.
4.2. Photocatalytic Dye Degradation
The widespread utilization of synthetic dyes in the food, paper, cosmetics, and textile sectors contributes to the discharge of massive quantities of colored wastewater into bodies of water. Even at low quantities, these dyes can have serious negative effects on the environment and human health since they are chemically stable and non-biodegradable [17]. Traditional treatment techniques, such as adsorption or biological degradation, are less effective due to the complex aromatic nature of these compounds. As a result, photocatalytic degradation involving semiconductor materials has become a viable and environmentally responsible method for dye removal from structures [18]. Two renowned anionic dyes that are acknowledged for their durability in aquatic settings are Orange II and Fast Green FCF [19]. Orange II (C16H11N2NaO4S), with a molecular weight of 248.71 g/mol, is an azo dye used in the leather, paper, and cosmetics sectors, whereas Fast Green FCF (C37H34N2Na2O10S3), with a molecular weight of 808.84 g/mol, is a triphenylmethane dye that is widely used as a food colorant and in textiles [20]. The structures of the dyes are illustrated in Figure 4a,b.
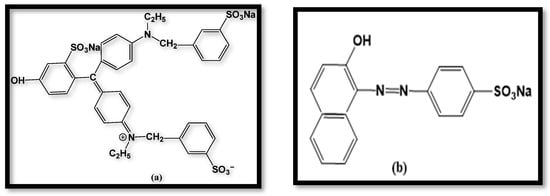
Figure 4.
Structure of dyes: (a) Fast Green FCF; (b) Orange II.
The absorbance of the untreated dye solution was recorded before treatment with the NPs. In the case of Fast Green FCF, the maximum absorbance was obtained at 623 nm, and the peak area was 170.299, whereas for Orange II the λmax was 485 nm (Figure 5) and the peak area was 84.925.
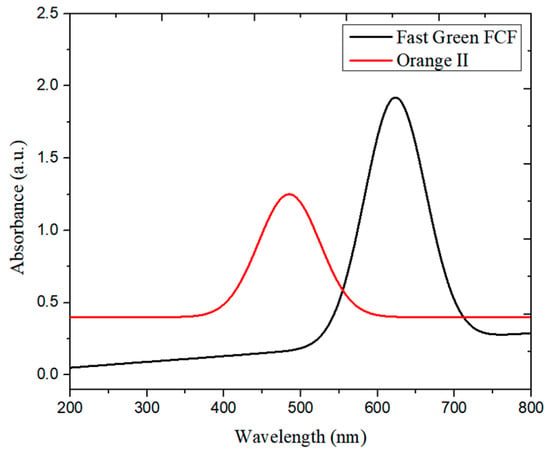
Figure 5.
Time-dependent UV–vis spectra of untreated Fast Green FCF and Orange II dyes.
In the present study, the photocatalytic degradation of Fast Green FCF and Orange II was studied using ZnFe2O4 NPs (Figure 6a,b) under visible light irradiation in 90 min. The dye degradation of Fast Green FCF with ZnFe2O4 NPs was found to be 60% and follows pseudo-first-order kinetics with a rate constant of 8.01 × 10−3 min−1 (R2 = 0.98737), as given in Figure 7a, while the photodegradation of Orange II with ZnFe2O4 NPs was found to be 73%, also following pseudo-first-order kinetics and the rate constant was found to be 6.08 × 10−3 min−1 (R2 = 0.94432), as given in Figure 7b. Figure 8 shows the % degradation versus time plot for both dyes.
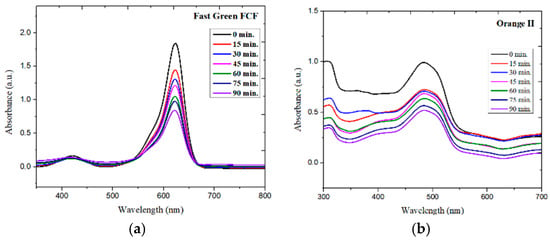
Figure 6.
Time-dependent UV–vis spectra of (a) Fast Green FCF and (b) Orange II treated with ZnFe2O4 NPs.
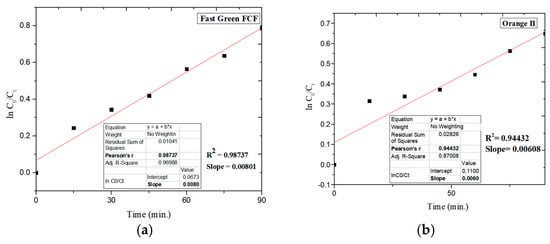
Figure 7.
Pseudo-first-order kinetic plot of ln(Co/Ct) vs. time for (a) Fast Green FCF and (b) Orange II treated with ZnFe2O4 NPs.
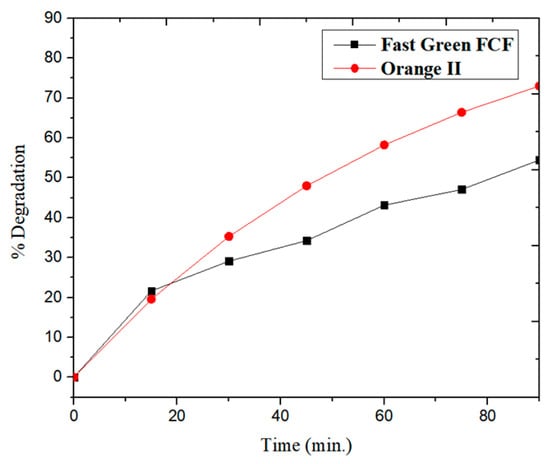
Figure 8.
Degradation (%) of Fast Green FCF and Orange II.
ZnFe2O4 has a narrow band gap that is easily stimulated by visible and UV light [21]. Because of its paramagnetic properties, adsorption capacity, narrow band gap, photocatalytic response, stability, reusability, and sensitivity to visible light, zinc ferrite is an important photocatalyst [22]. When the photocatalyst absorbs sufficient light energy, electron holes are formed in photocatalytic reactions. The mechanism of dye degradation can be illustrated through the following Equations (3)–(10):
Activation:
Catalyst + hν → e− + h+
ROS Generation:
h+ + H2O → •OH + H+
e− + O2 → •O2−
•O2− + H+ → HO2•
HO2• + HO2•→H2O2 + O2
e− + H2O2 → •OH + OH− (or H2O2 + hν → 2•OH)
Dye Degradation:
Dye (colored) + •OH/h+/•O2− → Organic Intermediates (Decolorized)
Mineralization:
Organic Intermediates + •OH/h+/•O2− → CO2 + H2O + Inorganic Ions
Upon exposure to photon energy on the surface of the NPs, the conduction band will excite electrons from the valence band, resulting in the formation of electron–hole pairs. After that, the photogenerated electron holes convert hydroxide ions (OH) or water molecules (H2O) into hydroxyl radicals (•OH). Subsequently, the photoexcited electrons will convert oxygen into superoxide radicals (•O2), which can then be protonated by H+ ions in water to generate hydroxyl radicals (HO2•). These radicals then transform into H2O2, which dissociates into the more reactive hydroxyl radical species OH•. The hydroxyl and superoxide radicals are crucial to dye degradation [23]. Previous research has also demonstrated the photocatalytic capability of such NPs. In a recent study, for example, MB dye was degraded in the presence of a ZnFe2O4/Ag2WO4 photocatalyst, which was made using a straightforward combustion process and a solution mixing approach. By adjusting the catalyst dosage and pH of the media used for the degradation process, the photocatalytic activity of the composite material was determined. The results indicated that 72.7% of the MB dye was degraded by the composite material [24]. In a similar study, Kumar S. et al. synthesized zinc ferrite NPs using the sol–gel auto-combustion (SG) technology in order to use photocatalysis and adsorption to remove the crystal violet dye present in wastewater from the textile sector. The nanomaterial had an 83% degradation efficiency, indicating that it is very advantageous for use in the environment [25]. A different study by Duraisamy K. et al. described an environmentally benign way to create ZnFe2O4 NPs using a hydrothermal process with Nyctanthes arbortristis leaf extract. This nanomaterial’s photocatalytic effectiveness was evaluated using MB dye, and the findings were encouraging. The results indicated that the photocatalyst showed degradation of more than 80% [26]. Some examples of nanocomposites exhibiting dye degradation are discussed in Table 1.

Table 1.
Some examples of nanomaterials exhibiting dye degradation.
5. Conclusions
ZnFe2O4 NPs were successfully synthesized via a co-precipitation method and showed significant photocatalytic activity for the breakdown of Fast Green FCF and Orange II in aqueous media. The photocatalyst showed a significant degradation capability of about 60% in the case of Fast Green FCF and 73% in the case of Orange II in 90 min, proving its versatility for wastewater remediation. Overall, ZnFe2O4 offers a promising strategy for the treatment of dyes in water, supporting the advancement of sustainable wastewater treatment technologies aligned with global environmental goals.
Author Contributions
Conceptualization, T.K.; methodology, N.F. and T.K.; data curation, N.F. and S.D.; writing—original draft preparation, writing—review and editing, N.F., E.V. and A.P.; supervision, T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors acknowledge the support extended by the Department of Chemistry, Integral University, Lucknow and the R&D cell of the university for the Manuscript Communication Number (IU/R&D/2025-MCN0004064). The corresponding author acknowledges the support extended to her through the Outstanding Researcher Award for the year 2024 by the university.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kodiya, M.A.; Modu, M.A.; Ishaq, K.; Yusuf, Z.; Wakili, A.Z.; Dayyabu, N.; Babangida, M.U. Environmental Pollution in Nigeria: Unlocking Integrated Strategies for Environmental Sustainability. Afr. J. Environ. Sci. Renew. Energy 2025, 18, 30–50. [Google Scholar] [CrossRef]
- Periyasamy, A.P. Recent Advances in the Remediation of Textile-Dye-Containing Wastewater: Prioritizing Human Health and Sustainable Wastewater Treatment. Sustainability 2024, 16, 495. [Google Scholar] [CrossRef]
- Tripathi, M.; Singh, S.; Pathak, S.; Kasaudhan, J.; Mishra, A.; Bala, S.; Garg, D.; Singh, R.; Singh, P.; Singh, P.K.; et al. Recent Strategies for the Remediation of Textile Dyes from Wastewater: A Systematic Review. Toxics 2023, 11, 940. [Google Scholar] [CrossRef] [PubMed]
- Kapanga, P.M.; Nyakairu, G.W.A.; Nkanga, C.I.; Lusamba, S.N.; Tshimanga, R.M.; Shehu, Z. A Review of Dye Effluents Polluting African Surface Water: Sources, Impacts, Physicochemical Properties, and Treatment Methods. Discov. Water 2024, 4, 85. [Google Scholar] [CrossRef]
- Kayani, K.F.; Mohammed, S.J.; Mustafa, M.S.; Aziz, S.B. Dyes and Their Toxicity: Removal from Wastewater Using Carbon Dots/Metal Oxides as Hybrid Materials—A Review. Mater. Adv. 2025. [CrossRef]
- Adesanmi, B.M.; Hung, Y.T.; Paul, H.; Huhnke, C. Comparison of Dye Wastewater Treatment Methods: A Review. GSC Adv. Res. Rev. 2022, 10, 126. [Google Scholar] [CrossRef]
- Sajith, M.; Hema, S.; Sambhudevan, S. A Comprehensive Review on Photocatalytic Degradation of Textile Dyes Using PANI–Semiconductor Composites. Water Air Soil Pollut. 2024, 235, 594. [Google Scholar] [CrossRef]
- Ganesan, S.; Kokulnathan, T.; Sumathi, S.; Palaniappan, A. Efficient Photocatalytic Degradation of Textile Dye Pollutants Using Thermally Exfoliated Graphitic Carbon Nitride (TE–g–C3N4). Sci. Rep. 2024, 14, 2284. [Google Scholar] [CrossRef]
- Jabeen, S.; Siddiqui, V.U.; Sharma, S.; Rai, S.; Bansal, P.; Bala, S.; Khan, T. A Novel Green Synthesis of CuFe2O4 Nanoparticles from Cissus rotundifolia for Photocatalytic and Antimicrobial Activity Evaluation. J. Alloys Compd. 2024, 984, 174020. [Google Scholar] [CrossRef]
- Jabeen, S.; Veg, E.; Bala, S.; Khan, T. Synthesis, Characterization, and Photocatalytic Activity of Sb2O3 Nanoparticles: A Step Towards Environmental Sustainability. Eng. Proc. 2024, 67, 8. [Google Scholar] [CrossRef]
- Khan, S.; Han, C.; Khan, H.M.; Boccelli, D.L.; Nadagouda, M.N.; Dionysiou, D.D. Efficient Degradation of Lindane by Visible and Simulated Solar Light-Assisted S-TiO2/Peroxymonosulfate Process: Kinetics and Mechanistic Investigations. Mol. Catal. 2017, 428, 9–16. [Google Scholar] [CrossRef]
- Ullah, R.; Khitab, F.; Gul, H.; Khattak, R.; Ihsan, J.; Khan, M.; Aouissi, H.A. Superparamagnetic Zinc Ferrite Nanoparticles as Visible-Light Active Photocatalyst for Efficient Degradation of Selected Textile Dye in Water. Catalysts 2023, 13, 1061. [Google Scholar] [CrossRef]
- Ahmed, A.I.; Siddig, M.A.; Mirghni, A.A.; Omer, M.I.; Elbadawi, A.A. Structural and Optical Properties of Mg1−xZnxFe2O4 Nano-Ferrites Synthesized Using Co-Precipitation Method. Adv. Nanopart. 2015, 4, 45–52. [Google Scholar] [CrossRef]
- Sarkar, T.; Kundu, S.; Ghorai, G.; Sahoo, P.K.; Reddy, V.R.; Bhattacharjee, A. Synthesis and Characterization of Zinc Ferrite Nanomaterials vis-à-vis Studies on Their Photocatalytic Application in Visible Light Dye Degradation. Appl. Phys. A 2025, 131, 1–24. [Google Scholar] [CrossRef]
- Hatami Kahkesh, K.; Baghbantaraghdari, Z.; Jamaledin, D.; Dabbagh Moghaddam, F.; Kaneko, N.; Ghovvati, M. Synthesis, Characterization, Antioxidant and Antibacterial Activities of Zinc Ferrite and Copper Ferrite Nanoparticles. Mater. Chem. Horiz. 2023, 2, 49–56. [Google Scholar] [CrossRef]
- Sami, W.A.; Sadeq, Z.S. Synthesis and Study of Calcination Effect of Zinc Ferrite on the Structure and Morphology of Nanoparticles. J. Phys. Conf. Ser. 2021, 1999, 012060. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic Organic Dyes as Contaminants of the Aquatic Environment and Their Implications for Ecosystems: A Review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef]
- Khan, K.A.; Shah, A.; Nisar, J.; Haleem, A.; Shah, I. Photocatalytic Degradation of Food and Juice Dyes via Photocatalytic Nanomaterials Synthesized Through Green Synthetic Route: A Systematic Review. Molecules 2023, 28, 4600. [Google Scholar] [CrossRef]
- Sudhakaran, G.; Priya, P.S.; Murugan, R.; Haridevamuthu, B.; Kannan, J.; Almutairi, S.M.; Hussein, D.S.; Eisa, Y.H.; Kumaradoss, K.M.; Namasivayam, S.K.R.; et al. Follicular and Neural Toxic Effect of Prolonged Exposure of Synthetic Dye Fast Green FCF (E143)—Insights from Zebrafish Model. Toxicol. Lett. 2025, 413, 111726. [Google Scholar] [CrossRef]
- Chahbane, N.; Popescu, D.L.; Mitchell, D.A.; Chanda, A.; Lenoir, D.; Ryabov, A.D.; Collins, T.J. Fe(III)–TAML-Catalyzed Green Oxidative Degradation of the Azo Dye Orange II by H2O2 and Organic Peroxides: Products, Toxicity, Kinetics, and Mechanisms. Green Chem. 2007, 9, 49–57. [Google Scholar] [CrossRef]
- Rasheed-Adeleke, A.A.; Olatunde, O.C.; Seheri, N.H.; Oyewo, O.A.; Ferjani, H.; Onwudiwe, D.C. Synthesis and Photocatalytic Performance of ZnFe2O4 on the Degradation of Tetracycline in Water. Appl. Phys. A 2025, 131, 625. [Google Scholar] [CrossRef]
- Ullah, R. Semiconductor ZnFe2O4 as Efficient Photocatalyst for the Degradation of Organic Dyes: An Update. J. Chem. Rev. 2023, 5, 466–476. [Google Scholar] [CrossRef]
- Saridewi, N.; Utami, D.J.; Zulys, A.; Nurbayti, S.; Putri, A.R.; Kamal, R. Utilization of Lidah mertua (Sansevieria trifasciata) Extract for Green Synthesis of ZnFe2O4 Nanoparticle as Visible-Light Responsive Photocatalyst for Dye Degradation. Case Stud. Chem. Environ. Eng. 2024, 9, 100745. [Google Scholar] [CrossRef]
- Gowda, G.K.; Vishnu, K.T.; Prashantha, K.; Ajeya, K.P. Enhancing the Photocatalytic and Antimicrobial Activity of ZnFe2O4 by Composite with Ag2WO4 Semiconducting Material. Colloids Surf. C Environ. Asp. 2024, 2, 100037. [Google Scholar] [CrossRef]
- Kumar, S.; Jasrotia, R.; Verma, A.; Kandwal, A.; Ahmed, J.; Alshehri, S.M.; Kumari, S.; Godara, S.K.; Sharma, P. Superparamagnetic Dy-Modified ZnFe2O4 Magnetic Nanophotocatalysts for the Photocatalytic Degradation of Crystal Violet Pollutant. Appl. Phys. A 2024, 130, 258. [Google Scholar] [CrossRef]
- Duraisamy, K.; Venkatesan, S.; Sivaji, I.; Kosuru, R.Y.; Palaniyappan, P.; Sureshkumar, M.; Dhakshinamurthy, D. Green Synthesis of Zinc Ferrite Nanoparticles from Nyctanthes arbor-tristis: Unveiling Larvicidal Potential, Protein Binding Affinity and Photocatalytic Activities. Environ. Sci. Pollut. Res. 2024, 31, 53026–53039. [Google Scholar] [CrossRef]
- Aljawrneh, B.; Ocak, Y.S.; Albiss, B.A.; Dwiri, A.; Tawalbeh, M.; Al-Othman, A. ZrO2 Nanoparticles for Effective Dye Degradation in Wastewater: Synthesis, Characterization, and Photocatalytic Performance Under Sunlight. J. Alloys Compd. 2024, 1008, 176522. [Google Scholar] [CrossRef]
- Ghotekar, S.; Mishra, S.R.; Gadore, V.; Roy, S.; Ahmaruzzaman, M.; Singh, K.R.; Mirzaei, M. Insights into the Expeditious Photocatalytic Performance of Greenly Fabricated CeVO4 Nanoparticles Using Polyalthia longifolia Leaf Extract. Inorg. Chem. Commun. 2025, 172, 113665. [Google Scholar] [CrossRef]
- Abed, S.H.; Reshak, A.H. Illuminating the Power of V2O5 Nanoparticles: Efficient Photocatalytic Degradation of Organic Dyes Under Visible Light. J. Fluoresc. 2025, 35, 4335–4345. [Google Scholar] [CrossRef]
- Mharsale, N.N.; More, P.S.; Khollam, Y.B.; Shaikh, S.F.; Al-Enizi, A.M.; Gadakh, S.R. Visible Light-Induced Photocatalytic Degradation of Methylene Blue Dye Using Pure Phase Bismuth Ferrite Nanoparticles. J. Phys. Chem. Solids 2024, 192, 112049. [Google Scholar] [CrossRef]
- Parida, S.; Sarangi, B.; Nanda, J.; Pany, B. Green-Synthesized BiFeO3 Nanoparticles for Efficient Photocatalytic Degradation of Organic Dyes, Antibiotic and Catalytic Reduction of 4-Nitrophenol. Inorg. Chem. Commun. 2024, 170, 113344. [Google Scholar] [CrossRef]
- Divakara, S.G.; Mahesh, B.; Jayanna, B.K.; Anil Kumar, H.G. Photocatalytic Degradation of Crystal Violet Dye Using Honey-Mediated Synthesis of NiFe2O4 Nanoparticles. Green Chem. Lett. Rev. 2025, 18, 2543931. [Google Scholar] [CrossRef]
- Kumar, J.V.; Karthika, D.; Rosaiah, P.; Devanesan, S.; Mythili, R.; Dhananjaya, M.; Joo, S.W. Fabrication of SnO2/NGO Hybrid Nanocomposite as an Effective Photocatalyst for Binary Dye Degradation Under Sunlight Illumination. Process Saf. Environ. Prot. 2024, 188, 398–405. [Google Scholar] [CrossRef]
- Jabeen, S.; Ganie, A.S.; Ahmad, N.; Hijazi, S.; Bala, S.; Bano, D.; Khan, T. Fabrication and Studies of LaFe2O3/Sb2O3 Heterojunction for Enhanced Degradation of Malachite Green Dye Under Visible Light Irradiation. Inorg. Chem. Commun. 2023, 152, 110729. [Google Scholar] [CrossRef]
- Veg, E.; Raza, A.; Rai, S.; Bansal, P.; Sharma, S.; Mishra, N.; Gupta, R.; Mishra, S.; Joshi, S.; Khan, A.R.; et al. Green synthesis of ZnO and α-Fe2O3 nanoparticles using Chinese palm leaf extract and their biological and photocatalytic evaluation. RSC Sustain. 2025, 3, 5609–5631. [Google Scholar] [CrossRef]
- Veg, E.; Raza, A.; Bansal, P.; Rai, S.; Sharma, S.; Gupta, R.; Dwivedi, S.; Joshi, S.; Khan, A.R.; Khan, T. Synthesis and characterization of ZnO–thiosemicarbazone nanoconjugates for enhanced biological and photocatalytic activity through surface synergy. Inorg. Chem. Commun. 2025, 181, 115265. [Google Scholar] [CrossRef]
- Veg, E.; Raza, A.; Bansal, P.; Rai, S.; Sharma, S.; Gupta, R.; Dwivedi, S.; Fatima, N.; Joshi, S.; Khan, A.R.; et al. Ultrasonically synthesized CuO–thiosemicarbazone nanoconjugates: A study on interfacial interactions and synergistic biological and photocatalytic effects. Surf. Interfaces 2025, 107, 107271. [Google Scholar] [CrossRef]
- Jabeen, S.; Siddiqui, V.U.; Rastogi, S.; Srivastava, S.; Bala, S.; Ahmad, N.; Khan, T. Fabrication of B–CuO nanostructure and B–CuO/rGO binary nanocomposite: A comparative study in the context of photodegradation and antimicrobial activity assessment. Mater. Today Chem. 2023, 33, 101712. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).