Graph Neural Networks for Drug–Drug Interaction Prediction—Predicting Safe Drug Pairings with AI †
Abstract
1. Introduction
- How can we leverage graph neural networks (GNNs) to more accurately predict drug–drug interactions (DDIs)?
- What are the key factors that contribute to the safe pairing of drugs, and how can these be incorporated into a predictive model?
2. Literature Review
3. Methodology
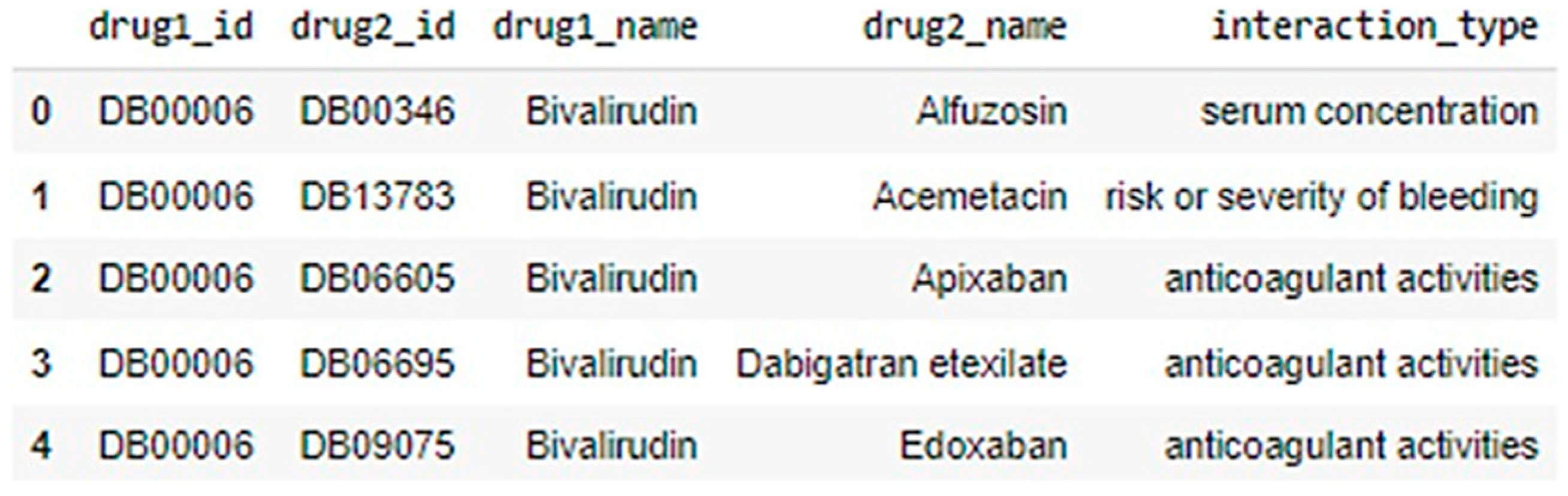
3.1. Data Collection
- DrugBank: A very popular and reliable database resource that has useful information on drugs including their chemical structure, their pharmacokinetics, and other drugs that are known to interact with them. DrugBank is a primary dataset for this study, as it offers the data needed to practically simulate drug interaction modeling.
- TWOSIDES: This resource specializes exclusively on adverse effects due to drug-polypharmacy and provides additional information on the safety profile of several drugs with TWOSIDES being provided. TWOSIDES is useful in determining possible dangers that may arise from the combination of drugs in polypharmacy and provides a predictive scope for both the likelihood and the level of adverse interactions.
- ChEMBL: The bioactivity database that ELIXIR API has includes drug interaction information, the biological effects of drugs, and drug targets. As mentioned in prior studies, ChEMBL contributes additional data related to pharmacology in this dataset, assisting the model in the comprehension of targeted system interaction at the molecular level.
3.2. Data Preprocessing
- 1.
- Data cleaning involves relatively more technical elements such as the rectification of errors and ensuring as much homogeneity as possible of within the dataset. As Figure 2 illustrates, raw datasets often contain inconsistencies that require careful cleaning before analysis.
- 2.
- Duplicate Removal: Duplicates of the samples are identified and removed to avoid any duplication that would yield incorrect results when training the model.
- 3.
- Inconsistency Resolution: There will always be variation in the names of drugs, measurement units, and types of interactions. These forms of variations are eliminated through standardization techniques.
- 4.
- Normalization: All volumetric and activity score continuous variables will be brought to standard ranges such as 0 and 1. This is important, as failure to apply normalization in models often causes bias to the model, as features with varying scales do not carry equal importance when constructing the model, which is the case in this model as well.
4. Results and Discussion
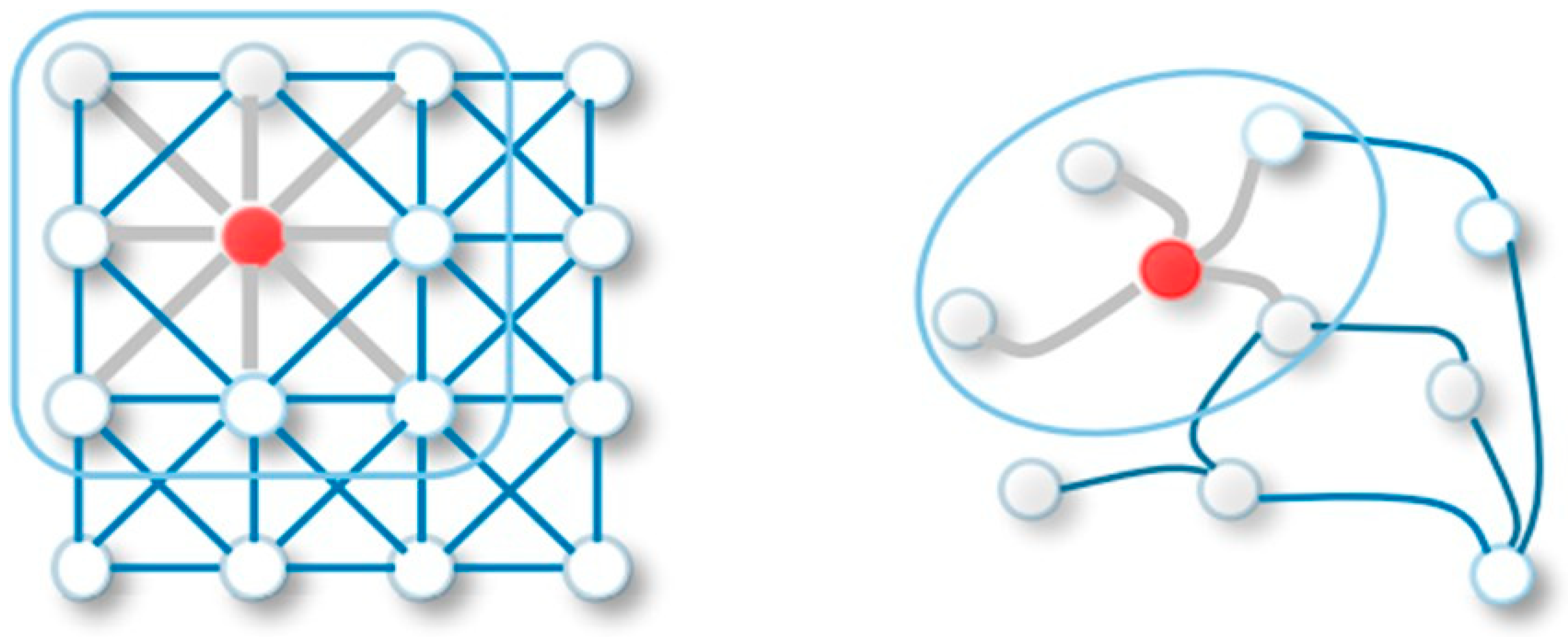
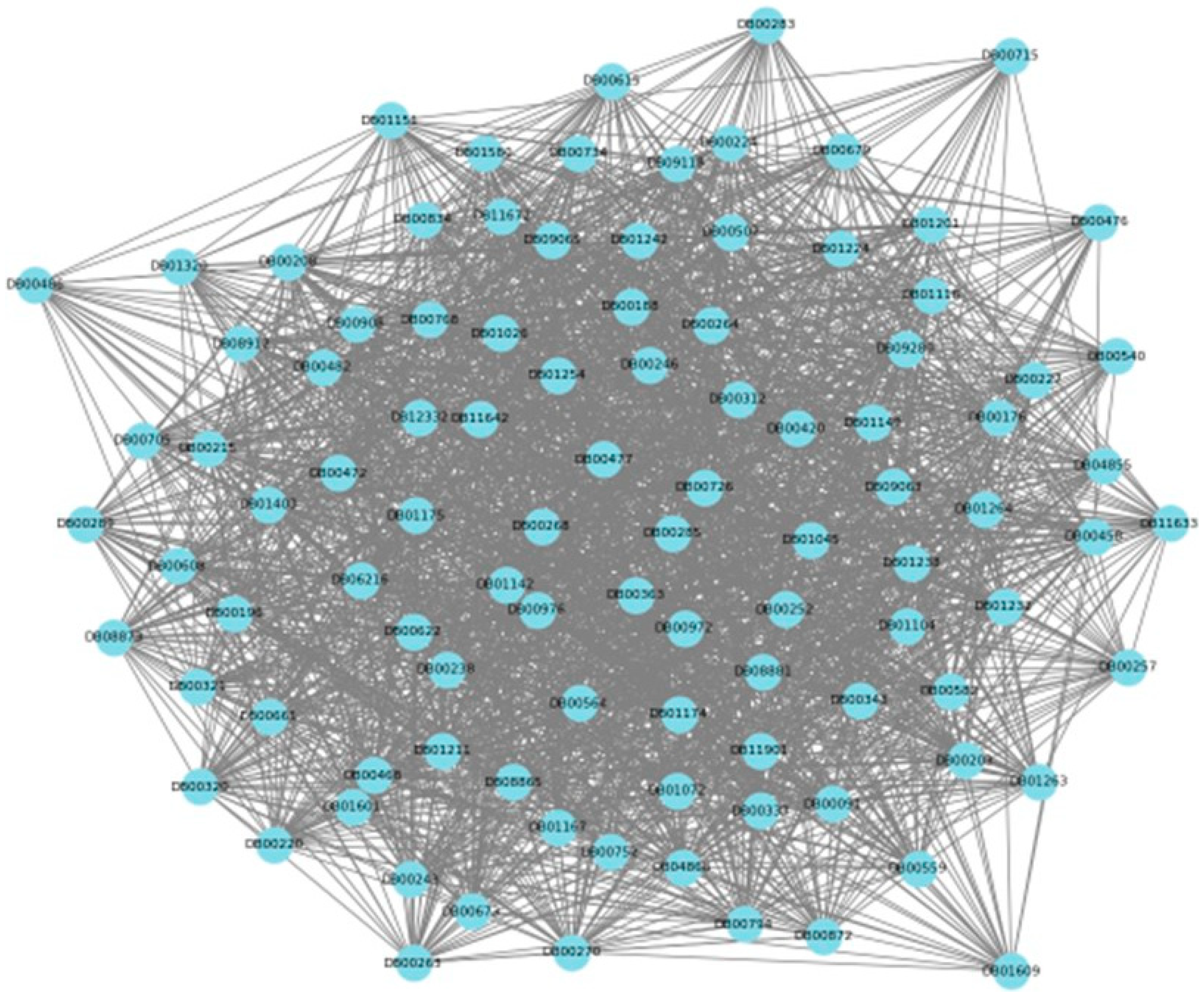
4.1. Representation of Inputs and Graph Construction
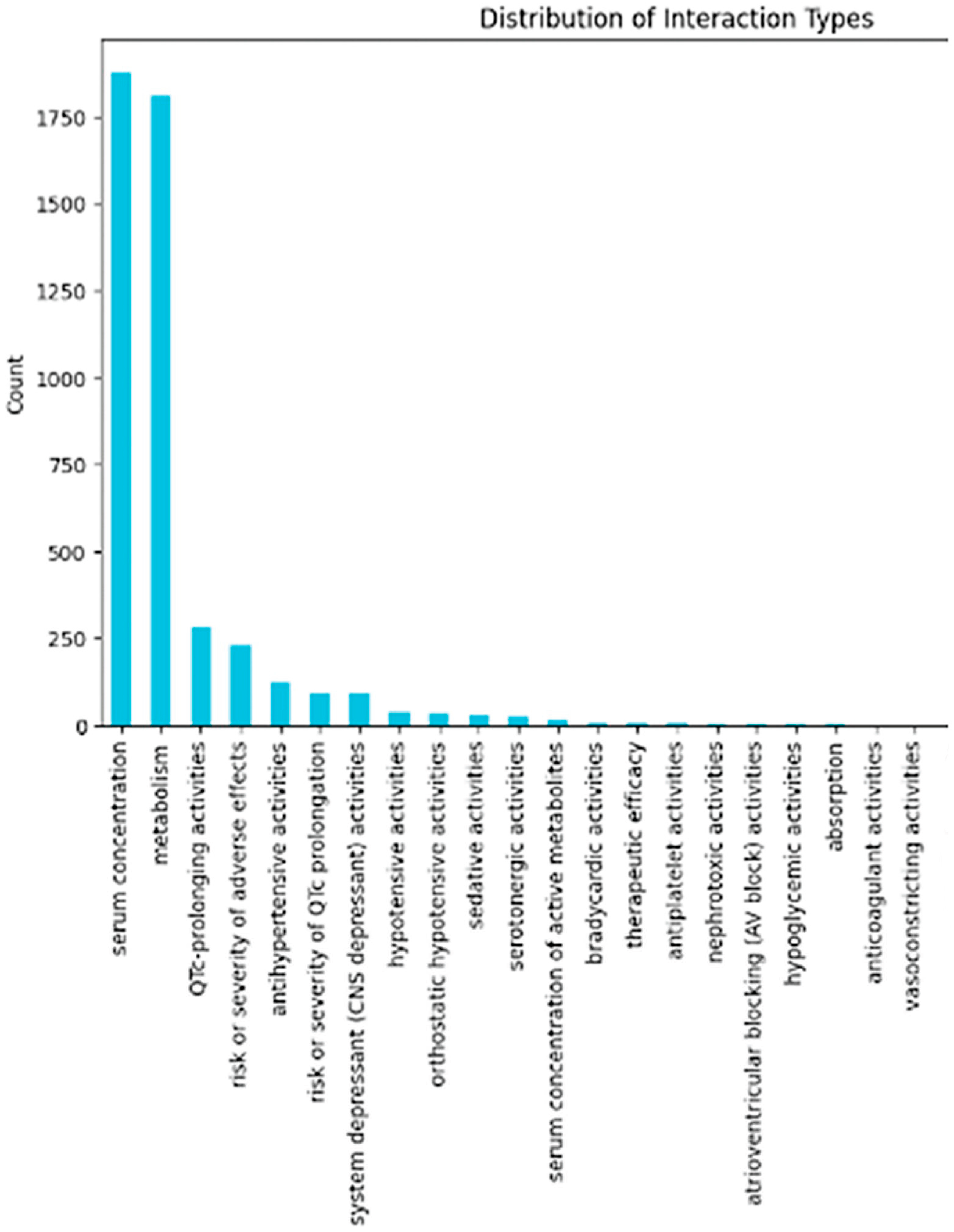
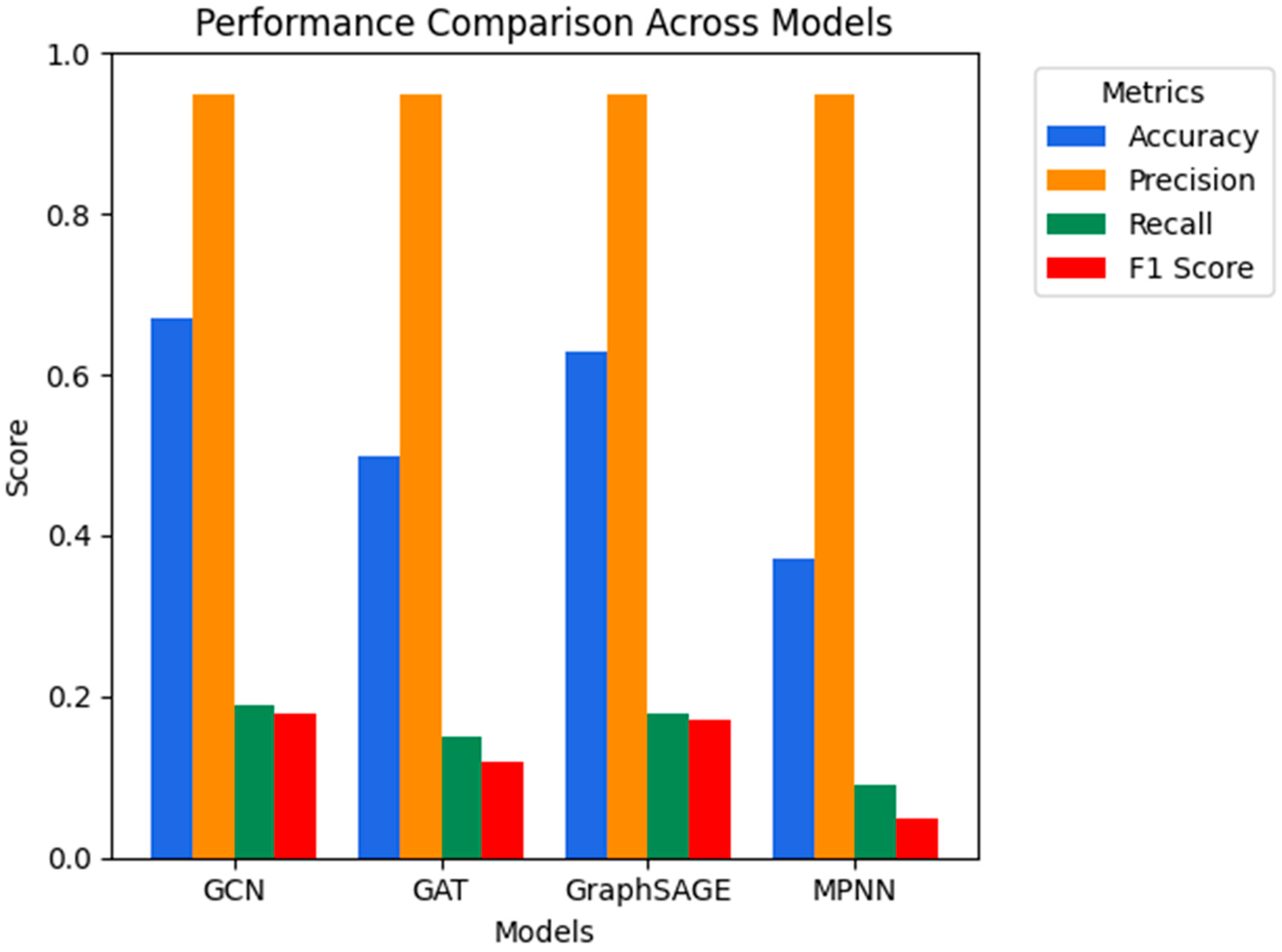
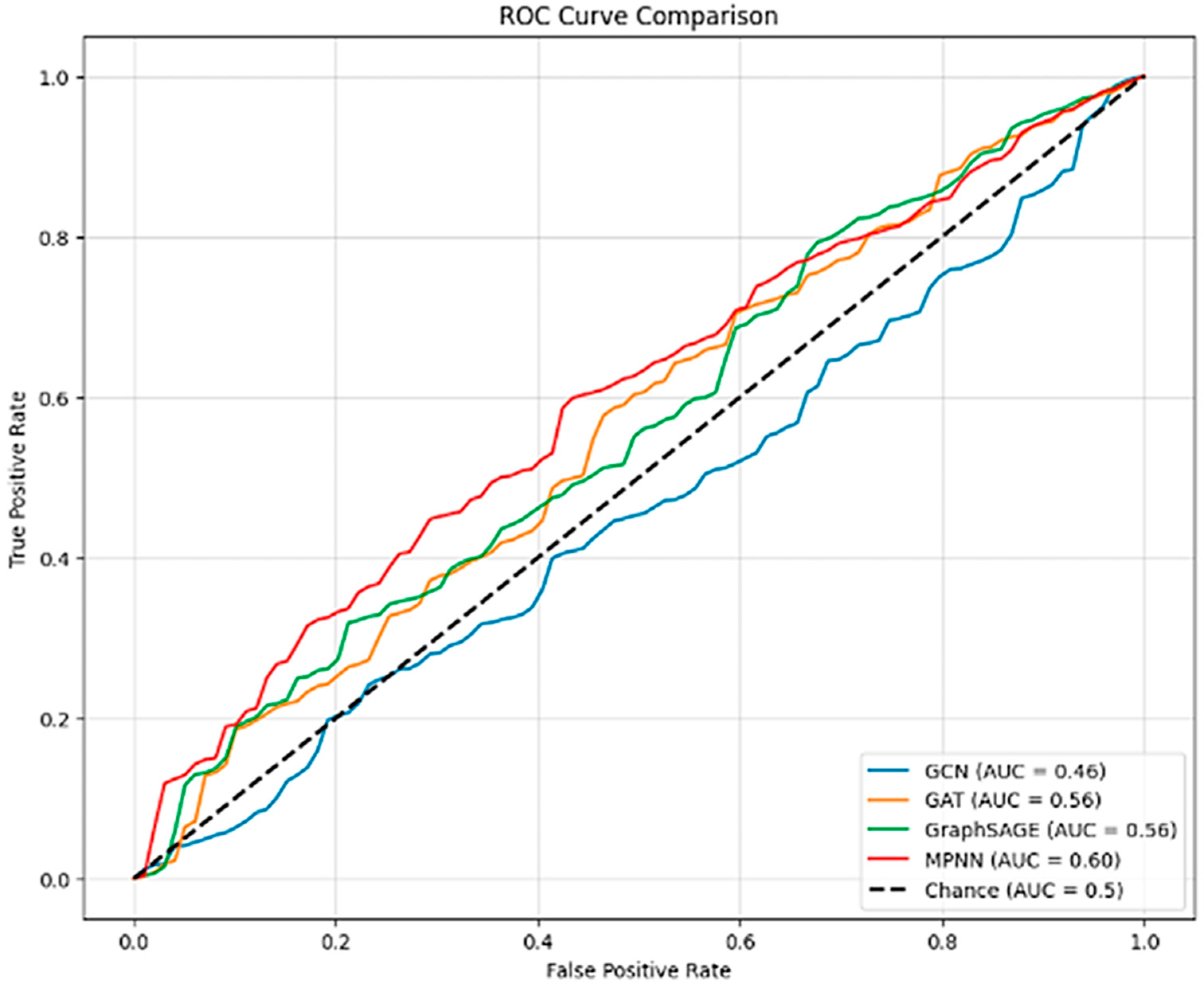
4.2. Evaluation Metrics and Results
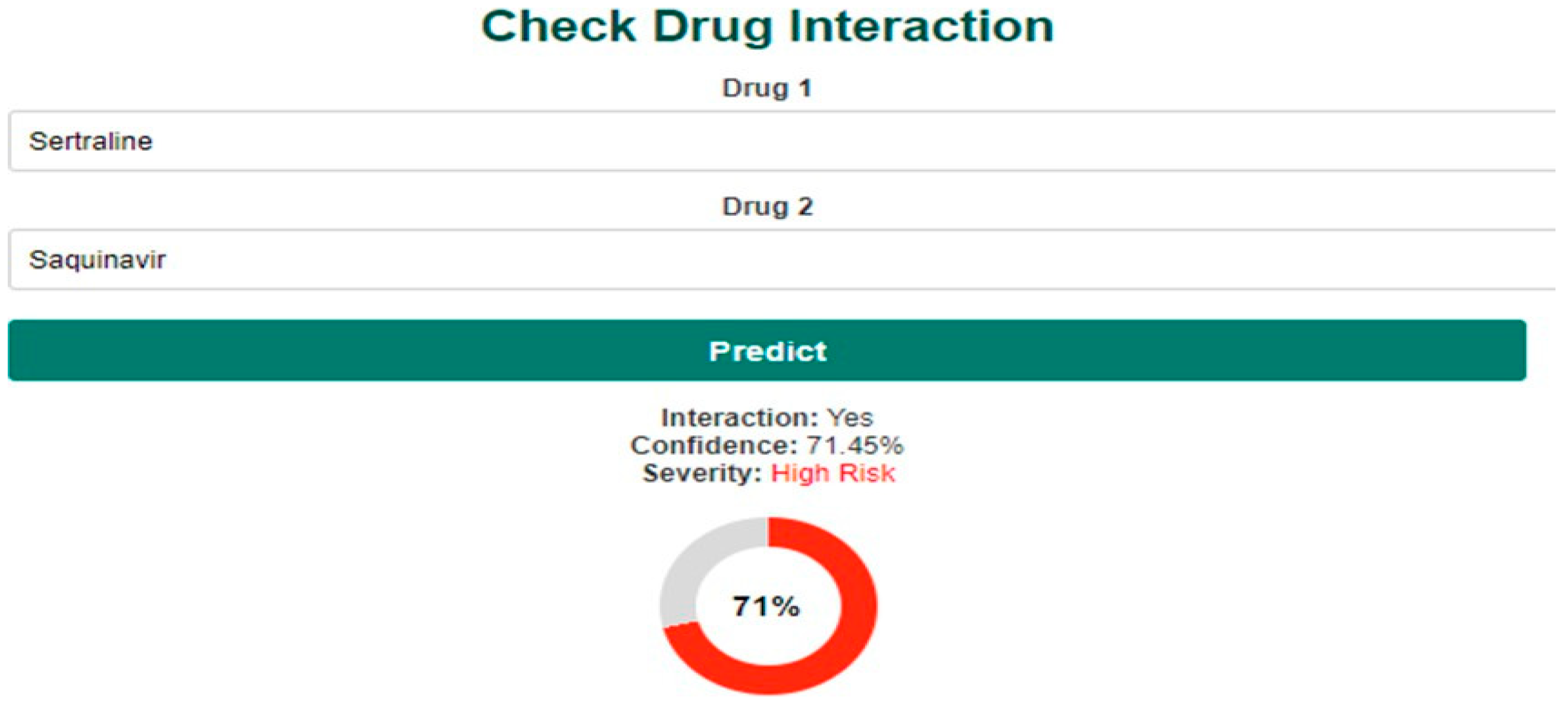
4.3. Frontend Design and User Interface
5. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baxter, K.; Stockley, I.; Varman, M. Stockley’s Drug Interactions: A Source book of Interactions, Their Mechanisms, Clinical Importance, and Management; Pharmaceutical Press: London, UK, 2021. [Google Scholar]
- Cheng, F.; Li, W.; Zhou, Y.; Shen, J. Machine learning approaches for drug–drug interaction prediction. J. Chem. Inf. Model. 2020, 60, 3129–3141. [Google Scholar]
- Cheng, F.; Li, W.; Zhou, Y.; Shen, J. Optimization techniques in graph neural networks for biomedical data. J. Biomed. Inform. 2021, 115, 103678. [Google Scholar]
- Doshi-Velez, F.; Kim, B. Towards a rigorous science of interpretable machine learning. arXiv 2017, arXiv:1702.08608. [Google Scholar] [CrossRef]
- Wang, S.; Yao, Z.; Zhang, S. Graph neural networks for multi-drug interaction prediction. Nat. Rev. Chem. 2022, 6, 245–260. [Google Scholar]
- Goodfellow, I.; Bengio, Y.; Courville, A. Deep Learning; MIT Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Wu, Z.; Ramsundar, B.; Feinberg, E.; Gomes, J.; Geniesse, C.; Pappu, A.; Leswing, K. A comprehensive survey on graph neural networks. IEEE Trans. Neural Netw. Learn. Syst. 2022, 32, 4–24. [Google Scholar] [CrossRef]
- Hamilton, W.; Ying, R.; Leskovec, J. Representation learning on graphs: Methods and applications. IEEE Data Eng. Bull. 2021, 40, 52–74. [Google Scholar]
- Hamilton, W.; Ying, R.; Leskovec, J. Graph representation learning for healthcare: Challenges and opportunities. IEEE Trans. Biomed. Eng. 2022, 69, 765–782. [Google Scholar]
- Hansen, C.; Follman, D.; Humes, A. Reducing drug-drug interactions with artificial intelligence: A clinical application of graph neural networks. J. Pharm. Sci. 2021, 110, 2879–2893. [Google Scholar]
- Huang, C.; Zhang, Z.; Wei, W. Multi-omics data integration for drug–drug interaction prediction using deep learning. Brief. Bioinform. 2021, 22, bbab192. [Google Scholar]
- Kipf, T.; Welling, M. Semi-supervised classification with graph convolutional networks. arXiv 2016, arXiv:1609.02907. [Google Scholar]
- Kingma, D.; Ba, J. Adam: A method for stochastic optimization. arXiv 2015, arXiv:1412.6980. [Google Scholar]
- Li, X.; Liu, Z.; Zhang, Z. A survey of machine learning approaches for drug–drug interaction prediction. J. Bioinform. Comput. Biol. 2022, 20, 123456. [Google Scholar]
- Lin, J.; Pan, Y.; Wang, Y. Graph neural networks for drug discovery and development. Nat. Rev. Drug Discov. 2021, 20, 547–560. [Google Scholar]
- Mendez, D.; Gaulton, A.; Bento, A.; Chambers, J.; De Veij, M.; Félix, E.; Hersey, A. ChEMBL: Towards direct deposition of bioassay data. Nucleic Acids Res. 2021, 47, D930–D940. [Google Scholar] [CrossRef] [PubMed]
- Mikolov, T.; Sutskever, I.; Chen, K.; Corrado, G.; Dean, J. Distributed representations of words and phrases and their compositionality. Adv. Neural Inf. Process. Syst. 2013, 26, 3111–3119. [Google Scholar]
- Tatonetti, N.; Ye, P.; Daneshjou, R.; Altman, R. Data-driven prediction of drug effects and interactions. Sci. Transl. Med. 2012, 4, 125ra31. [Google Scholar] [CrossRef] [PubMed]
- Zitnik, M.; Li, J.; Leskovec, J. Modeling polypharmacy side effects with graph convolutional networks. Bioinformatics 2019, 35, 4335–4342. [Google Scholar] [CrossRef]
- Velickovic, P.; Cucurull, G.; Casanova, A.; Romero, A.; Liò, P.; Bengio, Y. Graph attention networks. arXiv 2018, arXiv:1710.10903. [Google Scholar]
- Gilmer, J.; Schoenholz, S.; Riley, P.; Vinyals, O.; Dahl, G. Neural message passing for quantum chemistry. In Proceedings of the International Conference on Machine Learning 2017, Sydney, Australia, 6–11 August 2017; pp. 1263–1272. [Google Scholar]
- Hamilton, W.; Ying, R.; Leskovec, J. Inductive representation learning on large graphs. arXiv 2020, arXiv:1706.02216. [Google Scholar]
- Wu, Z.; Pan, S.; Chen, F.; Long, G.; Zhang, C.; Yu, P. MoleculeNet: A benchmark for molecular machine learning. Chem. Sci. 2020, 9, 513–530. [Google Scholar] [CrossRef]
- You, J.; Ying, R.; Leskovec, J. Design space for graph neural networks. Adv. Neural Inf. Process. Syst. 2020, 33, 17009–17021. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y. Drug-drug interaction prediction using graph neural networks. IEEE Access 2020, 8, 90380–90391. [Google Scholar] [CrossRef]

| Algorithm | Strengths | Limitations | Source |
|---|---|---|---|
| Graph Convolutional Networks (GCNs) | Molecular graph networks are able to obtain local neighborhood information efficiently and are therefore frequently applied to molecular prediction tasks. | Fairly limited in their scalability; they do however seem to struggle with very large datasets. | [19] |
| Graph Attention Networks (GATs) | Improved accuracy through attention mechanisms, better for complex relationships. | Lacking efficiency in computations because necessary weights must be incorporated for each attention neighbor. | [20] |
| Message-Passing Neural Networks (MPNNs) | Excellent at capturing complex chemical properties, flexible message-passing. | High computational cost; difficult to scale to large datasets. | [21] |
| GraphSAGE | Highly scalable, can handle large datasets efficiently, generalizes well to unseen graphs. | May lose information from distant nodes; focuses primarily on local structures. | [22] |
| DeepChem | Combines GNNs with deep learning techniques, designed for large pharmaceutical datasets. | Limited real-time application due to its high computational cost. | [23] |
| Model | Pre-Tuning Accuracy (%) | Post-Tuning Accuracy (%) | Pre-Tuning F1 (%) |
|---|---|---|---|
| GCN | 65.85 | 39.80 | 17.84 |
| GAT | 49.86 | 43.70 | 12.38 |
| GraphSAGE | 61.04 | 44.89 | 17.28 |
| MPNN | 37.66 | 7.09 | 3.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nisar, U.; Ashraf, H.; Jhanjhi, N.; Ashfaq, F.; Ihsan, U.; Lattu, A. Graph Neural Networks for Drug–Drug Interaction Prediction—Predicting Safe Drug Pairings with AI. Eng. Proc. 2025, 107, 42. https://doi.org/10.3390/engproc2025107042
Nisar U, Ashraf H, Jhanjhi N, Ashfaq F, Ihsan U, Lattu A. Graph Neural Networks for Drug–Drug Interaction Prediction—Predicting Safe Drug Pairings with AI. Engineering Proceedings. 2025; 107(1):42. https://doi.org/10.3390/engproc2025107042
Chicago/Turabian StyleNisar, Uzair, Humaira Ashraf, NZ Jhanjhi, Farzeen Ashfaq, Uswa Ihsan, and Arny Lattu. 2025. "Graph Neural Networks for Drug–Drug Interaction Prediction—Predicting Safe Drug Pairings with AI" Engineering Proceedings 107, no. 1: 42. https://doi.org/10.3390/engproc2025107042
APA StyleNisar, U., Ashraf, H., Jhanjhi, N., Ashfaq, F., Ihsan, U., & Lattu, A. (2025). Graph Neural Networks for Drug–Drug Interaction Prediction—Predicting Safe Drug Pairings with AI. Engineering Proceedings, 107(1), 42. https://doi.org/10.3390/engproc2025107042







