Tailoring the Optical and Sensing Properties of Sol–Gel Niobia Coatings via Doping with Silica and Noble Metal Nanoparticles †
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Doped Nb2O5 Thin Films
2.2. Characterization of Films
3. Results and Discussion
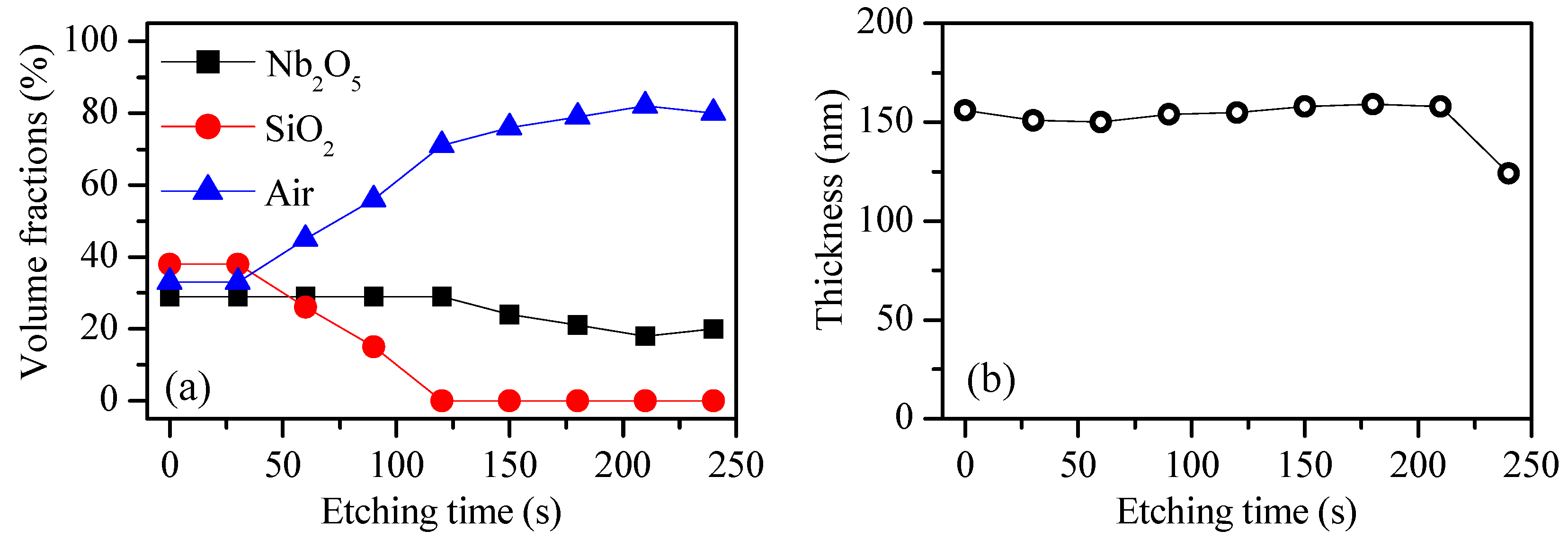
3.1. Introducing Porosity in Niobia Thin Films
3.2. Selection of Nanoparticle Type and Size
3.3. Doping with Au Nanoparticles (10 and 20 Nm) with Different Concentrations
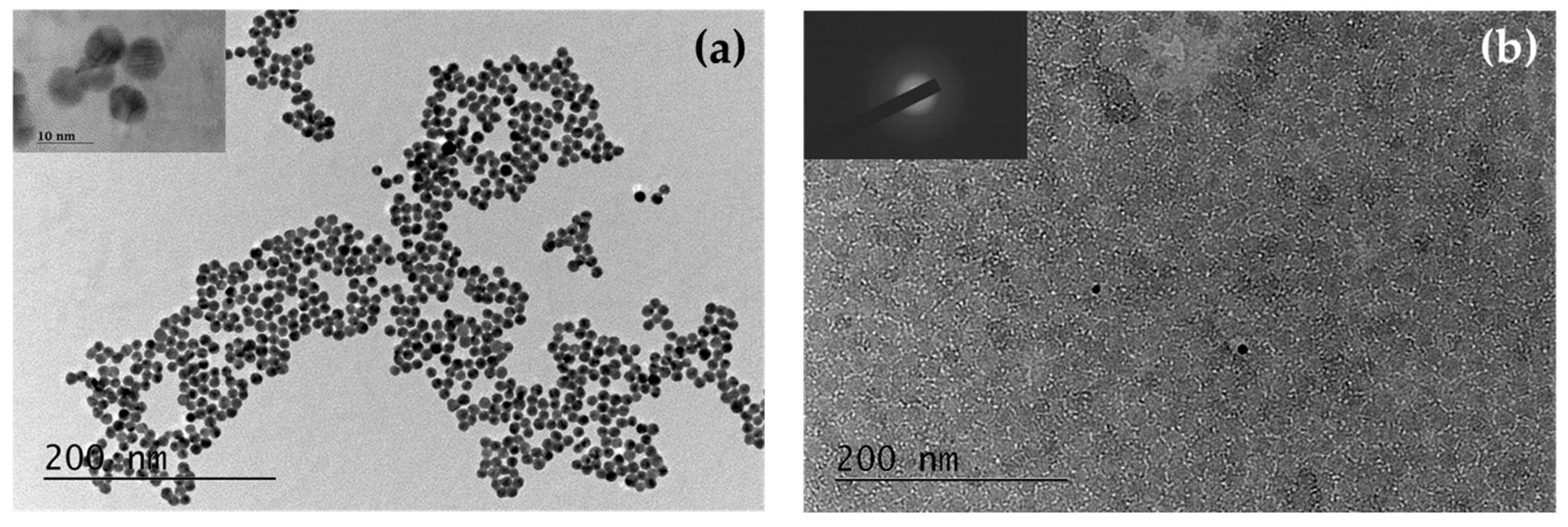
3.3.1. TEM Investigation
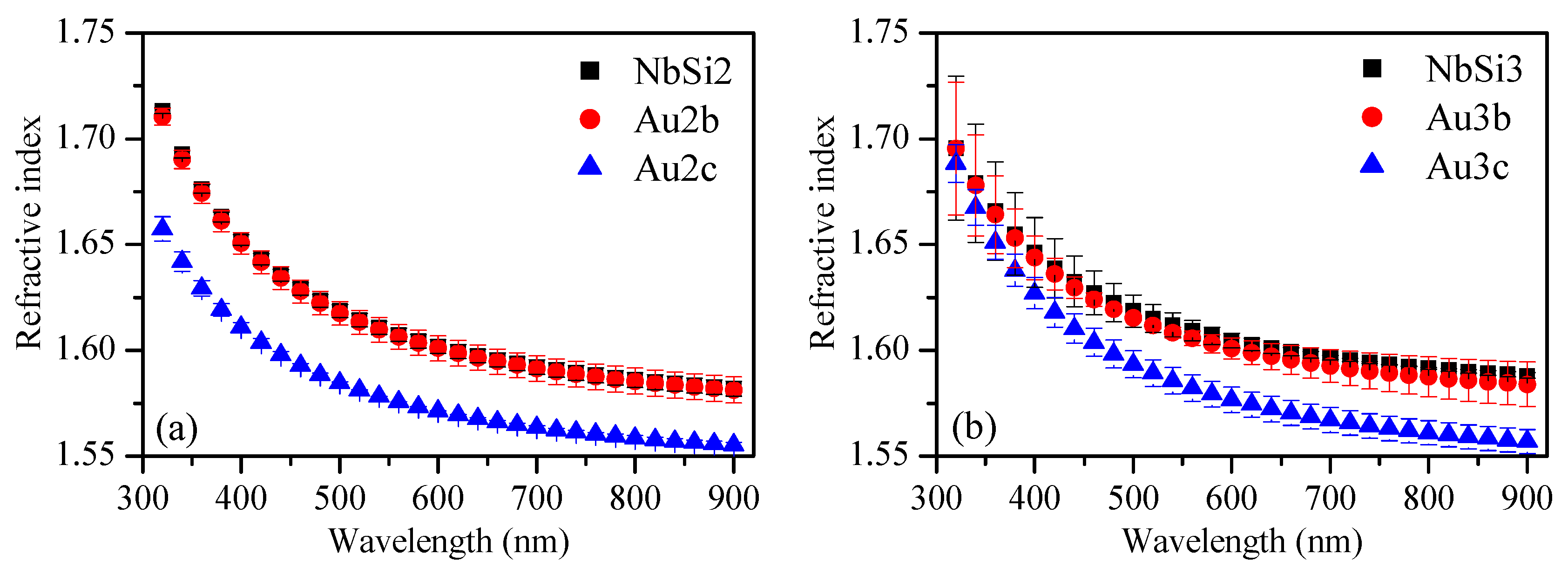
3.3.2. Refractive Index and Optical Band Gap
3.3.3. Sensing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahne, H.; Berger, L.; Martin, D.; Klemm, V.; Slesazeck, S.; Jakschik, S.; Rafaja, D.; Mikolajick, T. Filamentary resistive switching in amorphous and polycrystalline Nb2O5 thin films. Solid State Electron. 2012, 72, 73–77. [Google Scholar] [CrossRef]
- Saito, Y.; Shiosaki, T. Second harmonic generation in Nb2O5 thin-film optical waveguide deposited on LiTaO3 substrate by RF magnetron sputtering. Jpn. J. Appl. Phys. 1992, 31, 3164. [Google Scholar]
- Fredell, M.; Winchester, K.; Jarvis, G.; Locknar, S.; Johnson, R.; Keevers, M. Ultra-wide broadband dielectric mirrors for solar collector applications. In Proceedings of the SPIE OPTO, San Francisco, CA, USA, 24 February 2017. [Google Scholar]
- Ozer, N.; Rubin, M.D.; Lampert, C.M. Optical and electrochemical characteristics of niobium oxide films prepared by sol-gel process and magnetron sputtering A comparison. Sol. Energy Mater. Sol. Cells 1996, 40, 285–296. [Google Scholar] [CrossRef]
- Eduok, U. Niobia Nanofiber-Reinforced Protective Niobium Oxide/Acrylate Nanocomposite Coatings. ACS Omega 2020, 5, 30716–30728. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, Y.; Wang, W.; Zhang, X.; Wang, B.; Tian, H.; Wang, Y.; Guan, J.; Gu, H. Fast and highly-sensitive hydrogen sensing of Nb2O5 nanowires at room temperature. Int. J. Hydrogen Energy 2012, 37, 4526–4532. [Google Scholar] [CrossRef]
- Lombardo, L.; Grassini, S.; Parvis, M.; Donato, N.; Gullino, A. Ethanol breath measuring system. In Proceedings of the IEEE International Symposium on Medical Measurements and Applications (MeMeA), Bari, Italy, 1 June–1 July 2020. [Google Scholar]
- Lombardo, L.; Grassini, S.; Parvis, M.; Donato, N.; Gullino, A. Niobium Pentaoxide Thin-Film Gas Sensor for Portable Acetone Sensing. In Proceedings of the International Conference on Advanced Technologies, Systems and Services in Telecommunications (TELSIKS), Nis, Serbia, 20–22 October 2021. [Google Scholar]
- Liu, J.; Zuo, S.; Lin, S.; Shan, B.; Zhou, X.; Zhao, J.; Qi, C.; Yang, P. Promoting the performance of Nb2O5 by doping transition metal oxide for catalytic degradation of monochlorobenzene and toluene. J. Mater. Res. Technol. 2023, 25, 3642–3653. [Google Scholar] [CrossRef]
- Tao, H.; Li, J.; Ma, Q.; Chen, Z.; Zhang, X.; Quan, Y.; Peng, Y.; Qi, C. Synthesis of W-Nb-O solid acid for catalytic combustion of low-concentration monochlorobenzene. Chem. Eng. J. 2020, 382, 123045. [Google Scholar] [CrossRef]
- Jin, Y.; Quan, Y.; Liu, J.; Qi, C.; Pan, P.; Shan, B.; Luo, H.; Peng, Y. Controlled synthesis of niobium and rare earth mixed oxides for catalytic combustion of chlorinated VOCs in the synthesis process of polyether polyol and polyurethane. J. Solid State Chem. 2022, 313, 123318. [Google Scholar] [CrossRef]
- Georgiev, R.; Christova, D.; Georgieva, B.; Babeva, T. Organic framework engineering in mesoporous Nb2O5 thin films used as an active medium for organic vapors sensing. Proc. SPIE 2018, 10691, 1069128. [Google Scholar]
- Georgiev, R.; Chorbadzhiyska, Y.; Pavlov, V.; Georgieva, B.; Babeva, T. Optical Detection of VOC Vapors Using Nb2O5 Bragg Stack in Transmission Mode. Photonics 2021, 8, 399. [Google Scholar] [CrossRef]
- Pavlov, V.; Georgiev, R.; Lazarova, K.; Georgieva, B.; Babeva, T. Hard-Templated Porous Niobia Films for Optical Sensing Applications. Photonics 2023, 10, 167. [Google Scholar] [CrossRef]
- Raba, A.; Barba-Ortega, J.; Joya, M. Effects of the presence of a metal doping agent in the properties of the Nb2−xMxO5 (M = Mn, Fe, and Ni) system. Int. J. Appl. Ceram. Technol. 2018, 15, 1577–1583. [Google Scholar] [CrossRef]
- Zeng, M.; Wu, Y.; Wang, Y.; Zhang, Z.; Fu, X.; Dai, W. Silver nanoparticle-enhanced photocatalytic CO2 reduction over Ag/Nb2O5 under UV–vis light irradiation. Appl. Catal. A Gen. 2023, 666, 119412. [Google Scholar] [CrossRef]
- Dien, L.X.; Truong, Q.D.; Murayama, T.; Chinh, H.D.; Taketoshi, A.; Honma, I.; Haruta, M.; Ishida, T. Gold Nanoparticles Supported on Nb2O5 for Low-Temperature CO Oxidation and as Cathode Materials for Li-ion Batteries. Appl. Catal. A Gen. 2020, 603, 117747. [Google Scholar] [CrossRef]
- Boruah, B.; Gupta, R.; Modaka, J.M.; Madras, G. Enhanced photocatalysis and bacterial inhibition in Nb2O5 via versatile doping with metals (Sr, Y, Zr, and Ag): A critical assessment. Nanoscale Adv. 2019, 1, 2748–2760. [Google Scholar] [CrossRef]
- Antonini, L.M.; Adamski, J.; Bernardi, F.; Aguzzoli, C.; Hübler, R.; Malfatti, C.F. Photoactivity of nanostructured porous Nb2O5: Effect of Pt, Ta, Cu, and Ti impregnation. Int. J. Ceram. Eng. Sci. 2022, 4, 379–390. [Google Scholar] [CrossRef]
- Lazarova, K.; Vasileva, M.; Marinov, G.; Babeva, T. Optical characterization of sol-gel derived Nb2O5 thin films. Opt. Laser Technol. 2014, 58, 114–118. [Google Scholar] [CrossRef]
- Lazarova, K.; Awala, H.; Thomas, S.; Vasileva, M.; Mintova, S.; Babeva, T. Vapor Responsive One-Dimensional Photonic Crystals from Zeolite Nanoparticles and Metal Oxide Films for Optical Sensing. Sensors 2014, 14, 12207–12218. [Google Scholar] [CrossRef]
- Georgiev, R.; Georgieva, B.; Lazarova, K.; Vasileva, M.; Babeva, T. Sol–gel tantalum pentoxide thin films with tunable refractive index for optical sensing applications. Opt. Quant. Electron. 2020, 52, 437. [Google Scholar] [CrossRef]
- Babeva, T.; Kitova, S.; Konstantinov, I. Photometric methods for determining the optical constants and the thicknesses of thin absorbing films: Criteria for precise and unambiguous determination of n, k, and d in a wide spectral range. Appl. Opt. 2001, 40, 2682–2686. [Google Scholar] [CrossRef]
- Falk, G.; Borlaf, M.; Bendo, T.; Novaes de Oliveira, A.P.; Rodrigues Neto, J.B.; Moreno, R. Colloidal Sol–Gel Synthesis and Photocatalytic Activity of Nanoparticulate Nb2O5 Sols. J. Am. Ceram. Soc. 2016, 99, 1968–1973. [Google Scholar] [CrossRef]
- Moon, C.W.; Choi, M.-J.; Hyun, J.K.; Jang, H.W. Enhancing photoelectrochemical water splitting with plasmonic Au nanoparticles. Nanoscale Adv. 2021, 3, 5981–6006. [Google Scholar] [CrossRef]
- Ibrahem, M.A.; Rasheed, B.G.; Mahdi, R.I.; Khazal, T.M.; Omar, M.M.; O’Neill, M. Plasmonic-enhanced photocatalysis reactions using gold nanostructured films. RSC Adv. 2020, 10, 22324–22330. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, Y. Extinction effect of gold nanocatalysts on photocatalytic activities under plasmonic excitation. Catalysts 2021, 11, 413. [Google Scholar] [CrossRef]



| Sample | Nb Sol (μL) | Ludox (μL) | NPs (μL) | NPs Type | NPs Size (nm) | H2O (μL) | NbCl5 (g):Ludox (g):NPs (g) |
|---|---|---|---|---|---|---|---|
| NbSi1 | 909 | 45.5 | 0 | - | - | 45.5 | 4.27 × 10−2:1.37 × 10−2:0 |
| NbSi2 | 833.3 | 41.7 | 0 | - | - | 125 | 3.90 × 10−2:1.25 × 10−2:0 |
| NbSi3 | 800 | 40 | 0 | - | - | 160 | 3.74 × 10−2:1.20 × 10−2:0 |
| Au1a | 909 | 45.5 | 45.5 | Au | 5 | 0 | 4.27 × 10−2:1.37 × 10−2:9.1 × 10−7 |
| Au1b | 909 | 45.5 | 45.5 | Au | 10 | 0 | 4.27 × 10−2:1.37 × 10−2:9.1 × 10−7 |
| Au1c | 909 | 45.5 | 45.5 | Au | 20 | 0 | 4.27 × 10−2:1.37 × 10−2:9.1 × 10−7 |
| Au2b | 833.3 | 41.7 | 125 | Au | 10 | 0 | 3.90 × 10−2:1.25 ×10−2:2.5 × 10−6 |
| Au2c | 833.3 | 41.7 | 125 | Au | 20 | 0 | 3.90 × 10−2:1.25 × 10−2:2.5 × 10−6 |
| Au3b | 800 | 40 | 160 | Au | 10 | 0 | 3.74 × 10−2:1.20 × 10−2:3.2 × 10−6 |
| Au3c | 800 | 40 | 160 | Au | 20 | 0 | 3.74 × 10−2:1.20 × 10−2:3.2 × 10−6 |
| Ag1a | 909 | 45.5 | 45.5 | Ag | 10 | 0 | 4.27 × 10−2:1.37 × 10−2:9.1 × 10−7 |
| Ag1b | 909 | 45.5 | 45.5 | Ag | 20 | 0 | 4.27 × 10−2:1.37 × 10−2:9.1 × 10−7 |
| Ag1c | 909 | 45.5 | 45.5 | Ag | 40 | 0 | 4.27 × 10−2:1.37 × 10−2:9.1 × 10−7 |
| Pt1a | 909 | 45.5 | 45.5 | Pt | 5 | 0 | 4.27 × 10−2:1.37 × 10−2:4.6 × 10−8 |
| Pt1b | 909 | 45.5 | 45.5 | Pt | 30 | 0 | 4.27 × 10−2:1.37 × 10−2:4.6 × 10−8 |
| Sample | No AuNPs | AuNPs 10 nm | AuNPs 20 nm |
|---|---|---|---|
| 1:1 | 1.600 ± 0.008 | 1.600 ± 0.008 | 1.600 ± 0.004 |
| 1:3 | 1.602 ± 0.003 | 1.601 ± 0.006 | 1.571 ± 0.001 |
| 1:4 | 1.605 ± 0.003 | 1.601 ± 0.005 | 1.577 ± 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babeva, T.; Pavlov, V.; Zlatinov, G.; Georgieva, B.; Terziyska, P.; Alexieva, G.; Lazarova, K.; Georgiev, R. Tailoring the Optical and Sensing Properties of Sol–Gel Niobia Coatings via Doping with Silica and Noble Metal Nanoparticles. Eng. Proc. 2025, 105, 4. https://doi.org/10.3390/engproc2025105004
Babeva T, Pavlov V, Zlatinov G, Georgieva B, Terziyska P, Alexieva G, Lazarova K, Georgiev R. Tailoring the Optical and Sensing Properties of Sol–Gel Niobia Coatings via Doping with Silica and Noble Metal Nanoparticles. Engineering Proceedings. 2025; 105(1):4. https://doi.org/10.3390/engproc2025105004
Chicago/Turabian StyleBabeva, Tsvetanka, Venelin Pavlov, Georgi Zlatinov, Biliana Georgieva, Penka Terziyska, Gergana Alexieva, Katerina Lazarova, and Rosen Georgiev. 2025. "Tailoring the Optical and Sensing Properties of Sol–Gel Niobia Coatings via Doping with Silica and Noble Metal Nanoparticles" Engineering Proceedings 105, no. 1: 4. https://doi.org/10.3390/engproc2025105004
APA StyleBabeva, T., Pavlov, V., Zlatinov, G., Georgieva, B., Terziyska, P., Alexieva, G., Lazarova, K., & Georgiev, R. (2025). Tailoring the Optical and Sensing Properties of Sol–Gel Niobia Coatings via Doping with Silica and Noble Metal Nanoparticles. Engineering Proceedings, 105(1), 4. https://doi.org/10.3390/engproc2025105004







