Abstract
The present work is devoted to the synthesis of imidazo[2,1-b][1,3]thiazine derivatives as possible anti-inflammatory agents. The synthetic approach to (2-pyridinyloxy) substituted imidazo[2,1-b][1,3]thiazines based on the interaction of the polysubstituted 2-chloropyridines with 3-hydroxy-imidazo[2,1-b][1,3]thiazines was proposed. Selective nucleophilic substitution in position 2 of a pyridine ring was observed in the mentioned reaction. The synthesized (2-pyridinyloxy) substituted imidazo[2,1-b][1,3]thiazines drug-like properties were studied in silico using SwissADME and anti-inflammatory activity in the carrageenan test in vivo. Hit-compounds with satisfactory drug-like and pharmacological features were identified as promising objects for forthcoming structure optimization and in-depth studies.
1. Introduction
Imidazo[2,1-b][1,3]thiazine scaffold is the attractive matrix for the design of small molecules with a wide activity spectrum. The application of modern drug design methodologies and strategies allowed the identification of the mentioned heterocycles’ potential agents with trypanocidal [1,2], anti-tuberculosis [3,4,5], antioxidant [6] antiviral [7,8], antitumor [9] and antifungal [10] activities (Figure 1).
Figure 1.
Pharmacology profile of imidazo[2,1-b][1,3]thiazine scaffold.
Inflammation is an important part of many pathology processes and an attractive pathway/target in modern drug design for the modulation and obtaining of the appropriate and satisfactory therapeutic effects [11,12,13].
Taking into account the synthesis of the hybrid molecules containing two or more pharmacophores is a promising and interesting approach to the design of potential pharmacological active small molecules, it was interesting to work out the straightforward and convenient protocol for the synthesis of new hybrid molecules containing diversified imidazo[2,1-b][1,3]thiazine scaffolds linked with a potential pharmacophore–pyridine ring and evaluate their drug-like and anti-inflammatory properties.
2. Methods
2.1. General Information
Melting points were measured in open capillary tubes on a BÜCHI B-545 melting point apparatus (BÜCHI Labortechnik AG, Flawil, Switzerland) and are uncorrected. The elemental analyses (C, H, N) were performed using the Perkin–Elmer 2400 CHN analyzer (PerkinElmer, Waltham, MA, USA) and were within ±0.4% of the theoretical values. The 400 MHz-1H and 126 MHz-13C spectra were recorded on a Varian Unity Plus 400 (400 MHz) spectrometer (Varian Inc., Paulo Alto, CA, USA). All spectra were recorded at room temperature except where indicated otherwise and were referenced internally to solvent reference frequencies. Chemical shifts (δ) are quoted in ppm and coupling constants (J) are reported in Hz. LC–MS spectra were obtained on a Finnigan MAT INCOS-50 (Thermo Finnigan LLC, San Jose, CA, USA). The reaction mixture was monitored by thin layer chromatography (TLC) using commercial glass-backed TLC plates (Merck Kieselgel 60 F254). Solvents and reagents that are commercially available were used without further purification. The 3-hydroxy-imidazo[2,1-b][1,3]thiazines 2a–c were prepared using the similar protocol described in [5].
2.2. Synthesis and Characterization of Compounds 3a–m
To the mixture of compounds 2a–c and a 60% NaH in mineral oil (10 mmol) in the dry DMF (4 mL), 10 mmol of the appropriate substituted derivate of 2-chloropiridine was added and stirred at room temperature for 24 h. Then, the mixture was poured onto ice, the sediment was filtered off, washed with water, dried and recrystallized from MeOH.
6-[(5-Chloropyridin-2-yl)oxy]-6,7-dihydro-5H-imidazo[2,1-b][1,3]thiazine (3a). M.p.: 150–151 °C. 1H NMR: δ = 8.25 (s, 1H, Ar), 7.83 (d, 3J = 8.8 Hz, 1H, Ar), 7.16 (s, 1H, Ar), 6.90 (d, 3J = 8.8 Hz, 1H, Ar), 6.87 (s, 1H, Ar), 5.69–5.70 (m, 1H, CH), 4.32–4.33 (m, 2H, NCH2), 3.57–3.60 (m, 1H, SCH2), 3.47 (dd, 2J = 13.2 Hz, 3J = 5.4 Hz, 1H, SCH2). 13C NMR: δ = 160.80 (Py), 145.32 (Py), 140.04 (Py), 135.83 (C8a), 128.20 (C2), 124.54 (Py), 121.80 (C3), 113.35 (Py), 65.33 (C6), 48.56 (C5), 28.86 (C7). LC-MS: m/z = 268 [M + 1] (100%). Anal. Calcd. for C11H10ClN3OS, %: C, 49.35; H, 3.76; N, 15.69. Found, %: C, 49.48; H, 3.77; N, 15.54.
6-{[5-(Trifluoromethyl)pyridin-2-yl]oxy}-6,7-dihydro-5H-imidazo[2,1-b][1,3]thiazine (3b). M.p.: 130–131 °C. 1H NMR: δ = 8.64 (s, 1H, Ar), 8.09 (d, 3J = 8.8 Hz, 1H, Ar), 7.18 (s, 1H, Ar), 7.05 (d, 3J = 8.4 Hz, 1H, Ar), 6.88 (s, 1H, Ar), 5.82–5.85 (m, 1H, CH), 4.37–4.38 (m, 2H, NCH2), 3.61–3.65 (m, 1H, SCH2), 3.52 (dd, 2J = 13.4 Hz, 3J = 5.4 Hz, 1H, SCH2). 13C NMR: δ = 168.58 (Py), 145.31 (q, 3JCF = 4.5 Hz, Py), 137.42 (q, 4JCF = 3.0 Hz, Py), 135.80 (C8a), 128.21 (C2), 124.42 (d, 1JCF = 270.0 Hz, CF3), 121.82 (C3), 119.93 (q, 2JCF = 33.0 Hz, Py), 112.45 (Py), 65.73 (C6), 48.52 (C5), 28.80 (C7). LC-MS: m/z = 302 [M + 1] (100%). Anal. Calcd. for C12H10F3N3OS, %: C, 47.84; H, 3.35; N, 13.95. Found, %: C, 48.02; H, 3.32; N, 13.89.
6-[(6,7-Dihydro-5H-imidazo[2,1-b][1,3]thiazin-6-yl)oxy]nicotinonitrile (3c). M.p.: 182–183 °C. 1H NMR: δ = 8.74 (s, 1H, Ar), 8.18 (d, 3J = 8.8 Hz, 1H, Ar), 7.17 (s, 1H, Ar), 7.04 (d, 3J = 8.8 Hz, 1H, Ar), 6.87 (s, 1H, Ar), 5.81–5.85 (m, 1H, CH), 4.35–4.36 (m, 2H, NCH2), 3.60–3.64 (m, 1H, SCH2), 3.44 (dd, 2J = 13.6 Hz, 3J = 5.2 Hz, 1H, SCH2). 13C NMR: δ = 164.24 (Py), 152.49 (Py), 143.20 (Py), 135.76 (C8a), 128.24 (C2), 121.82 (C3), 117.59 (Py), 112.66 (Py), 103.11 (CN), 65.97 (C6), 48.50 (C5), 28.80 (C7). LC-MS: m/z = 259 [M + 1] (100%). Anal. Calcd. for C12H10N4OS, %: C, 55.80; H, 3.90; N, 21.69. Found, %: C, 56.02; H, 3.92; N, 21.60.
6-[(3,5-Dichloropyridin-2-yl)oxy]-6,7-dihydro-5H-imidazo[2,1-b][1,3]thiazine (3d). M.p.: 163–164 °C. 1H NMR: δ = 8.24 (s, 1H, Ar), 8.17 (s, 1H, Ar), 7.17 (s, 1H, Ar), 6.87 (s, 1H, Ar), 5.75–5.77 (m, 1H, CH), 4.36–4.38 (m, 2H, NCH2), 3.58–3.61 (m, 1H, SCH2), 3.46–3.50 (m, 1H, SCH2). 13C NMR: δ = 156.32 (Py), 143.54 (Py), 139.34 (Py), 135.82 (C8a), 128.24 (C2), 124.35 (Py), 121.78 (C3), 118.58 (Py), 66.85 (C6), 48.42 (C5), 28.84 (C7). LC-MS: m/z = 302 [M + 1] (100%). Anal. Calcd. for C11H9Cl2N3OS, %: C, 43.72; H, 3.00; N, 13.91. Found, %: C, 43.88; H, 2.97; N, 14.04.
6-{[3-Chloro-5-(trifluoromethyl)pyridin-2-yl]oxy}-6,7-dihydro-5H-imidazo[2,1-b][1,3]thiazine (3e). M.p.: 113–114 °C. 1H NMR: δ = 8.57 (s, 1H, Ar), 8.37 (s, 1H, Ar), 7.16 (s, 1H, Ar), 6.86 (s, 1H, Ar), 5.85–5.88 (m, 1H, CH), 4.38–4.40 (m, 2H, NCH2), 3.61–3.64 (m, 1H, SCH2), 3.51 (dd, 2J = 10.6 Hz, 3J = 4.6 Hz, 1H, SCH2). 13C NMR: δ = 159.97 (Py), 143.26 (q, 3JCF = 3.75 Hz, Py), 136.87 (q, 4JCF = 2.5 Hz, Py), 135.78 (C8a), 128.23 (C2), 123.52 (d, 1JCF = 270.0 Hz, CF3), 121.79 (C3), 120.83 (q, 2JCF = 33.75 Hz, Py), 118.67 (Py), 67.34 (C6), 48.37 (C5), 28.77 (C7). LC-MS: m/z = 336 [M + 1] (100%). Anal. Calcd. for C12H9ClF3N3OS, %: C, 42.93; H, 2.70; N, 12.52. Found, %: C, 43.08; H, 2.67; N, 12.64.
2,3-Diphenyl-6-{[5-(trifluoromethyl)pyridin-2-yl]oxy}-6,7-dihydro-5H-imidazo[2,1-b][1,3]thiazine (3f). M.p.: 154–155 °C. 1H NMR: δ = 8.54 (s, 1H, Ar), 8.05 (d, 3J = 9.0 Hz, 1H, Ar), 7.43–7.44 (m, 3H, Ar), 7.33–7.34 (m, 2H, Ar), 7.28–7.29 (m, 2H, Ar), 7.14–7.17 (m, 2H, Ar), 7.07–7.10 (m, 1H, Ar), 7.05 (d, 3J = 8.4 Hz, 1H, Ar), 5.80–5.82 (m, 1H, CH), 4.13–4.16 (m, 1H, NCH2), 3.92–3.95 (m, 1H, NCH2), 3.62–3.64 (m, 1H, SCH2), 3.53–3.57 (m, 1H, SCH2). 13C NMR: δ = 164.49 (Py), 145.22 (q, 3JCF = 4.5 Hz, Py), 137.38 (q, 4JCF = 3.0 Hz, Py), 137.01 (C8a), 136.83 (C3), 134.62, 130.97, 130.19 (Ar), 129.85 (C2), 129.54, 129.22, 128.51, 126.67, 126.40 (Ar), 124.39 (d, 1JCF = 270.0 Hz, CF3), 119.95 (q, 2JCF = 33.0 Hz, Py), 112.47 (Py), 65.92 (C6), 47.33 (C5), 28.40 (C7). LC-MS: m/z = 454 [M + 1] (100%). Anal. Calcd. for C24H18F3N3OS, %: C, 63.57; H, 4.00; N, 9.27. Found, %: C, 63.75; H, 3.97; N, 9.19.
6-[(2,3-Diphenyl-6,7-dihydro-5H-imidazo[2,1-b][1,3]thiazin-6-yl)oxy]nicotinonitrile (3g). M.p.: 235–236 °C. 1H NMR: δ = 8.69 (s, 1H, Ar), 8.16–8.19 (m, 1H, Ar), 7.45–7.49 (m, 5H, Ar), 7.33–7.35 (m, 4H, Ar), 7.17–7.20 (m, 1H, Ar), 7.06–7.13 (m, 1H, Ar), 5.79–5.85 (m, 1H, CH), 4.14–4.17 (m, 1H, NCH2), 3.90–3.94 (m, 1H, NCH2), 3.63–3.66 (m, 1H, SCH2), 3.52–3.57 (m, 1H, SCH2). 13C NMR: δ = 164.22 (Py), 152.50 (Py), 143.19 (Py), 136.99 (C8a), 136.88 (C3), 134.65, 131.05, 130.22 (Ar), 129.90 (C2), 129.65, 129.32, 128.61, 126.77, 126.47 (Ar), 117.64 (CN), 112.76, 103.19 (Py), 66.15 (C6), 47.41 (C5), 28.39 (C7). LC-MS: m/z = 411 [M + 1] (100%). Anal. Calcd. for C24H18N4OS, %: C, 70.22; H, 4.42; N, 13.65. Found, %: C, 70.32; H, 4.44; N, 13.58.
6-[(3,5-Dichloropyridin-2-yl)oxy]-2,3-diphenyl-6,7-dihydro-5H-imidazo[2,1-b][1,3]thiazine (3h). M.p.: 165–166 °C. 1H NMR: δ = 8.20 (s, 2H, Ar), 7.46–7.49 (m, 3H, Ar), 7.30–7.35 (m, 5H, Ar), 7.16–7.20 (m, 2H, Ar), 7.11–7.13 (m, 1H, Ar), 5.72–5.76 (m, 1H, CH), 4.09–4.12 (m, 1H, NCH2), 3.93–3.98 (m, 1H, NCH2), 3.61–3.64 (m, 1H, SCH2), 3.50–3.55 (m, 1H, SCH2). 13C NMR: δ = 155.86, 143.23, 138.86 (Py), 136.65 (C8a), 136.39 (C3), 134.23, 130.57, 129.84 (Ar), 129.45 (C2), 129.16, 128.83, 128.10, 126.24, 125.92 (Ar), 124.00, 118.17 (Py), 66.98 (C6), 46.63 (C5), 28.17 (C7). LC-MS: m/z = 455 [M + 1] (100%). Anal. Calcd. for C23H17Cl2N3OS, %: C, 60.80; H, 3.77; N, 9.25. Found, %: C, 60.94; H, 3.73; N, 9.16.
6-{[3-Chloro-5-(trifluoromethyl)pyridin-2-yl]oxy}-2,3-diphenyl-6,7-dihydro-5H-imidazo[2,1-b][1,3]thiazine (3i). M.p.: 159–160 °C. 1H NMR: δ = 8.49 (s, 1H, Ar), 8.36 (s, 1H, Ar), 7.42–7.44 (m, 3H, Ar), 7.29–7.34 (m, 4H, Ar), 7.08–7.15 (m, 3H, Ar), 5.83–5.87 (m, 1H, CH), 4.12–4.14 (m, 1H, NCH2), 3.98–4.00 (m, 1H, NCH2), 3.64–3.66 (m, 1H, SCH2), 3.54–3.56 (m, 1H, SCH2). 13C NMR: δ = 159.47 (Py), 142.79 (q, 3JCF = 3.75 Hz, Py), 136.65 (C8a + C3), 136.45 (q, 4JCF = 2.5 Hz, Py), 134.20, 130.55, 129.81 (Ar), 129.47 (C2), 129.14, 128.83, 128.09, 126.24, 125.94 (Ar), 123.03 (d, 1JCF = 270.0 Hz, CF3), 120.47 (q, 2JCF = 33.75 Hz, Py), 118.28 (Py), 67.50 (C6), 46.61 (C5), 28.15 (C7). LC-MS: m/z = 488 [M + 1] (100%). Anal. Calcd. for C24H17ClF3N3OS, %: C, 59.08; H, 3.51; N, 8.61. Found, %: C, 59.25; H, 3.47; N, 8.49.
3-{[5-(Trifluoromethyl)pyridin-2-yl]oxy}-3,4-dihydro-2H-benzo[4,5]imidazo[2,1-b][1,3]thiazine (3j). M.p.: 140–141 °C. 1H NMR: δ = 8.66 (s, 1H, Ar), 8.08 (d, 3J = 9.2 Hz, 1H, Ar), 7.48 (d, 3J = 7.6 Hz, 1H, Ar), 7.43–7.45 (m, 1H, Ar), 7.13–7.19 (m, 2H, Ar), 7.05 (d, 3J = 8.4 Hz, 1H, Ar), 6.87 (s, 1H, Ar), 6.00–6.04 (m, 1H, CH), 4.57–4.61 (m, 1H, NCH2), 4.48–4.52 (m, 1H, NCH2), 3.75–3.78 (m, 1H, SCH2), 3.66 (dd, 2J = 13.4 Hz, 3J = 5.4 Hz, 1H, SCH2). 13C NMR: δ = 164.50 (Py), 146.24 (C10a), 145.33 (q, 3JCF = 4.5 Hz, Py), 143.05 (C9a), 137.47 (q, 4JCF = 3.0 Hz, Py), 136.20 (C5a), 124.42 (d, 1JCF = 270.0 Hz, CF3), 122.42 (C8), 121.47 (C7), 120.02 (q, 2JCF = 33.0 Hz, Py), 117.61 (Py), 112.47 (C9), 109.25 (C6), 65.06 (C3), 46.59 (C4), 28.48 (C2). LC-MS: m/z = 352 [M + 1] (100%). Anal. Calcd. for C16H12F3N3OS, %: C, 54.70; H, 3.44; N, 11.96. Found, %: C, 54.88; H, 3.47; N, 11.84.
6-[(3,4-Dihydro-2H-benzo[4,5]imidazo[2,1-b][1,3]thiazin-3-yl)oxy]nicotinonitrile (3h). M.p.: 161–162 °C. 1H NMR: δ = 8.74 (s, 1H, Ar), 7.46 (s, 1H, Ar), 7.40 (s, 1H, Ar), 7.00–7.13 (m, 4H, Ar), 5.97–6.00 (m, 1H, CH), 4.55–4.57 (m, 1H, NCH2), 4.46–4.48 (m, 1H, NCH2), 3.73–3.75 (m, 1H, SCH2), 3.61–3.63 (m, 1H, SCH2). 13C NMR: δ = 164.14 (Py), 152.49 (Py), 146.19 (C10a), 143.14 (Py), 143.01 (C9a), 136.16 (C5a), 122.45 (C8), 121.51 (C7), 117.62 (Py), 117.60 (Py), 112.66 (C9), 109.24 (C6), 103.19 (CN), 65.26 (C3), 46.56 (C4), 28.48 (C2). LC-MS: m/z = 309 [M + 1] (100%). Anal. Calcd. for C16H12N4OS, %: C, 62.32; H, 3.92; N, 18.17. Found, %: C, 62.45; H, 3.89; N, 18.29.
3-[(3,5-dichloropyridin-2-yl)oxy]-3,4-dihydro-2H-benzo[4,5]imidazo[2,1-b][1,3]thiazine (3l). M.p.: 203–204 °C. 1H NMR: δ = 8.25 (s, 1H, Ar), 8.14 (s, 1H, Ar), 7.41–7.46 (m, 2H, Ar), 7.11–7.16 (m, 2H, Ar), 5.90–5.94 (m, 1H, CH), 4.48–4.50 (m, 1H, NCH2), 4.54–4.56 (m, 1H, NCH2), 3.70–3.73 (m, 1H, SCH2), 3.58–3.62 (m, 1H, SCH2). 13C NMR: δ = 155.81 (Py), 145.83 (C10a), 143.32 (Py), 142.64 (C9a), 138.89 (Py), 135.78 (C5a), 124.04 (Py), 121.99 (C8), 121.05 (C7), 118.19 (Py), 117.20 (C9), 108.87 (C6), 65.63 (C3), 46.07 (C4), 28.06 (C2). LC-MS: m/z = 352 [M + 1] (100%). Anal. Calcd. for C15H11Cl2N3OS, %: C, 51.15; H, 3.15; N, 11.93. Found, %: C, 51.36; H, 3.11; N, 11.82.
3-{[3-Chloro-5-(trifluoromethyl)pyridin-2-yl]oxy}-3,4-dihydro-2H-benzo[4,5]imidazo[2,1-b][1,3]thiazine (3m). M.p.: 165–166 °C. 1H NMR: δ = 8.61 (s, 1H, Ar), 8.39 (s, 1H, Ar), 7.42–7.47 (m, 2H, Ar), 7.11–7.16 (m, 2H, Ar), 6.04–6.07 (m, 1H, CH), 4.58–4.61 (m, 1H, NCH2), 4.51–4.54 (m, 1H, NCH2), 3.74–3.77 (m, 1H, SCH2), 3.63–3.67 (m, 1H, SCH2). 13C NMR: δ = 159.87 (Py), 146.19 (C10a), 143.34 (q, 3JCF = 3.75 Hz, Py), 143.05 (C9a), 136.97 (q, 4JCF = 2.5 Hz, Py), 136.19 (C5a), 123.52 (d, 1JCF = 270.0 Hz, CF3), 122.42 (C8), 121.47 (C7), 120.92 (q, 2JCF = 33.75 Hz, Py), 118.68 (Py), 117.63 (C9), 109.31 (C6), 66.54 (C3), 46.50 (C4), 28.27 (C2). LC-MS: m/z = 386 [M + 1] (100%). Anal. Calcd. for C16H11ClF3N3OS, %: C, 49.81; H, 2.87; N, 10.89. Found, %: C, 50.01; H, 2.89; N, 10.97.
2.3. Anti-Inflammatory (Anti-Exudative) Activity
The male albino rats, weighing 180–220 g, were used for the anti-exudative activity studies. The animals were treated humanely throughout the study period, adhering to the guideline for the use and care of animals in the declaration of Helsinki (National Research Council, 2011). The experiment design and study protocol were approved by the Animal Ethics Committee of the Danylo Halytsky Lviv National Medical University, Lviv, Ukraine, protocol No.10, 17 March 2021. The carrageenin-induced hind paw edema was produced by the method of Winter et al. [14]. The synthesized compounds were intraperitoneally injected in a dose of 50 mg/kg (in saline solution with one drop of Tween-80™). Diclofenac (tablets “Diclofenac sodium”, “Zdorovja narodu”, Ukraine) in a dose of 8 mg/kg was used as reference drug. The antiexudative activity (inflammation inhibition) was expressed as a decrease in the rats’ paw edema and was calculated using the equation and was given in a percentage:
where, ΔVcontrol and ΔVexperiment—the mean values of the volume difference for control and experimental animal hinds, respectively.
Inhibition = (∆Vcontrol − ∆Vexperiment)/∆Vcontrol × 100%
3. Results and Discussion
Used in the present work, the synthetic approach is based on the utilization of structure-modified imidazolinthiones as starting the building blocks for the formation of the imidazo[2,1-b][1,3]thiazine core. The interaction of the last ones in the soft conditions with epichlorohydrin led to the key 3-hydroxy-imidazo[2,1-b][1,3]thiazines 2a–c [5]. The various polysubstituted 2-chloropyridines were studied in the alkylation reaction with early synthesized compounds 2a–c (Scheme 1). As a result, the target (2-pyridinyloxy) substituted imidazo[2,1-b][1,3]thiazines 3a–m were obtained with satisfied yields (in the presence of equimolar amounts of 60% sodium hydride in an anhydrous DMF medium) at room temperature, and the selective nucleophilic substitution in position 2 of the pyridine ring was observed.
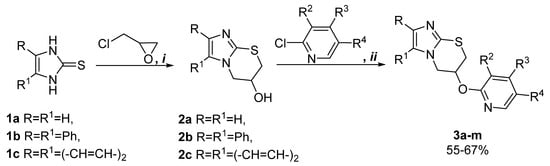
Scheme 1.
Synthesis of compounds 3a–m. Reagents and conditions: (i) 1a–c (10 mmol), 2-(chloromethyl)oxirane (10 mmol), NaOH (10 mmol), MeOH (25 mL), stirring, r.t. 24 h; (ii) 2a–c (10 mmol), 60% NaH in mineral oil (10 mmol), appropriate derivate of 2-chloropiridine (10 mmol), DMF (4 mL), stirring, r.t. 24 h.
The control of reaction process and products formation was monitored by TLC. The compounds’ structure characterization and yield are presented in the Table 1.

Table 1.
Structure characterization and yeilds of synthesized compounds 3a–m.
The structure of compounds was studied and confirmed using 1H, 13C NMR spectroscopy and LC-MS spectrometry.
3.1. In Silico Evaluation of Drug-Likeness Properties
The drug-likeness properties of the derivatives 3a–m were determined based on Lipinski and Veber rules and evaluated in silico using the SwissAdme of the Swiss Institute of Bioinformatics website [15] (Table 2).

Table 2.
Drug-likeness parameters of derivatives 3a-m according to Lipinski and Veber rules.
All tested compounds comply with Lipinski’s rules of five and Veber’s rules, except derivatives 3h and 3i, for which the calculated MlogP values were higher (4.69 and 4.28, accordingly) than limited for the Mlog P parameter (accepted ≤ 4.15), in line with the Lipinski’s rules.
3.2. Study of Anti-Inflammatory (Anti-Exudative) Activity of Synthesized Compounds 3a–m
The anti-inflammatory (anti-exudative) activity of all synthesized compounds 3a–m was investigated on the in vivo carrageenin model of the total edema of the hind paws of albino rats [14]. The study results are presented in Table 3.

Table 3.
In vivo anti-inflammatory activity of compounds 3a–m on carrageenin-induced paw edema in white rats (intraperitoneally use; doses: carrageenin 1%, 0.1 mL; Diclofenac sodium—8 mg/kg, tested compounds—50 mg/kg; M ± m; n = 6 in each group).
The synthesized compounds 3a–m possess different levels of anti-inflammatory activity (inhibition index was in the range of 3.7 to 39.1%). From the point of view of the “structure—anti-inflammatory activity” derivatives 3a–d with an unsubstituted imidazole ring in the imidazo[2,1-b][1,3]thiazine core, they are characterized with a total higher activity level. The compound 3c containing cyano-group in the pyridine ring was the most active among derivatives 3a–d, whereas the change of cyano-group on the chlorine or threefluormethyl-group led to an insignificant activity decrease. Derivative 3l was found to be the most active inside the tested group, with an inflammation inhibition value of 39.1%, which is only 15.5% less compared to the same data for the reference drug, diclofenac.
4. Conclusions
In the present work, a synthetic approach to (2-pyridinyloxy) substituted imidazo[2,1-b][1,3]thiazines is described. The polysubstituted 2-chloropyridines were studied in the alkylation reaction with some 3-hydroxy-imidazo[2,1-b][1,3]thiazines, and the selective nucleophilic substitution in position 2 of pyridine ring was observed. The synthesized (2-pyridinyloxy) substituted imidazo[2,1-b][1,3]thiazines comply with Lipinski’s rules of five and Veber’s rules and possess promising anti-inflammatory properties in the carrageenan test in vivo. Such drug-like and pharmacological features of synthesized derivatives argue for forthcoming studies as potential non-steroidal anti-inflammatory agents.
Author Contributions
Conceptualization, N.S. and M.V.; methodology and experimental work, N.S., S.H., L.S. and M.V.; data analysis, N.S., S.H., L.S. and M.V.; writing—review and editing, N.S., S.H., L.S. and M.V.; project administration and supervision, N.S. and M.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethic Committee of the Danylo Halytsky Lviv National Medical University, Lviv, Ukraine (protocol No. 10 from 17 March 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
This research was supported by the Lesya Ukrainka Volyn National University, Danylo Halytsky Lviv National Medical University and Institute of Organic Chemistry of National Academy of Sciences of Ukraine, which is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Volkov, O.A.; Cosner, C.C.; Brockway, A.J.; Kramer, M.; Booker, M.; Zhong, S.; Ketcherside, A.; Wei, S.; Longgood, J.; McCoy, M.; et al. Identification of Trypanosoma brucei AdoMetDC Inhibitors Using a High-Throughput Mass Spectrometry-Based Assay. ACS Infect. Dis. 2017, 3, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.M.; Marshall, A.J.; Maes, L.; Yarlett, N.; Bacchi, C.J.; Gaukel, E.; Wringd, S.A.; Launaye, D.; Braillarde, S.; Chatelaine, E.; et al. Assessment of a pretomanid analogue library for African trypanosomiasis: Hit-to-lead studies on 6-substituted 2-nitro-6,7-dihydro-5H-imidazo[2,1-b][1,3]thiazine 8-oxides. Bioorg. Med. Chem. Lett. 2018, 28, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Tawari, N.R.; Degani, M.S. Pharmacophore modeling and density functional theory analysis for a series of nitroimidazole compounds with antitubercular activity. Chem. Biol. Drug. Des. 2011, 78, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.M.; Robert, A.B.; Anderson, F.; Shinde, S.S.; Franzblau, S.G.; Ma, Z.; Denny, W.A.; Palmer, B.D. Synthesis, Reduction Potentials, and Antitubercular Activity of Ring A/B Analogues of the Bioreductive Drug (6S)-2-Nitro-6-{[4-(trifluoromethoxy)benzyl]oxy}-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazine (PA-824) J. Med. Chem. 2009, 52, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.-X.; He, Y.; Cui, Z.-L.; Guo, Y.-W. Synthesis, spectral characterization, and antituberculosis activity of thiazino[3,2-A]benzimidazole derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 1036–1041. [Google Scholar] [CrossRef]
- Rodríguez, O.A.R.; Vergaraa, N.E.M.; Sánchez, J.P.M.; Martínez, M.T.S.; Sandoval, Z.G.; Cruz, A.; Organillo, A.R. Synthesis, crystal structure, antioxidant activity and dft study of 2-aryl-2,3-dihydro-4H-[1,3]thiazino[3,2-a]benzimidazol-4-One. J. Mol. Struct. 2020, 1199, 127036. [Google Scholar] [CrossRef]
- Mickleburgh, I.; Geng, F.; Tiley, L. Mesoionic heterocyclic compounds as candidate messenger RNA cap analogue inhibitors of the influenza virus RNA polymerase cap-binding activity. Antivir. Chem. Chemother. 2009, 19, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, I.; Slavchev, I.; Ravutsov, M.; Dangalov, M.; Nikolova, Y.; Zagranyarska, I.; Stoyanova, A.; Nikolova, N.; Mukova, L.; Grozdanov, P.; et al. Anti-enteroviral activity of new MDL-860 analogues: Synthesis, in vitro/in vivo studies and QSAR analysis. Bioorg Chem. 2019, 85, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Hamama, W.S.; Waly, M.A.; El-Hawary, I.I.; Zoorob, H.H. Utilization of 2-Chloronicotinonitrile in the Syntheses of Novel Fused Bicyclic and Polynuclear Heterocycles of Anticipated Antitumor Activity. J. Heterocycl. Chem. 2016, 53, 953–957. [Google Scholar] [CrossRef]
- LaFleur, M.D.; Lucumi, E.; Napper, A.D.; Diamond, S.L.; Lewis, K. Novel high-throughput screen against Candida albicans identifies antifungal potentiators and agents effective against biofilms. J. Antimicrob. Chemother. 2011, 66, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Hori, H.; Kim, Y. Inflammation and post-traumatic stress disorder. Psychiatry. Clin. Neurosci. 2019, 73, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-Inflammatory Activity of Natural Products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef] [PubMed]
- Mishchenko, M.; Shtrygol’, S.; Lozynskyi, A.; Khomyak, S.; Novikov, V.; Karpenko, O.; Holota, S.; Lesyk, R. Evaluation of Anticonvulsant Activity of Dual COX-2/5-LOX Inhibitor Darbufelon and Its Novel Analogues. Sci. Pharm. 2021, 89, 22. [Google Scholar] [CrossRef]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef] [PubMed]
- SwissADME. Available online: http://www.swissadme.ch/ (accessed on 15 September 2021).
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Moriguchi, I.; Hirono, S.; Nakagome, I.; Hirano, H. Comparison of Reliability of log P Values for Drugs Calculated by Several Methods. Chem. Pharm. Bull. 1994, 42, 976–978. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).