Abstract
In acidic environments, acid phosphatases (EC 3.1.3.2) play a crucial role in hydrolyzing phosphate ester linkages. Two isoforms of acid phosphatases, namely AP-I and AP-II, were purified to homogeneity from the seeds of Erythrina indica using a combination of gel filtration and affinity chromatography techniques. The purification process involved multiple steps to ensure the enzymes were free from other seed components, thereby facilitating detailed characterization. We report in this study the active site characterization of acid phosphatase form AP-I. The active site of purified AP-I was characterized in detail through chemical modification studies, which revealed the presence of one residue each of carboxylate, tryptophan, and serine amino acid. Substrate protection experiments using p-nitrophenyl phosphate effectively prevented the modification of all three residues, suggesting their essential role in the enzyme’s active site. These experiments provided strong evidence that these residues are directly involved in the catalytic process. Kinetic studies of the partially inactivated enzyme, achieved through the use of the specific modifying agents dicyclohexylcarbodiimide (DCCD) for carboxylate, N-bromosuccinimide (NBS) for tryptophan, and phenylmethylsulfonyl fluoride (PMSF) for serine further confirmed the involvement of these residues in the catalytic mechanism. The results demonstrated that the inactivation of any of these residues significantly impaired the enzyme’s activity, highlighting their critical roles in the catalytic process. The results provide a comprehensive understanding of the active site architecture and the catalytic mechanism of AP-I function.
1. Introduction
Erythrina indica, commonly known in English as the Indian coral tree, is also referred to as the tropical coral tree, tiger’s claw, moochy wood tree, or variegated coral tree. In Sanskrit, it is called Paribadra, and in Hindi, it is known by names such as Ferrud, Dadap, and Pharad [1]. Erythrina indica is a prickly shrub with medicinal value found in Indian forests. It is used in Ayurveda, Siddha, Unani, and Homeopathy. Its bark, roots, leaves, and fruits treat fever, skin diseases, and act as astringents [2]. The seeds contain non-volatile oil, fatty acids, and lectins. Although the plant is traditionally used in various geographical regions, there is a lack of scientific evidence to support its folkloric applications [3]. Phytochemical studies have resulted in the isolation, purification, and identification of lectins from the seeds and leaves of Erythrina indica [4]. Additionally, enzyme analysis of seed extracts has revealed the presence of four glycosidases—α-galactosidase, β-galactosidase, α-mannosidase, and N-acetyl-β-D-glucosaminidase—and acid phosphatase [5]. Acid phosphatases (EC 3.1.3.2) are key enzymes involved in phosphate metabolism, responsible for catalyzing transphosphorylation and breaking down a wide range of orthophosphate esters in acidic environments [6]. The active site of an enzyme is the region responsible for substrate binding and the catalytic process. It usually consists of a small number of crucial amino acid residues that interact specifically with the substrate, enabling the chemical reaction [7].
The aim of this study is to examine the active site of AP-I from Erythrina indica seeds in order to offer a better understanding of its substrate specificity, structural function, catalytic performance, and potential regulatory pathways.
2. Materials
Phenylmethylsulphonyl fluoride, phenyl glyoxal, N-acetyl imidazole, p-nitrophenyl phosphate, N-bromosuccinimide, diethyl pyrocarbonate, 5,5′-dithiobis (2-nitrobenzoic acid), and dicyclohexyl carbodiimide were sourced from Sigma (Saint Louis, MD, USA). All other chemicals and reagents were obtained from commercial suppliers and were of the highest available purity.
3. Methods
3.1. Acid Phosphatase Assay
Enzyme activity was measured using p-nitrophenyl phosphate as the substrate at 37 °C for 30 min, following the procedure of Campbell et al. [8]. The released p-nitrophenol was quantified at 405 nm. One unit of activity corresponds to the release of 1 µmol of p-nitrophenol per minute.
3.2. Protein Estimation
Protein concentration was measured by Lowry’s method [9] using BSA as the standard. Absorbance at 280 nm was also recorded, applying an extinction coefficient of A1%₍280₎ = 6.45 to calculate protein levels.
3.3. Fluorescence Measurements
Fluorescence spectroscopy was performed at 25 °C using a Hitachi F-4500 Fluorescence Spectrophotometer manufactured by Hitachi High-Tech, made in Tokyo, Japan, with 5 nm slit widths. Native and modified AP-I enzymes (5 µM in 0.05 M citrate buffer, pH 4.6) were analyzed with and without 50 µM p-nitrophenol. Excitation was set at 295 nm to target tryptophan residues, and emission was recorded from 300 to 400 nm. Tryptophan environment was probed through quenching studies using acrylamide, cesium chloride, and potassium iodide [10].
3.4. Chemical Modification Studies
Specific amino acid-modifying reagents were used to identify active site residues in the purified isoenzyme [10].
3.5. Modification of Tryptophan Residues
AP-I (0.5 µM) in 0.05 M citrate buffer (pH 4.6) was titrated with 1–5 µM N-bromosuccinimide, added in five 5 µL increments, followed by recording fluorescence spectra and absorbance at 280 nm, after each addition. Enzyme activity was monitored over 0–12 min, and reactions were quenched with 0.1 M borate buffer. The extent of tryptophan modification was calculated using an extinction coefficient of 5500 M−1 cm−1. Controls lacked NBS [10].
3.6. Detection of Carboxylate Residues
To assess the role of carboxylate groups in catalysis, Km and Vmax values for purified AP-I were measured across pH 3.0 to 6.0. pKa1 and pKa2 values were determined from a plot of log(Vmax/Km) versus pH [11].
3.7. Modification of Carboxylate and Serine Residues
AP-I (0.5 µM) was treated with 1–5 µM DCHC and PMSF in methanol to modify carboxylate and serine residues. Enzyme activity was monitored over 0–12 min, and the reaction was arrested with 0.1 M borate buffer. Inactivation was analyzed based on time and reagent concentration. The control lacked DCHC and PMSF [11].
3.8. Determination of Tryptophan Environment
AP-I (5 µM) in 0.05 M citrate buffer (pH 4.6) was titrated with acrylamide, potassium iodide, and cesium chloride. Fluorescence at λmax was recorded after each addition (5 µL for acrylamide and KI; 2 µL for CsCl) until quenching plateaued. Stern–Volmer and modified Stern–Volmer plots were used to calculate Ksv and the fraction of accessible tryptophan residues [10].
3.9. Estimation of Surface and Buried Tryptophan Residues
AP-I (5 µM) in 0.05 M citrate buffer (pH 4.6) was titrated with NBS (1.73–5.51 µM) in five 5 µL increments. Absorbance at 280 nm was measured after each addition, and 100 µL samples were withdrawn to assess enzyme activity. Surface-exposed tryptophanwere estimated using an extinction coefficient of 5500 M−1 cm−1. The same procedure was applied to enzymes denatured with 7 M guanidine hydrochloride to determine total tryptophan content, from which the accessible fraction was calculated [10].
3.10. Thermal and Chemical Denaturation Studies
Structural changes in AP-I induced by guanidine hydrochloride and elevated temperatures were monitored through enzyme activity and fluorescence spectroscopy [12].
4. Results and Discussion
Table 1 shows that NBS, PMSF, and DCHC treatments caused near-total loss of AP-I activity, indicating that tryptophan, serine, and carboxylate residues are critical for its active site.

Table 1.
Effect of modifying agents on acid phosphatase activity.
4.1. pH Dependence of AP-I Activity
DCHC reduced AP-I activity by 90%, highlighting the role of a carboxylate residue with peak activity at pH 4.8. Lineweaver–Burk analysis across pH 3–6 using pNPP yielded pKa values of 4.2 and 5.25, indicating involvement of carboxyl groups in catalysis (Figure 1).
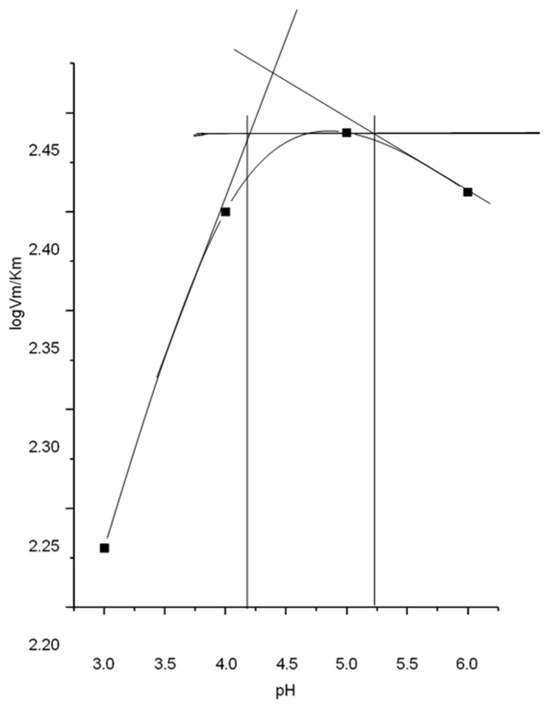
Figure 1.
pH dependence of the kinetic parameter.
4.2. Substrate Protection Studies
The loss of enzyme activity caused by the modification of tryptophan (NBS), serine (PMSF), and carboxylate (DCHC) residues was significantly reduced when 2 mM substrate was added before the modification. Substrate protection results are summarized in Table 2.

Table 2.
Substrate protection studies.
4.3. Kinetics of Partially Modified Enzyme
Partial inactivation with NBS, PMSF, and DCHC reduced Vmax without affecting Km, indicating that tryptophan, serine, and carboxylate residues are involved in catalysis, not substrate binding as shown in Table 3. Figure 2 shows the number of tryptophan residue essential for activity.

Table 3.
Kinetics of partially modified enzymes.
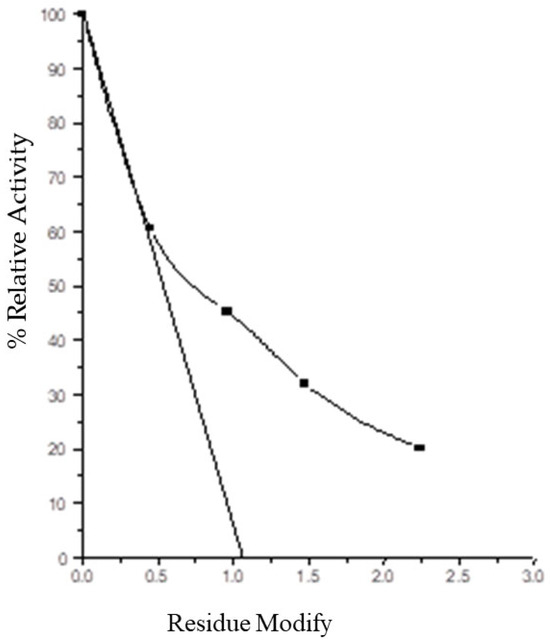
Figure 2.
Plot of percent residual activity versus number of tryptophan residues modified in AP-I.
4.4. Modification of Carboxylate Residue
Modification of AP-I with DCHC indicates the involvement of carboxylate residues in catalysis. Inactivation was both time- and concentration-dependent, with a log K vs. log [DCHC] plot showing a slope of one, suggesting that the binding of one mole of DCHC per enzyme leads to inactivation (Figure 3). Further evidence comes from pH-dependent kinetic studies: Lineweaver–Burk plots show constant Km values for pNPP across pH 3.0–6.0, while Vmax varies, indicating that substrate binding remains unaffected by pH, but catalytic efficiency changes.
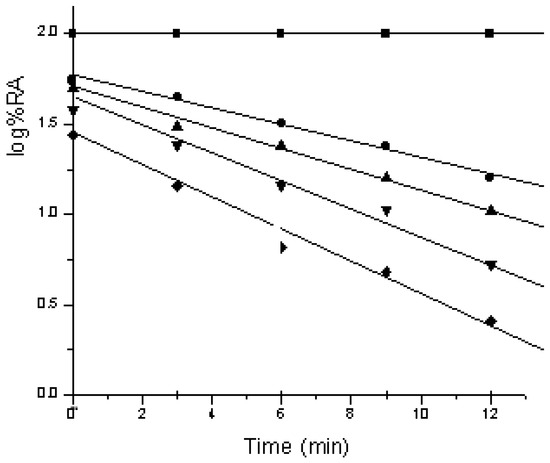
Figure 3.
Kinetics of inactivation of AP-I by DCHC.
4.5. Fluorescence Measurements
Fluorescence studies showed that Trp residues, buried in the hydrophobic core, contribute to AP-I emission (λmax 310–313 nm). NBS quenched Trp fluorescence without shifting the peak, while pNPP partially protected against quenching, indicating tryptophan’s involvement in the active site (Figure 4).
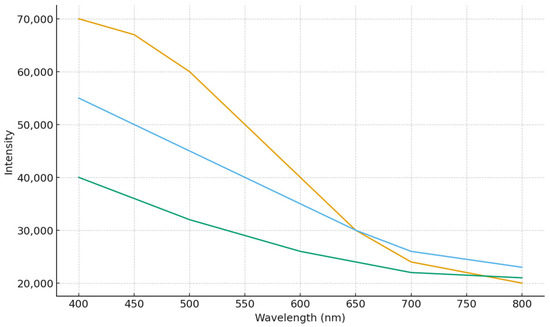
Figure 4.
Fluorescence spectra of native enzyme AP-I in the presence of NBS and in the presence of substrate and NBS.
4.6. Determination of Tryptophan Environment by Quenching Experiments
Fluorescence characteristics of tryptophan residues depend upon the microenvironment surrounding the tryptophan residues. Fluorescence quenching was observed only with acrylamide and cesium chloride, as shown below (Figure 5, Figure 6 and Figure 7), indicating a strongly negatively charged environment, which is supported by the modified Stern–Volmer plot (Figure 8) and Table 4.
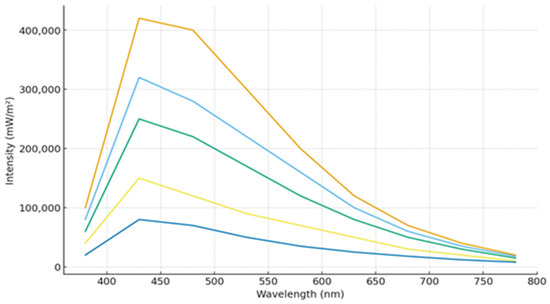
Figure 5.
Spectra of fluorescence quenching by acrylamide of AP-I.
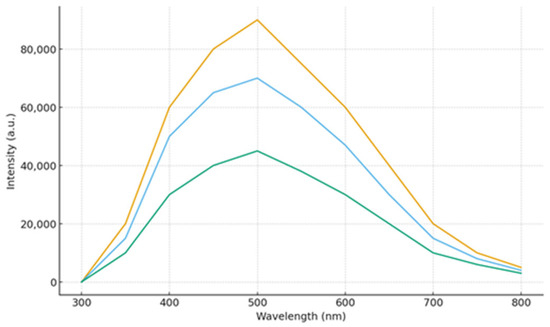
Figure 6.
Spectra of fluorescence quenching by cesium chloride.
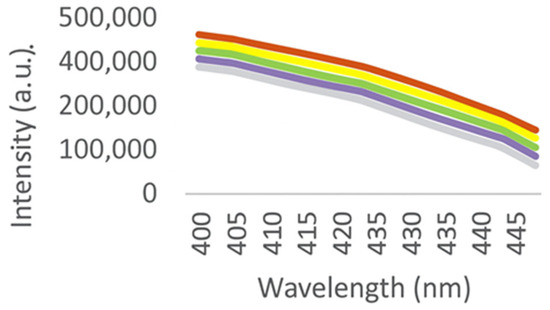
Figure 7.
Spectra of fluorescence quenching by potassium iodide.
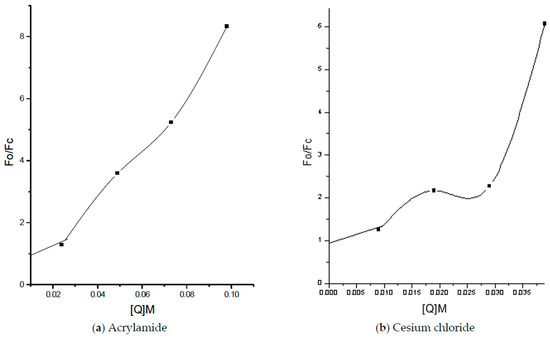
Figure 8.
Stern–Volmer plots of fluorescence quenching.

Table 4.
Values of Stern–Volmer and modified Stern–Volmer constants for AP-I.
The upward curvature in the Stern–Volmer plots (Figure 9) suggests both dynamic and static quenching, with CsCl showing two tryptophan populations—one initially quenched, followed by rapid quenching of the other. While full accessibility to acrylamide is common, the unusually high accessibility to Cs+ ions further supports the presence of a negatively charged microenvironment.
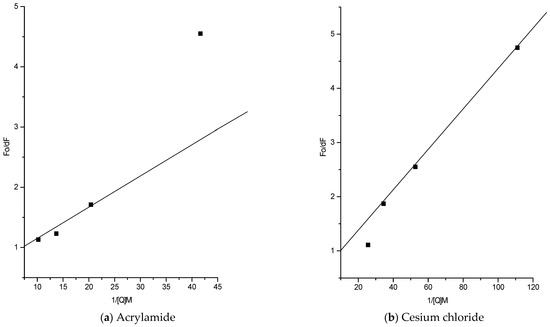
Figure 9.
Modified Stern–Volmer plots of fluorescence quenching studies of AP-I.
4.7. Thermal and Chemical Denaturation Studies
Acid phosphatase underwent denaturation through heat stress and guanidine HCl–induced unfolding. Thermal denaturation results correlated with activity loss observed during temperature studies, where increasing temperature led to decreased fluorescence intensity (310–313 nm), with a red shift in emission maximum to 333 nm. Chemical denaturation with guanidine HCl caused a sharp drop in activity even at 0.2 M, highlighting the importance of carboxylate residues in catalysis. Titration of the denatured enzyme with NBS, monitored by absorbance decrease at 280 nm, was used to estimate total tryptophan content using an extinction coefficient of 5500 M−1 cm−1. For AP-I, four tryptophan residues were modified two surface-exposed and two buried—indicating that denaturation exposes previously inaccessible residues.
5. Discussion
Distinct isoforms of Erythrina indica acid phosphatase persist after purification, suggesting genetic or post-translational origins [13]. Chemical modification identified a conserved catalytic triad—carboxylate, tryptophan, and serine essential for catalysis, consistent with findings in other plant phosphatases [14]. PMSF inhibition and substrate protection confirmed their direct catalytic role [15]. Fluorescence quenching studies indicated a strongly negatively charged environment around tryptophan residues. These features suggest that form AP-I contributes to phosphate mobilization, with isoform diversity enhancing adaptability during seed germination and stress [6,16].
6. Conclusions
This study on Erythrina indica acid phosphatase AP-I revealed a conserved catalytic mechanism involving essential carboxylate, tryptophan, and serine residues. Selective modification and kinetic analysis confirmed their role in catalysis without affecting substrate binding. These findings enhance understanding of plant acid phosphatases and support future structural and applied research.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
I place on record the technical support provided by Fakeha Mohammed Rehan Shaikh in giving final shape to this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kumar, B.S.; Prasad, D.N.; Sandhya, S.; Rao, K.N.V.; Banji, D. Cultivation, phytochemistry, pharmacological actions and therapeutic applications of Erythrina indica Lam. Int. J. Appl. Biol. Pharm. Technol. 2010, 1, 858–863. [Google Scholar]
- Avhad, S.; Tiwari, K.; Ushir, Y.V. Ethnobotanical studies of Erythrina indica plants. Int. J. Pharmacogn. Phytochem. Outlook (IJPO) 2019, 1, 67–70. [Google Scholar]
- Haque, R.; Ali, M.S.; Saha, A.; Alimuzzaman, M. Analgesic activity of methanolic extract of the leaf of Erythrina variegata. Dhaka Univ. J. Pharm. Sci. 2006, 5, 77–79. [Google Scholar] [CrossRef]
- Konozy, E.H.E.; Mulay, R.; Faca, V.; Ward, R.J.; Greene, L.J.; Roque-Barriera, M.C.; Sabharwal, S.; Bhide, S.V. Purification, Some Properties of a D-Galactose-Binding Leaf Lectin from Erythrina indica and Further Characterization of Seed Lectin. Biochimie 2002, 84, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Kestwal, R.M.; Bhide, S.V. Purification of β-galactosidase from Erythrina indica: Involvement of tryptophan in the active site. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2007, 1770, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Kahandal, A.; Kanagare, A.; Kulkarni, A.; Tagad, C.K. The multifaceted nature of plant acid phosphatases: Purification, biochemical features, and applications. J. Enzym. Inhib. Med. Chem. 2023, 38, 2282379. [Google Scholar] [CrossRef] [PubMed]
- Fraser, L.; Wysocki, P.; Ciereszko, A.; Plucienniczak, G.; Kotlowska, M.; Kordan, W.; Wojtczak, M.; Dietrich, G.; Strezezek, J. Application of biochemical markers for identification of biological properties of animal semen. Reprod. Biol. 2006, 6, 5–20. [Google Scholar] [PubMed]
- Campbell, H.D.; Dionysius, D.A.; Keough, D.T.; Wilson, B.E.; de Jersey, J.; Zerner, B. Containing Acid-Phosphatases—Comparison of Enzymes from Beef Spleen and Pig Allantoic Fluid. Biochem. Biophys. Res. Commun. 1978, 82, 615. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Roseborough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagen. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Fossum, C.J.; Johnson, B.O.V.; Golde, S.T.; Kielman, A.J.; Finke, B.; Smith, M.A.; Lowater, H.R.; Laatsch, B.F.; Bhattacharyya, S.; Hati, S. Insights into the mechanism of tryptophan fluorescence quenching due to synthetic crowding agents: A combined experimental and computational study. ACS Omega 2023, 8, 44820–44830. [Google Scholar] [CrossRef] [PubMed]
- Granjeiro, P.; Ferreira, C.V.; Cavagis, A.; Granjeiro, J. Essential sufhydryl groups in the active site of castor bean (Ricinus communis) seed acid phosphatase. Plant Sci. 2003, 164, 629–633. [Google Scholar] [CrossRef]
- Jana, S.; Chaudhuri, T.K.; Deb, J.K. Effects of guanidine hydrochloride on the conformation and enzyme activity of streptomycin adenylyltransferase monitored by circular dichroism and fluorescence spectroscopy. Biochemistry 2006, 71, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, Y.; Kawabe, H.; Tanaka, H.; Fujimoto, S.; Ohara, A. Purification, enzymatic properties, and active site environment of a novel manganese(III)-containing acid phosphatase. J. Biol. Chem. 1981, 256, 10664–10670. [Google Scholar] [CrossRef] [PubMed]
- Antonyuk, S.V.; Olczak, M.; Olczak, T.; Ciuraszkiewicz, J.; Strange, R.W. The structure of a purple acid phosphatase involved in plant growth and pathogen defence exhibits a novel immunoglobulin-like fold. IUCrJ 2014, 1, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.W.; Hoffman, A.D.; Swintek, J.A.; Droge, S.T.J.; Fitzsimmons, P.N. Addition of phenylmethylsulfonyl fluoride increases the working lifetime of the trout liver S9 substrate depletion assay resulting in improved detection of low intrinsic clearance rates. Environ. Toxicol. Chem. 2020, 40, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Uzgare, A.S.; Bhide, A. Purification and partial characterization of two forms of acid phosphatase from seeds of Erythrina indica. Res. J. Chem. Environ. 2021, 25, 125–130. Available online: https://worldresearchersassociations.com/Archives/RJCE/Vol(25)2021/March%202021/Purification%20and%20partial%20characterization%20of%20two%20forms.pdf (accessed on 9 December 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).