Abstract
This work investigates the physicochemical characterization of two poly(styrene-co-divinylbenzene) copolymer supports, containing 6.7% and 15% divinylbenzene, functionalized with glycine. The resulting copolymers were characterized using Fourier transform infrared spectroscopy, thermogravimetric analysis, energy-dispersive X-ray spectroscopy, and scanning electron microscopy. The degree of amino acid functionalization was estimated by statistical modeling of the repeating structural units and through analysis of nitrogen content. Thermogravimetric analysis (TGA) was further employed to investigate the impact of grafted amino acid groups on the thermal stability and decomposition behavior of the copolymers.
1. Introduction
Styrene-divinylbenzene (S-DVB) resins are synthetic copolymers valued for their chemical stability, mechanical strength, and adjustable porosity [1]. They can be used in ion exchange, chromatography, and catalysis. These resins can be synthesized with varying degrees of crosslinking to tailor their physical properties. By adjusting the degree of crosslinking, their physical characteristics can be tailored for specific uses [2]. Amino acids contain functional groups such as amines (-NH2) and carboxylic acids (-COOH). Many of them are natural and non-toxic, making them ideal for applications in biomedical devices, drug delivery, and tissue engineering [3].
In the present article, two poly(styrene-co-divinylbenzene) supports, having functionalization degrees of 6.7% and 15%, have reacted with glycine to obtain amino acid groups grafted onto the support. The synthesized copolymers were characterized using various techniques, namely Fourier transform infrared (FTIR), thermogravimetric analysis, energy dispersive X-ray analysis (EDX), and scanning electron microscopy. Using statistical modeling of the repeating structural unit of the functionalized copolymers and the nitrogen content, the degree of functionalization with amino acid-type groups was determined. Thermogravimetric analysis was used to evaluate how specific functional groups, introduced via amino acid grafting, influence the thermal decomposition behavior of the copolymers.
2. Experimental Part
2.1. Materials and Methods
The chloromethylated copolymer styrene-15% divinylbenzene is a raw material received from the Institute of Macromolecular Chemistry “Petru Poni” Iasi, and chloromethylated copolymer styrene-6.7% divinylbenzene was supplied by Purolite (Victoria, Romania).
The thermal properties of the new polymeric products were characterized through thermogravimetric analysis on a TGA/SDTA 851-LF1100—Mettler machine (Greifensee, Switzerland) at a heating rate of 10 °C/min and a temperature range from 25 to 900 °C. The experiments were carried out in a nitrogen atmosphere at a flow rate of 50 mL.min−1. Energy-dispersive X-ray analysis (EDX) and study of the surface morphology of the functionalized copolymers were performed using a Quanta FEG 250 microscope (FEI Company, Eindhoven, The Netherlands) at an accelerating voltage of 20 kV.
2.2. Obtaining Amino Acid Groups Grafted onto Styrene-Divinylbenzene Copolymer
The syntheses for obtaining AP1 and AP2 (Scheme 1) were carried out according to the method that we have previously published [4]. In a flask equipped with a thermometer, stirrer and refrigerant for reflux, 6 g of the styrene-15%divinylbenzenecopolymer (%Cl = 11.93; GF = 3.36 mmoles/g copolymer) (Code: S15DVBCH2Cl)/styrene-6.7%divinylbenzene (%Cl = 14.22; GF = 4.01 mmoles/g copolymer) (Code: S6.7DVBCH2Cl) and glycine (NH2CH2COOH) were introduced at a molar ratio of 1:1 compared to the pendant group (–CH2Cl). Glycine was previously dissolved in a 100 mL/75 mL ethanol/distilled water solution. The synthesis took place for 30 h at a temperature of 70 °C. The final product was filtered, washed with hot distilled water and ethanol, and dried at 50 °C for 24 h. The samples were noted AP1 and AP2.
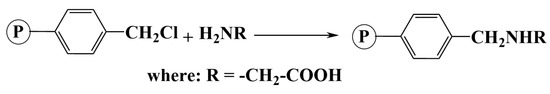
Scheme 1.
Obtaining AP1 and AP2 samples.
3. Results and Discussion
3.1. Characterization of Amino Acid Functionalized onto Styrene-Divinylbenzene Copolymers
The two samples, AP1 and AP2, were characterized by EDX and SEM analysis.
The EDX spectrum in Figure 1 and the data in Table 1 confirm the reaction between the styrene-divinylbenzene copolymers and the amino acid.
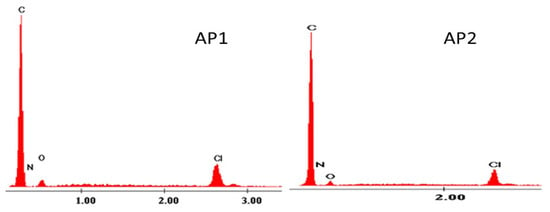
Figure 1.
EDX image for AP1 and AP2.

Table 1.
Semi-quantitative EDX-analysis of AP1 and AP2.
Figure 2 shows that the surface of the microspheres remained clean and largely unchanged after the reaction to obtain AP1 and AP2.
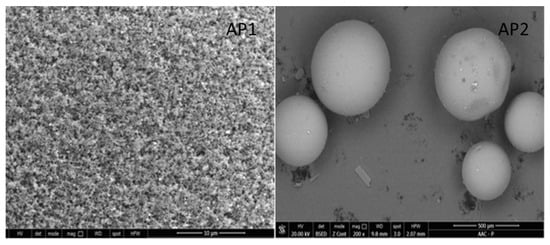
Figure 2.
SEM image for AP1 and AP2.
Figure 3 shows the FTIR spectra of the starting copolymer and compound AP1. The FTIR spectrum of AP1 shows a slight shift in the C=O stretching band observed at 1615 cm−1, suggesting a change following functionalization [5]. It is important to note that in this region (1700–1600 cm−1), the interpretation is slightly hampered due to the overlap with the vibrations of the water molecule. Water, even in residual amounts, exhibits a H–O–H bending vibration at around 1640 cm−1, which overlaps with the carbonyl band, potentially masking or distorting real differences between the precursor and API. In addition, the broad band observed at 3510 cm−1 is attributed to the O–H and N–H stretching vibrations but may also be partially influenced by adsorbed moisture. Also, the general similarity between the FTIR spectra of the precursor and AP1 can be explained by the fact that the basic structure of the copolymer is preserved, and the chemical modification (introduction of an amino acid group) does not significantly affect the other major functional groups. However, an intensification of the band in the region 1510–1365 cm−1, attributed to the C–N, C–H, and C=O vibrations of the amino acid moieties [6,7], indicates the presence of the new functionalization.
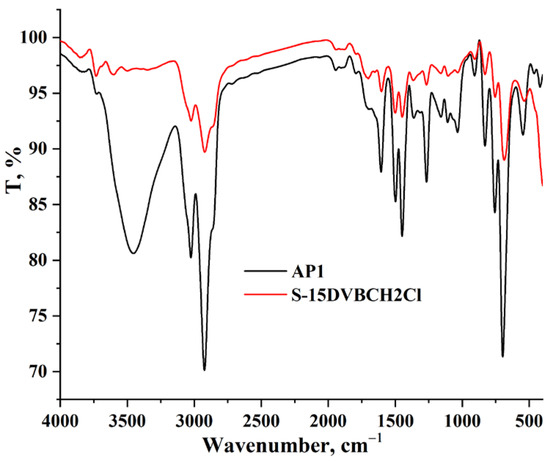
Figure 3.
FTIR spectra for AP1 and initial support.
Using statistical modeling of the repeating structural unit of the functionalized copolymers and the nitrogen content, the degree of functionalization with amino acid-type groups was determined. Scheme 2 shows the statistical structure of the repeating unit of the copolymers (AP1 and AP2) with functional groups of the amino acid type.
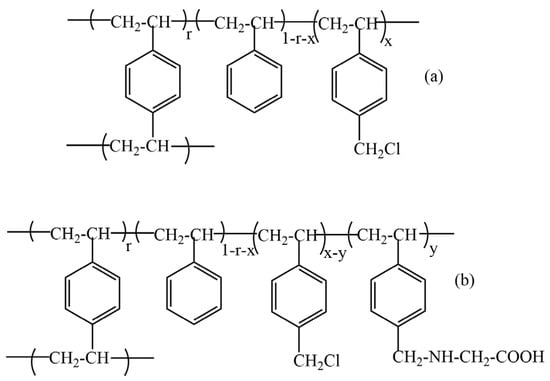
Scheme 2.
Statistical structure of the repetitive unit of the (S15DVBCH2Cl and S6.7DVBCH2Cl) initial copolymer (a) and (AP1; AP2) final functionalized copolymer (b).
The notations used in Scheme 2 and Table 2 are as follows: Ff, amino acid groups; Fi, CH2Cl groups; x, fraction of styrene units bearing pendant-CH2Cl initial groups; r, fraction of divinylbenzene (DVB) units; y, fraction of styrene units bearing pendant-amino acid groups (Ff); %N, nitrogen percentage in the final copolymer; AN, atomic weight of nitrogen; nN, number of nitrogen atoms in the pendant groups; Mmi, average molecular weight of the repetitive unit of the initial copolymer; Mmf, average molecular weight of the repetitive unit of the final copolymer; MS, molecular weight of the repetitive unit of styrene; MDVB, molecular weight of the repetitive unit of divinylbenzene; MSFi, molecular weight of the repetitive unit of the styrene functionalized with Fi (CH2Cl) groups; MSFf, molecular weight of the repetitive unit of the styrene functionalized with Ff (amino acid) groups; GF, functionalization degree.

Table 2.
The characteristics of amino acid groups functionalized onto copolymers.
3.2. Thermal Stability of Amino Acid Functionalized onto Styrene-Divinylbenzene Copolymers
The TGA data for the pure and amino acid-modified copolymers reveal notable differences in thermal stability and decomposition behavior (see Figure 4 and Figure 5). The TG (thermogravimetric) analysis (see Figure 4 and Figure 5) for the pure copolymers S-15DVBCH2Cl and AP1 clearly indicates differences in thermal stability and decomposition behavior, based on residue and weight loss data at 900 °C:—S-15%DVB exhibits higher thermal stability than AP1, as indicated by a higher residue (19.18% vs. 15.52%). This suggests that the incorporation of the amino acid group in the AP1 sample leads to a more complete thermal decomposition. AP1 loses more mass (84.48%) than S-15%DVB (80.82%), supporting the idea that the AP1 structure degrades more significantly or contains components with less thermal stability. Analyzing the TG (sees Figure 4 and Figure 5) for the copolymers S-6.7DVBCH2Cl and AP2 clearly indicates differences in thermal stability and decomposition behavior, based on residue and weight loss data at 900 °C:—The raw material S-6.7DVB copolymer leaves a higher residue (31.58%) compared to AP2 (21.72%), suggesting that the raw material sample is more thermally resistant. The higher weight loss (78.28%) in AP2 indicates greater degradation, possibly due to changes in the polymer structure with amino acid groups. The differences in total weight loss and final residues suggest that the AP2 copolymer may decompose more completely.
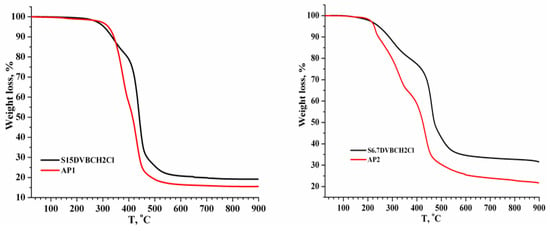
Figure 4.
Thermogravimetric analysis of pristine and modified copolymers (AP1 and AP2) in a nitrogen atmosphere.
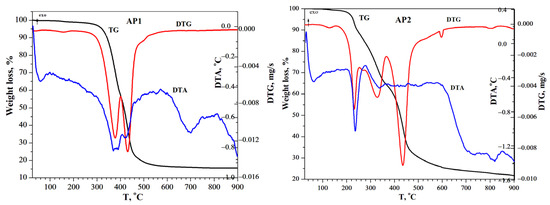
Figure 5.
TG–DTG–DTA curves for AP1 and AP2 recorded in a nitrogen atmosphere.
Thermogravimetric analysis (see Figure 5) was used to evaluate the thermal behavior of two styrene-divinylbenzene copolymers functionalized with an amino acid, denoted AP1 and AP2. From the weight loss percentages, information can be obtained about the structural stability of the polymer backbone. The TGA curves for both AP1 and AP2 showed three main weight loss stages. The initial weight loss observed below 150 °C was attributed to the loss of adsorbed moisture, which is amplified by the hydrophilic nature of the amino and carboxyl groups of glycine. The second and most significant decomposition stage occurred between approximately 150 °C and 400 °C, corresponding to the thermal degradation of the glycine functionalities. The third stage, which occurs above 400 °C, was associated with the decomposition of the S-DVB polymer backbone. The total mass losses recorded were 84.48% for AP1 and 78.28% for AP2, indicating substantial thermal decomposition.
The thermal stability, for all analyzed samples, indicated by the residual mass at 900 °C, follows the following sequence: S-6.7%DVBCH2Cl > AP2 (21.72%) > S-15%DVBCH2Cl > AP1 (15.52%). This sequence highlights that AP2 is more thermally stable than AP1, probably due to structural differences, such as the degree of crosslinking (see Figure 6). Divinylbenzene (DVB) also plays a key role in the crosslink density.
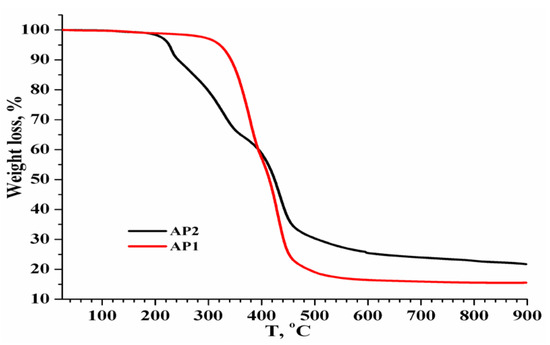
Figure 6.
Thermal stability comparison (between AP1 and AP2), in a nitrogen atmosphere.
4. Conclusions
The functionalization of amino acid groups onto styrene-divinylbenzene copolymers (AP1 and AP2) reduces the copolymers’ thermal stability, as evidenced by increased weight loss and lower char residues. These modifications likely introduce thermally less stable moieties and alter the decomposition pathways. Thermal stability (based on residue) follows: S-6.7DVBCH2Cl > AP2 (copolymer residue = 21.72%) > S-15%DVBCH2Cl > AP1 (copolymer residue = 15.52%). This suggests AP2 is more thermally stable than AP1. The pristine copolymers exhibit significantly higher thermal resistance, suggesting that both AP1 and AP2 have a more degradable structure due to functionalization. The amino acid content confirms the potential applicability of these materials for metal ion chelation, ion exchange, or bioconjugation applications.
Author Contributions
Conceptualization, A.P. and L.C.; methodology, A.P., L.C. and A.V.; formal analysis, L.C., L.L. and E.S.D.; investigation A.P., L.C. and A.V.; data curation, A.P., L.C., L.L. and A.V.; validation, L.C., A.P. and E.S.D.; writing—review and editing, A.P., M.Ț.-L.M. and L.C.; manuscript revisions, A.P., L.L. and E.S.D.; supervision, A.P. and L.C.; project administration, A.P. and L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Teixeira, V.G.; Coutinho, F.M.B. Morphological study on the reactivity of styrene-divinylbenzene copolymers in a chloromethylation reaction. J. Appl. Polym. Sci. 2010, 118, 2389–2396. [Google Scholar] [CrossRef]
- Ramos, G.S.M.; Mendes, M.S.L.; Neves, M.A.F.S.; Pedrosa, M.S.; da Silva, M.R. Experimental design to evaluate the efficiency of maghemite nanoparticles incorporation in styrene-divinylbenzene copolymers. J. Appl. Polym. Sci. 2021, 138, 50318. [Google Scholar] [CrossRef]
- Khan, W.S.; Muthupandian, S.; Farah, N.; Kumar, A.J. Domb, Biodegradable Polymers Derived From Amino Acids Macromol. Biomaterials 2011, 11, 1625–1636. [Google Scholar]
- Cocheci, L.; Visa, A.; Maranescu, B.; Lupa, L.; Pop, A.; Dragan, E.S.; Popa, A. Glycine-group-functionalized polymeric materials impregnated with Zn(II) used in the photocatalytic degradation of congo red dye. Polymers 2025, 17, 641. [Google Scholar] [CrossRef] [PubMed]
- Karabacak, M.; Kurt, M. The spectroscopic (FT-IR and FT-Raman) and theoretical studies of 5-bromo-salicylic acid. J. Mol. Struct. 2009, 919, 215–222. [Google Scholar] [CrossRef]
- Arjunan, V.; Balamourougane, P.S.; Mythili, C.V.; Mohan, S. Experimental spectroscopic (FTIR, FT-Raman, FT-NMR, UV–Visible) and DFT studies of 2-amino-5-chlorobenzoxazole. J. Mol. Struct. 2011, 1003, 92–102. [Google Scholar] [CrossRef]
- Boukaoud, A.; Chiba, Y.; Sebbar, D. A periodic DFT study of IR spectra of amino acids: An approach toward a better understanding of the N-H and O-H stretching regions. Vib. Spectrosc. 2021, 116, 103280. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).