Abstract
The importance of 1,2,4-triazole derivatives in modern pharmaceuticals is very high. They find their application in drug therapy as antifungal, antifungal agents (fluconazole, intraconazole). It is worth noting that some 1,2,4-triazole compounds are used in therapy for the treatment of Alzheimer’s disease, and new, more effective pharmacophores are being sought to create drugs for neurodegenerative diseases. We propose a modernized method for obtaining a new bis(1,2,4-triazole) derivative using the recyclization reaction of 4-hydroxy-2,5-disubstituted-1,3-6H-oxazin-6-ones with a bisnucleophilic reagent, which was m-phenylenedihydrazine. The method of preparation described in the literature did not lead to the expected products, so it was necessary to select new reaction conditions. 1,1′-(benzene-1,3-diyl)bis[5-benzyl-3-(4-nitrophenyl)-1H-1,2,4-triazole] was obtained by recyclization of 4-hydroxy-2-(4-nitrophenyl)-5-phenyl-6H-1,3-oxazin-6-one with m-phenylenedihydrazine dihydrochloride in absolute methanol in the presence of sodium methoxide for 48 h. The structure of the compound was confirmed by 1H, 13C NMR spectroscopy, and mass spectrometry. Antifungal and antibacterial activities were determined by serial dilutions using meat-peptone broth and Sabouraud medium. The yield based on 4-hydroxy-2-(4-nitrophenyl)-5-phenyl-6H-1,3-oxazin-6-one was 76%. The obtained compound exhibited antimicrobial activity against Staphylococcus aureus with a minimum inhibitory concentration of 62.5 μg/mL and 125 μg/mL against Candida albicans.
1. Introduction
The study of compounds related to bis(1H-1,2,4-triazole) derivatives occupies an important place in the chemistry of heterocyclic compounds, and the significance of these derivatives in modern pharmaceuticals is great. These structures are active drugs with antifungal and antimicrobial effects (fluconazole, itraconazole) [1]. A wide range of their biological activity has also been noted in the literature: antitumor [2], analgesic [3], antineurodegenerative [4]. It is known that they can inhibit tumor DNA methylation in sarcoma 180 [5]. It is worth noting that some bis(1H-1,2,4-triazole) compounds can be used in the treatment of Alzheimer’s disease [6], and a search is underway for new, more effective pharmacophores to create drugs for the treatment of neurodegenerative diseases. Thus, the study of bis(1H-1,2,4-triazole) derivatives is an urgent task in the chemistry of heterocyclic compounds and modern pharmaceuticals.
2. Materials & Methods
Synthesis of 1,1′-(benzene-1,3-diyl)bis [5-benzyl-3-(4-nitrophenyl)-1H-1,2,4-triazole] and m-phenylenedihydrazine dihydrochloride was carried out according to known methods [7,8]. Commercially available solvents were used: “Vecton” methanol, chemically pure; “EKOS-1” ethyl acetate, chemically pure; and “Vecton” dimethyl sulfoxide, chemically pure.
Thin-layer chromatography to confirm reaction completion was performed on Silica gel 60 F254 plates (Merck), eluting with a 4:1 mixture of ethyl acetate and methanol, visualization under UV light.
1H and 13C NMR spectroscopy were used to confirm the structure of the synthesized compounds. 1H and 13C NMR spectra were recorded on a Bruker Advance II spectrometer (400 and 100 MHz, respectively), using the residual signal of deuterated dimethyl sulfoxide as an internal standard.
The molecular weights of the synthesized compounds were determined by mass spectrometry. Mass spectra were recorded on a Flexar FX15 liquid chromatograph equipped with a quadrupole mass spectrometer and an SQ300 electrospray ionization source (Perkin Elmer, Springfield, IL, USA).
Antibacterial activity was determined by serial dilution. A sample of the test compound was weighed on an analytical balance and dissolved in dimethyl sulfoxide, with the initial concentration of the compound in the resulting solution being 2000 μg/mL. One ml of the resulting solution was then added to the first of eight test tubes containing 1 mL of meat-peptone broth. By sequentially transferring 1 mL of the mixture of the nutrient medium and the test substance from one test tube to another, concentrations of the test substances ranged from 1000 μg/mL (in the first test tube) to 7.8 μg/mL (in the eighth test tube). One of the test tubes (control) did not contain the test compound. The microbial suspension was prepared in sterile saline using a turbidity standard containing 109 microbial cells. The cell suspension was diluted, and 100 μL of the diluted suspension was added to each test tube. The final microbial load was 107 cells/mL. The test tubes containing the microorganisms were kept in a thermostat at 36–37 °C for two days. To determine the minimum inhibitory concentration (MIC), the turbidity of the test tube contents was compared with the control. Test tubes that showed no signs of growth, or showed weaker growth than the control tube, were streaked with a loop onto a solid nutrient medium (meat-peptone agar). After streaking, the Petri dishes were incubated in a thermostat for two days.
Antifungal activity was determined by serial dilution. A sample of the test substance was taken on an analytical balance and dissolved in dimethyl sulfoxide, with the initial concentration of the substance in the resulting solution being 2000 μg/mL. Then, 1 mL of the resulting solution was added to the first of eight test tubes containing 1 mL of Sabouraud’s medium for culturing Candida albicans. By sequentially transferring 1 mL of the nutrient medium and test substance mixture from one test tube to another, concentrations of the test substances ranged from 1000 μg/mL (in the first tube) to 7.8 μg/mL (in the eighth tube). One of the test tubes (control) did not contain the test compound. A microbial suspension was prepared in sterile saline using a turbidity standard containing 109 microbial cells. The cell suspension was diluted, and 100 µL of the diluted suspension was added to each test tube. The final microbial load was 107 cells/mL. The test tubes containing the microorganisms were incubated in a thermostat at 36–37 °C for two days. To determine the minimum inhibitory concentration (MIC), the turbidity of the test tube contents was compared with the control. Test tubes that showed no signs of growth, or showed weaker growth than the control tube, were streaked onto Sabouraud agar medium using a loop. After streaking, the Petri dishes were incubated in a thermostat for two days. The minimum fungicidal concentration (MFC) was determined by the absence of microorganism growth on a solid nutrient medium.
3. Results & Discussion
3.1. Synthesis
To obtain 1,1′-(benzene-1,3-diyl)bis[5-benzyl-3-(4-nitrophenyl)-1H-1,2,4-triazole], we studied the reaction of 4-hydroxy-2-(4-nitrophenyl)-5-phenyl-6H-1,3-oxazin-6-one and m-phenylenedihydrazine dihydrochloride (Scheme 1). These compounds were stirred in methanol with sodium methoxide at room temperature for 48 h. Reaction completion was determined by TLC, as the absence of a spot of the starting oxazine in the reaction mixture. Upon completion of the synthesis, the target product was isolated by distillation of the solvent and addition of 15% aqueous sodium hydroxide solution to the residue. The precipitate of the target compound was washed with water on a filter and dried in an oven at 60 °C. The yield was 76% (Appendix A.1).
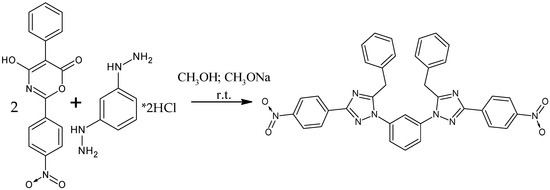
Scheme 1.
Reaction of 4-hydroxy-2-(4-nitrophenyl)-5-phenyl-6H-1,3-oxazin-6-one and m-phenylenedihydrazine dihydrochloride.
3.2. Chemical Structure
The 1H NMR (Appendix A.2) spectrum (Figure 1) of the obtained compound contains resonance signals of the protons of the “-CH2-” group (4.34 (s, 4H)), protons of the phenyl ring (positions 5–8) (7.82 (s, 3H), 7.93 (s, 1H)), aromatic protons of the benzyl radical (7.16–7.27 (m, 10H)), and aromatic protons of the p-nitrophenyl radical (8.29–8.39 (q, 8H)).
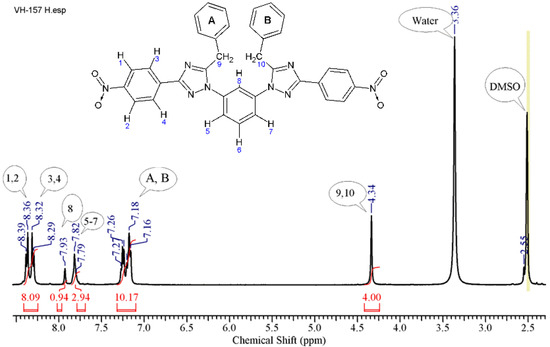
Figure 1.
1H NMR spectrum of 1,1′-(benzene-1,3-diyl)bis[5-benzyl-3-(4-nitrophenyl)-1H-1,2,4-triazole].
In the 13C NMR (Appendix A.2) spectrum (Figure 2) of the obtained compound, the following signals were observed: those of the nodal carbon atom of the 4-nitrophenyl radical: 159.40 ppm—at the nitro group and 157.10 ppm—at the triazole ring; 148.37 and 137.97 ppm—correspond to the carbon atoms of the triazole ring; the signals of 136.77—122.48 ppm—correspond to the carbon atoms of the benzene rings; and the signal of 32.48 ppm—to the methylene groups.
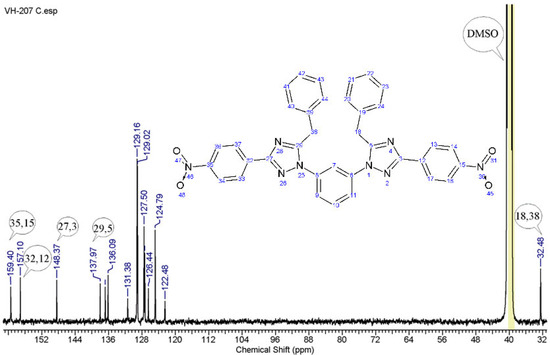
Figure 2.
13C NMR spectrum of 1,1′-(benzene-1,3-diyl)bis[5-benzyl-3-(4-nitrophenyl)-1H-1,2,4-triazole].
The mass spectrum (Figure 3) (Appendix A.2), obtained in the positive ionization mode by the electrospray method using a quadrupole analyzer, contained an intense signal corresponding to a molecular ion of the [M+Na]+ type with an m/z value of 657.26.
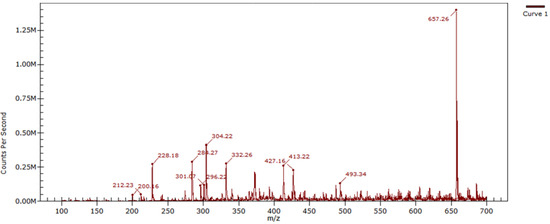
Figure 3.
Mass spectrum of 1,1′-(benzene-1,3-diyl)bis[5-benzyl-3-(4-nitrophenyl)-1H-1,2,4-triazole].
3.3. Evaluation of Antibacterial and Antifungal Activities
Based on the results of the in vitro tests, it was established that the compound exhibits moderate inhibitory activity against Staphylococcus aureus and weak antifungal activity against Candida albicans (Table 1).

Table 1.
Results of determination of the minimum inhibitory concentration (MIC), minimum bactericidal (MBC), and fungicidal (MFC) concentrations of the synthesized compound against Staphylococcus aureus and Candida albicans.
4. Conclusions
Thus, an effective laboratory method for the synthesis of 1,1′-(benzene-1,3-diyl)bis [5-benzyl-3-(4-nitrophenyl)-1H-1,2,4-triazole] was developed by studying the cleavage reaction of 4-hydroxy-6H-1,3-oxazin-6-one and m-phenylenedihydrazine dihydrochloride.
The structure of the resulting compound was confirmed using physicochemical analysis methods—1H and 13C NMR spectroscopy and mass spectrometry.
An experimental in vitro evaluation of its antibacterial and antifungal activity against opportunistic microorganisms—Staphylococcus aureus and Candida albicans—was conducted. It was found that the resulting compound exhibited moderate inhibitory activity only against Staphylococcus aureus.
Author Contributions
Authors E.V.M., D.A.K., I.P.Y., M.V.S. and O.A.K. contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| MIC | Minimum inhibitory concentration |
| MBC | Minimum bactericidal concentration |
| MFC | Minimum fungicidal concentration |
| NMR | Nuclear magnetic resonance |
Appendix A
Appendix A.1. Synthesis
A 25 mL round-bottomed flask was charged with 2.2 mmol (464.4 mg) of m-phenylenedihydrazine dihydrochloride, 4.4 mmol (237.6 mg) of sodium methoxide, and 6 mL of methanol. The mixture was stirred for 30 min, then 4.4 mmol (1364.0 mg) of 4-hydroxy-5-(4-nitrophenyl)-6H-1,3-oxazin-6-one was added, and the mixture was stirred for 48 h at room temperature. Reaction completion was determined by TLC, as evidenced by the absence of a spot of the starting oxazine in the reaction mixture. Upon completion of the synthesis, the methanol was distilled off, 15% aqueous sodium hydroxide solution was added to the residue, and the mixture was stirred for 30 min. The suspension was then filtered. The precipitate was washed with water on the filter. Drying was carried out in a drying oven at 60 °C. The yield of the product was 76%.
Appendix A.2. 1,1′-(Benzene-1,3-diyl)bis [5-benzyl-3-(4-nitrophenyl)-1H-1,2,4-triazole]
Light orange powder. m.p. = 131–132 °C.
1H NMR spectrum data (400.17 MHz, DMSO-d6) δ, ppm (J, Hz): 4.34 s (4H, -CH2-); 7.18 m (10H, PhA,B); 7.82 s (3H, H5–7); 7.93 s (1H, H8); 8.32 d (4H, H3,4,3′,4′, J = 8.53 Hz); 8.36 d (4H, H1,2,1′,2′, J = 8.66 Hz).
13C NMR spectrum data (101.63 MHz, DMSO), δ, ppm: 32.48 (-CH2-), 122.48–136.77 (C6–11, C33,34,36,37, C13,14,17,16, C19–24, C39–44); 148.37 (C27,3); 137.97 (C29,5); 157.10 (C35,15); 159.40 (C32,12).
LRMS (ESI, pos), m/z, Calculated: 657.20 [M+Na]+; Found: 657.26 [M+Na]+.
References
- Mashkovskiy, M.D. Medicines, 15th ed.; Novaya Volna: Moscow, Russia, 2009; ISBN 5786402037. [Google Scholar]
- Lockhart, N.R.; Waddell, J.A.; Schrock, N.E. Itraconazole Therapy in a Pancreatic Adenocarcinoma Patient: A Case Report. J. Oncol. Pharm. Pract. 2015, 22, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Cheretaev, I.V.; Chuyan, E.N.; Ravaeva, M.Y.; Shulgin, V.F. Vliyanie 1-gidroksi-1,1-etilidendifosfonovoj kisloty i bis(2-piridil-1,2,4-triazolil-3)propana na bolevuyu chuvstvitel’nost’ samok krys. Mezhdunar. Nauchno-Issled. Zh. 2019, 85, 104–109. [Google Scholar]
- Bulut, N.; Kocyigit, U.M.; Gecibesler, I.H.; Dastan, T.; Karci, H.; Taslimi, P.; Dastan, S.D.; Gulcin, I.; Cetin, A. Synthesis of Some Novel Pyridine Compounds Containing Bis-1,2,4-Triazole/Thiosemicarbazide Moisty and Investigation of Their Antioxidant Properties, Carbonic Anhydrase, and Acetylcholinesterase Enzymes Inhibition Profiles. J. Biochem. Mol. Toxicol. 2017, 32, e22006. [Google Scholar] [CrossRef] [PubMed]
- Dilanyan, S.V.; Hovsepyan, T.R.; Nersesyan, L.E.; Agaronyan, A.S.; Danielyan, I.S.; Minasyan, N.S.; Harutyunyan, A.A. New Bis-4H-1,2,4-Triazoles and Their In Vitro Study as DNA Methylation Inhibitors. Russ. J. Gen. Chem. 2020, 90, 787–793. [Google Scholar] [CrossRef]
- Xu, M.; Peng, Y.; Zhu, L.; Wang, S.; Ji, J.; Rakesh, K.P. Triazole Derivatives as Inhibitors of Alzheimer’s Disease: Current Developments and Structure-Activity Relationships. Eur. J. Med. Chem. 2019, 180, 656–672. [Google Scholar] [CrossRef] [PubMed]
- Chernov, N.M. Sintez, Stroenie, Svojstva i Biologicheskaya Aktivnost’ 2-Funkcional’no Zameshchennyh 4-Gidroksi-6H-1,3-Oksazin-6-Onov i ih Proizvodnyh. Ph.D. Thesis, Sankt-Peterburgskaya Gosudarstvennaya Himiko-Farmacevticheskaya Akademiya Minzdrava RF, St. Petersburg, Russia, 2016. [Google Scholar]
- Lee, W.Y. Studies on the Aromatic Dihydrazines (IV). A New Synthesis of m-Phenylenedihydrazine via Tetrazonium Salt. J. Korean Chem. Soc. 1978, 22, 326–333. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).