Efficient Synthesis of Substituted 2-Nitrochalcone Derivatives †
Abstract
1. Introduction
2. Materials and Methods
2.1. Physical Measurements
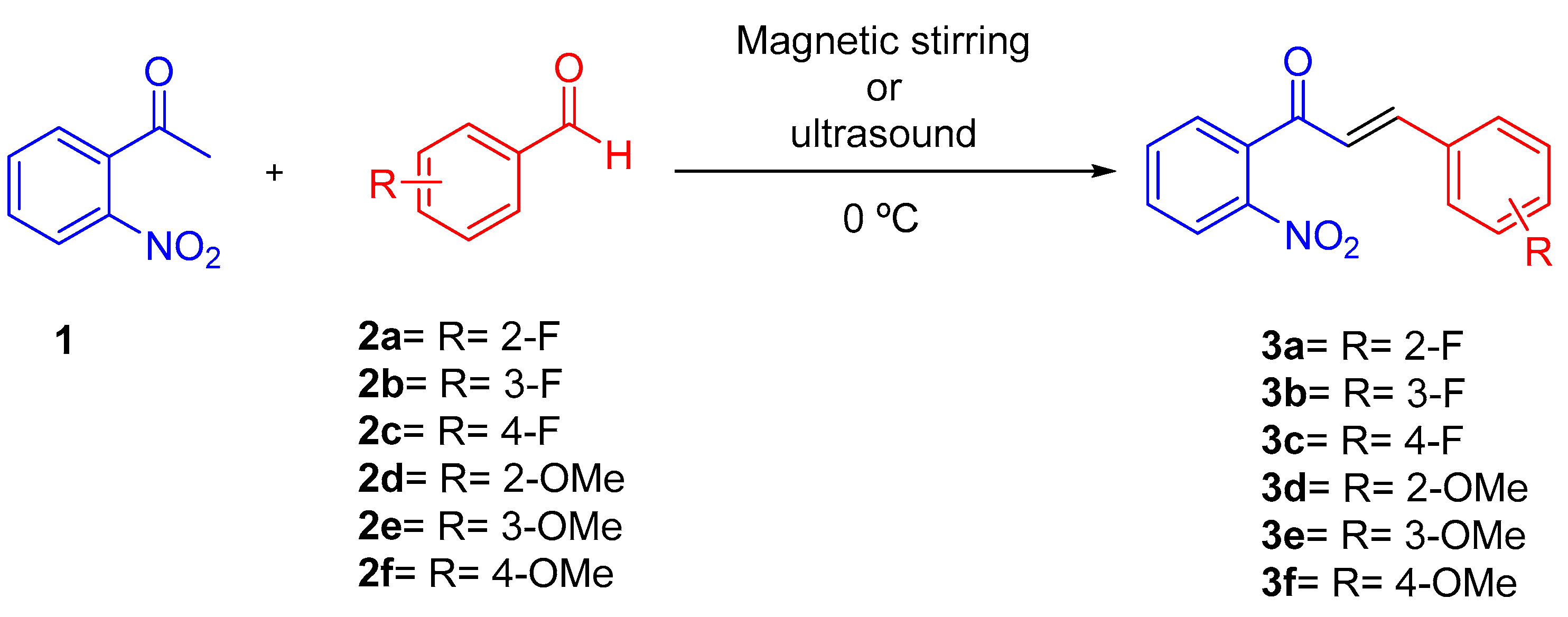
2.2. Synthesis and Characterization of Compounds 3a–e
2.2.1. Conventional Conditions (Method A)
2.2.2. Ultrasound Irradiation (Method B)
3. Results and Discussion
Chemistry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blakemore, D.C.; Castro, L.; Churcher, I.; Rees, D.C.; Thomas, A.W.; Wilson, D.M.; Wood, A. Organic Synthesis Provides Opportunities to Transform Drug Discovery. Nat. Chem. 2018, 10, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.A.; Krithiga, T.; Manigandan, S.; Sathish, S.; Renita, A.A.; Prakash, P.; Prasad, B.S.N.; Kumar, T.R.P.; Rajasimman, M.; Hosseini-Bandegharaei, A. A Focus to Green Synthesis of Metal/Metal Based Oxide Nanoparticles: Various Mechanisms and Applications towards Ecological Approach. J. Clean. Prod. 2021, 324, 129198. [Google Scholar] [CrossRef]
- Martínez, R.F.; Cravotto, G.; Cintas, P. Organic Sonochemistry: A Chemist’s Timely Perspective on Mechanisms and Reactivity. J. Org. Chem. 2021, 86, 13833–13856. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, A.Y.; Romero-Ceronio, N.; Lobato-García, C.E.; Herrera-Ruiz, M.; Vázquez-Cancino, R.; Peña-Morán, O.A.; Vilchis-Reyes, M.Á.; Gallegos-García, A.J.; Medrano-Sánchez, E.J.; Hernández-Abreu, O. Position Matters: Effect of Nitro Group in Chalcones on Biological Activities and Correlation via Molecular Docking. Sci. Pharm. 2024, 92, 54. [Google Scholar] [CrossRef]
- Martins, T.; Fonseca, B.M.; Rebelo, I. Antioxidant Effects of Chalcones during the Inflammatory Response: An Overall Review. Curr. Med. Chem. 2021, 28, 7658–7713. [Google Scholar] [CrossRef] [PubMed]
- Khanapure, S.; Jagadale, M.; Bansode, P.; Choudhari, P.; Rashinkar, G. Anticancer Activity of Ruthenocenyl Chalcones and Their Molecular Docking Studies. J. Mol. Struct. 2018, 1173, 142–147. [Google Scholar] [CrossRef]
- Chaouiki, A.; Lgaz, H.; Salghi, R.; Chafiq, M.; Oudda, H.; Bhat, K.S.; Cretescu, I.; Ali, I.H.; Marzouki, R.; Chung, I.M. Assessing the Impact of Electron-Donating-Substituted Chalcones on Inhibition of Mild Steel Corrosion in HCl Solution: Experimental Results and Molecular-Level Insights. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 588, 124366. [Google Scholar] [CrossRef]
- Olender, D.; Sowa-Kasprzak, K.; Pawełczyk, A.; Skóra, B.; Zaprutko, L.; Szychowski, K.A. Curcuminoid Chalcones: Synthesis and Biological Activity against the Human Colon Carcinoma (Caco-2) Cell Line. Curr. Med. Chem. 2024, 31, 5397–5416. [Google Scholar] [CrossRef] [PubMed]
- Gaonkar, S.L.; Vignesh, U.N. Synthesis and Pharmacological Properties of Chalcones: A Review. Res. Chem. Intermed. 2017, 43, 6043–6077. [Google Scholar] [CrossRef]
- Goyal, K.; Kaur, R.; Goyal, A.; Awasthi, R. Chalcones: A Review on Synthesis and Pharmacological Activities. J. Appl. Pharm. Sci. 2021, 11, 1–14. [Google Scholar]
| Compounds | Method A | Method B | ||||
|---|---|---|---|---|---|---|
| Base | Time (h) | Yield (%) | Base | Time (h) | Yield (%) | |
| 3a * | NaOH | 1 | 71 | K2CO3 | 2 | 62 |
| 3b * | NaOH | 1 | 90 | K2CO3 | 2 | 71 |
| 3c * | NaOH | 1 | 95 | K2CO3 | 2 | 73 |
| 3d * | NaOH | 2 | 74 | K2CO3 | 2 | 70 |
| 3e * | NaOH | 2 | 70 | K2CO3 | 2 | 61 |
| 6f * | NaOH | 2 | 93 | K2CO3 | 2 | 78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidalgo, A.Y.; Torres-Sauret, Q.; Lobato-García, C.E.; Vilchis-Reyes, M.Á.; Gómez-Rivera, A.; Hernandez-Abreu, O.; Romero-Ceronio, N. Efficient Synthesis of Substituted 2-Nitrochalcone Derivatives. Chem. Proc. 2024, 16, 85. https://doi.org/10.3390/ecsoc-28-20109
Hidalgo AY, Torres-Sauret Q, Lobato-García CE, Vilchis-Reyes MÁ, Gómez-Rivera A, Hernandez-Abreu O, Romero-Ceronio N. Efficient Synthesis of Substituted 2-Nitrochalcone Derivatives. Chemistry Proceedings. 2024; 16(1):85. https://doi.org/10.3390/ecsoc-28-20109
Chicago/Turabian StyleHidalgo, Alam Yair, Quirino Torres-Sauret, Carlos Ernesto Lobato-García, Miguel Ángel Vilchis-Reyes, Abraham Gómez-Rivera, Oswaldo Hernandez-Abreu, and Nancy Romero-Ceronio. 2024. "Efficient Synthesis of Substituted 2-Nitrochalcone Derivatives" Chemistry Proceedings 16, no. 1: 85. https://doi.org/10.3390/ecsoc-28-20109
APA StyleHidalgo, A. Y., Torres-Sauret, Q., Lobato-García, C. E., Vilchis-Reyes, M. Á., Gómez-Rivera, A., Hernandez-Abreu, O., & Romero-Ceronio, N. (2024). Efficient Synthesis of Substituted 2-Nitrochalcone Derivatives. Chemistry Proceedings, 16(1), 85. https://doi.org/10.3390/ecsoc-28-20109







