Abstract
In this work we present, a kinetic study of the Knoevenagel condensation reaction between 5-methoxy-1-tetralone and glyoxylic acid, catalyzed by sulfuric acid, to produce (E)-2-(5-methoxy-1-oxo-3,4-dihydronaphthalen-2(1H)-ylidene)acetic acid. The reaction was carried out in a batch system at 400 rpm for 24 h at temperatures of 75, 80 and 85 °C. The yields obtained at these temperatures were 90.30, 93.75 and 94.16%, respectively. The reaction was monitored by TLC and HPLC. For the kinetic analysis, three mathematical methods were used: integral, differential and non-linear regression. The results showed an excellent fit of the experimental data to the pseudo-first-order kinetic model.
1. Introduction
1-Tetralones (3,4-dihydro-1H-naphthalene-1-ones), have been the subject of much interest in organic synthesis and natural products, as they possess a structure with the appropriate functional groups for building the skeletons of a wide range of products, making them a key starting material (Figure 1), particularly due to their potential as lead compounds in the pharmaceutical industry. Chemists worldwide have been interested in the isolation, synthesis, and structural modification of 1-tetralone and its derivatives due to their importance in the synthesis of bioactive compounds such as steroids, prostaglandin analogs, dyes, heterocycles and pharmaceuticals, and new drug candidates [1,2,3,4,5,6,7], as well as their use as fluorescent dyes or sensors [8].
Figure 1.
1-tetralones moiety with various activities.
In this study, we synthesized and characterized (E)-2-(5-methoxy-1-oxo-3,4-dihydronaphthalen-2(1H)-ylidene)acetic acid by a modification in acid medium of the Knoevenagel condensation reaction.
2. Materials and Methods
2.1. Materials and General Methods
All reagents were analytic grade and solvents were previously purified by conventional methods. The reaction monitoring was carried out by thin layer chromatography (TLC) with silica plates. Products were purified by recrystallization using ethanol. Infrared spectra were obtained using a Perkin-Elmer Spectrum two FTIR spectrometer in a range of 500–4000 cm−1 and UV-Vis spectra were obtained using an Agilent Technologies Cary 60 UV-Vis spectrometer. Concentration data were obtained using an Agilent Technologies 1260 Series Infinity High Performance Liquid Chromatograph (RP-18 column, 150 × 4.6 mm). (1H, 13C) NMR spectra were recorded in CDCl3 on Bruker Avance-400 ((400.13 MHz (1H), 100.62 MHz (13C)) instruments.
2.2. Synthesis and Characterization
The compound (1) (400 mg, 2.27 mmol) was added to diglyme (8 mL) and water (0.22 mL) and left in agitation, then, the glyoxylic acid (0.4 mL, 9.94 mmol) and a catalytic amount of sulfuric acid (0.03 mL) were added. The reaction mixture was heated to temperatures of 75 °C, 80 °C and 85 °C, with constant stirring at 400 rpm, for 24 h. For reaction monitoring, TLC (Rf = 0.62 (dichloromethane, methanol and glacial acetic acid; 4.5: 0.45: 0.05) and HPLC (UV-vis (Ethanol) [λmax (nm)]: 260.1) were performed at times ranging from the beginning at 0 h to the end at 24 h. The final product obtained (2) was recrystallized in ethanol, giving yellow crystals with a yield of 94 %, and characterized by FTIR, GC-MS, 1H and 13C NMR: IR (KBr) υmax (cm−1): 3460. 61 (O-H), 1696.8 (C=O), 1673.6 (C=O α,β-unsaturated) MS (EI) m/z: 214 [M-H2O]+, 186 [M-CO]+, 171 [M-CH2]+. 1H-NMR (400 MHz, CDCl3, δ/ppm): 7.51 (d, 1Haromatic, J = 7.85 Hz), 7.32 (t, 1Haromatic, J = 7.95 Hz), 7.06 (d, 1Haromatic, J = 8,01 Hz ), 6.86 (s, 1H, =CH-), 3.87 (s, 3H, O-CH3 ), 3.39 (t, 2H, -CH2), 2.99 (t, 2H, -CH2) and 13C-NMR (400 MHz, CDCl3, δ/ppm): 21.59, 26.82, 55.79, 115.01, 119.96, 122.15, 127.57, 133.34, 144.61, 152.10, 156.50, 171.61, 187.09.
3. Results and Discussion
Synthesis
The modified Knoevenagel condensation reaction in acidic medium generated the unsaturated acid of 1-tetralone (3) (Figure 2), obtaining results similar to those reported by Cheung et al. [9].
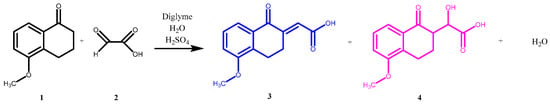
Figure 2.
Scheme of the reaction synthesis of (3).
In this study, the optimal conditions that allowed for the desired compound (3) to be obtained with a yield of 94.16% were a reaction time of 24 h, stirring at 400 rpm, and a temperature of 85 °C. These conditions were determined by monitoring the reaction using thin-layer chromatography (TLC) (Figure 3), where the formation of a collateral product was observed, which was also analyzed by high-performance liquid chromatography (HPLC) (Figure 4).
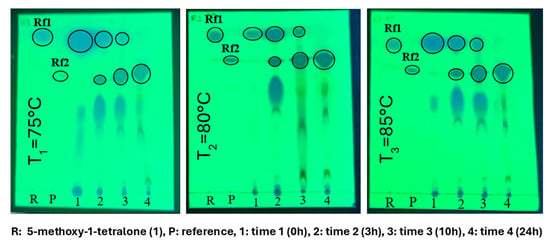
Figure 3.
Monitoring the reaction using thin-layer chromatography (TLC).
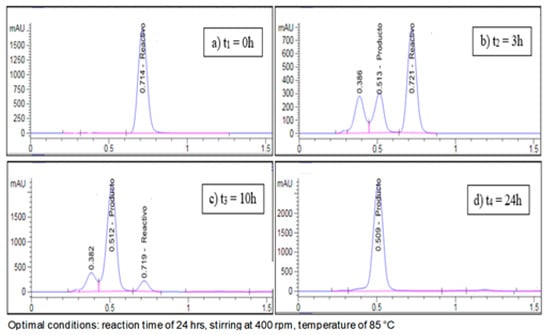
Figure 4.
Monitoring the reaction using high-performance liquid chromatography (HPLC).
According to Costantino et al. [10], during the reaction in basic medium (NaOH), the hydroxylated acid (4) is formed; however, its physical properties, characterization, and separation are not reported. In this study, it was possible to determine, by monitoring the reaction using HPLC, the time required for all starting material (1) and the intermediate (4) to be completely converted into the desired compound (3).
A variety of techniques were employed to ascertain the kinetic parameters, including the integral method, the differential method and non-linear regression. Of these, the non-linear regression method proved the most effective, demonstrating positive statistical metrics with a minimum deviation and acceptable coefficients between the experimental and calculated values.
The study determined a kinetic behavior that approximates a pseudo-first-order in respect to 1. Additionally, the activation energy was calculated (Ea = 43.1014 kJ/mol) and, thus, a high-sensitivity temperature dependence of the reaction for small changes was found. Finally, the yields obtained for the studied temperatures of 75, 80 and 85 °C were 90.30, 93.75, and 94.16%, respectively (Table 1).

Table 1.
Results of the kinetic parameters.
4. Conclusions
In this work, the kinetic study of the Knoevenagel condensation reaction between 5-methoxy-1-tetralone and glyoxylic acid, catalyzed by sulfuric acid to produce (E)-2-(5-methoxy-1-oxo-3,4-dihydronaphthalen-2(1H)-ylidene)acetic acid was carried out. The ideal reaction parameters were 24 h time, 400 rpm, and 85 °C for 94.16% yield, which were followed by TLC and HPLC. A pseudo-first-order reaction, kinetic constant (k = 0.0196 mL/(mmol∗min)), pre-exponential factor (A = 8.4608 × 103 (mL/(mmol∗min)) and activation energy (Ea = 43.1014 kJ/mol) were determined for the rate law using the non-linear regression method, which provided the best model fit and statistical parameters. Through FTIR, GC-MS and 1H and 13C NMR, the product and the purity obtained were clearly identified.
Author Contributions
Conceptualization, M.E.H., E.V.C. and R.S.G.; methodology, R.S.G. and M.E.H.; investigation, E.V.C., M.E.H. and R.S.G.; writing—original draft preparation, E.V.C. and U.S.; writing—review and editing, E.V.C., R.S.G. and U.S.; supervision, EVC and M.E.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from the Universidad Central del Ecuador via the Senior Research Project DI-CONV-2022-049.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to express their gratitude to the Universidad Central del Ecuador for financing the Senior Project “Síntesis de nuevos compuestos de amidolidenos-1-tetralonas: análisis estructural, de interacción molecular y de actividad antineoplásica.” with code DI-CONV-2022-049 and Laboratorios de Investigación y servicios especializados from the Faculty of Chemical Engineering of the Universidad Central del Ecuador.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chumpradit, S.; Kung, M.P.; Vessotskie, J.; Foulon, C.; Mu, M.; Kung, H.F. Iodinated 2-Aminotetralins and 3-Amino-1-benzopyrans: Ligands for Dopamine D2 and D3 Receptors. J. Med. Chem. 1994, 37, 4245–4250. [Google Scholar] [CrossRef] [PubMed]
- Foulon, C.; Kung, M.P.; Kung, H.F. Synthesis of (R,S)-2′-trans-7-hydroxy-2-[N-n-propyl-N-(3′-iodo-2′-propenyl)-amino]tetralin(trans-7-OH-PIPAT): A new D3 dopamine receptor ligand. J. Med. Chem. 1993, 36, 1499–1500. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.H.; Bartlett, P.A. Macrocyclic Inhibitors of Penicillopepsin Design, Synthesis, and Evaluation of an Inhibitor Bridged between P1 and P3. J. Am. Chem. Soc. 1998, 120, 4600–4609. [Google Scholar] [CrossRef]
- Matsumoto, T.; Ohsaki, M.; Suzuki, M.; Kimura, Y.; Terashima, S. Synthesis of 4-demethoxyanthracyclines carrying a lipophilic alkanoyl group at the C9-position. Chem. Pharm. Bull. 1986, 34, 4605–4612. [Google Scholar] [CrossRef] [PubMed]
- Stout, D.M.; Gorczynski, R.J. N-Aralkyl substitution of 2-amino-5,6- and -6,7-dihydroxy-1,2,3,4-tetrahydronaphthalenes. 2. Derivatives of a hypotensive-positive inotropic agent. J. Med. Chem. 1982, 25, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Tagmatarchis, N.; Thermos, K.; Katerinopoulos, H.E. N-(Iodopropenyl)-octahydrobenzo[f]-and -[g]quinolines: Synthesis and adrenergic and dopaminergic activity studies. J. Med. Chem. 1998, 41, 4165–4170. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Reines, E.H.; Montgomery, S.A. A comparative review of escitalopram, paroxetine, and sertraline: Are they all alike? Int. Clin. Psychopharm. 2014, 29, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Sheng, K.; Song, Y.; Lei, F.; Zhao, W.; Fan, L.; Wu, L.; Liu, Y.; Wu, S.; Zhang, Y. Research progress in pharmacological activities and structure-activity relationships of tetralone scaffolds as pharmacophore and fluorescent skeleton. Eur. J. Med. Chem. 2022, 227, 113964. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.; Tangirala, R.S.; Bethi, S.R.; Joshi, H.V.; Ariazi, J.L.; Tirunagaru, V.G.; Kumar, S. Discovery of Tetralones as Potent and Selective Inhibitors of Acyl-CoA:Diacylglycerol Acyltransferase 1. ACS Med. Chem. Lett. 2018, 9, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Costantino, L.; Rastelli, G.; Cignarella, G.; Barlocco, D. Synthesis and aldose reductase inhibitory activity of a new series of benzo[h]cinnolinone derivatives. II Farmaco 2000, 55, 544–552. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).