Abstract
An efficient catalytic system consisting of 20 mol. % FeCl3·6H2O and 20 mol. % trifluoromethanesulfonic acid for the amidation of binor-S in a solution of toluene with organic nitriles under the action of microwave irradiation at 100 °C for 30 min was developed.
1. Introduction
The classical method for the synthesis of N-substituted amides is the Ritter reaction of alcohols, haloalkanes, or olefins with nitriles and water, catalyzed by H2SO4 [1] and HF [2]. The Ritter reaction can also be catalyzed by various Lewis acids based on salts of such transition metals as cobalt [3], copper [4], and iron [5]. Of particular interest is the Ritter reaction with cyclopropane derivatives. Only a few examples of such interactions are known. Thus, iron compounds, and in particular FeCl3·6H2O, catalyze the amidation of binor-S with a number of organic nitriles at a temperature of 140–150 °C during the reaction in an autoclave for 6 h. Only a few examples of the reaction of nitriles with cyclopropyl ketones [6], cyclopropanols [7], and arylcyclopropanes [8] are known. Previously, it was found that FeCl3·6H2O catalyzed amidation of binor-S [9] and deltacyclene [10], which are cyclopropane-containing polycyclic hydrocarbons, with a number of organic nitriles at a temperature of 140–150 °C during the reaction in an autoclave for 6–12 h. A significant disadvantage of these reactions is the need to use a 16-fold excess of organic nitrile to carry out the reaction at elevated pressures.
In order to develop more efficient catalytic systems for the amidation of cyclopropane-containing polycyclic hydrocarbons, we studied the reaction of binor-S with organic nitriles in the presence of a catalytic system consisting of FeCl3·6H2O and trifluoromethanesulfonic acid. We hoped that the use of a binary catalytic system would significantly reduce the reaction temperature and allow it to be carried out at atmospheric pressure.
2. Results and Discussion
We found that the catalytic system, consisting of 20 mol. % FeCl3·6H2O and 20 mol. % trifluoromethanesulfonic acid makes it possible to reduce the reaction time of amidation of binor-S in a toluene solution with organic nitriles (propionitrile, cyclopropyl nitrile) to 30 min with a simultaneous decrease in the reaction temperature to 100 °C. The yield of the corresponding amides 1, and 2 was 92–94% (Scheme 1). The use of toluene as a solvent made it possible to reduce the amount of excess organic nitrile used by four times relative to the original method. However, reducing the amount of organic nitrile used from 4 mmol to 2 mmol leads to a decrease in conversion to 45%. When additional water was used (1 equivalent with respect to binor-S), we observed the formation of the hydroxy derivative of binor-S as a by-product in an amount of up to 20%, which could be a consequence of the interaction of binor-S with water in the reaction conditions.
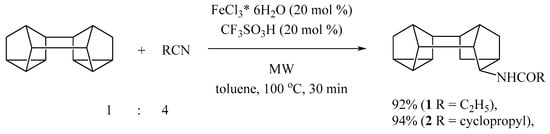
Scheme 1.
Microwave activation in Fe-catalyzed reaction of Binor-S with nitriles.
Thus, the use of a binary system of catalysts made it possible to achieve a fourfold decrease in the amount of organic nitrile used and a decrease in the reaction temperature to 100 °C compared with the previously developed amidation procedure. The latter circumstance made it possible to carry out the binor-S amidation reaction in glass reactors at atmospheric pressure.
3. Conclusions
Thus, we have developed an efficient catalytic system consisting of 20 mol. % FeCl3·6H2O and 20 mol. % trifluoromethanesulfonic acid for the amidation of binor-S in a solution of toluene with organic nitriles under the action of microwave irradiation at 100 °C for 30 min.
4. Experimental Part
Propionitrile and cyclopropyl cyanide (Acros) were commercial reagents. 1H and 13С NMR spectra were recorded on a Bruker Avance-II 400 Ascend instrument (400 MHz for 1Н and 100 MHz for 13С in CDCl3). Mass spectra were run on a Shimadzu GCMS-QP2010Plus mass spectrometer (SPB-5 capillary column, 30 m × 0.25 mm, helium as the carrier gas, temperature programming from 40 to 300 °C at 8 °C/min, an evaporation temperature of 280 °C, ion source temperature of 200 °C, and ionization energy of 70 eV). The course of the reaction and the purity of the products were monitored by gas-liquid chromatography on a Shimadzu GC-9A, GC-2014 instrument [2 m × 3 mm column, SE-30 silicone (5%) on Chromaton N-AW-HMDS as the stationary phase, temperature programming from 50 to 270 °C at 8 °C/min, helium as the carrier gas (47 mL/min)].
Binor-S amidation. General methodology. To a solution of 0.188 g (1 mmol) of binor-S (1) in 1 mL of toluene, 0.054 g (0.2 mmol) of FeCl3·6H2O, 0.03 g (0.2 mmol) of trifluoromethanesulfonic acid, nitrile (4 mmol): 0.23 g of propionitrile, 0.27 g cyclopropyl nitrile were added. The reaction was carried out at 100 °C for 0.5 h. After completion, the reaction mixture was washed with water, extracted with ethyl acetate (3 × 1 mL), and the solvent was distilled off under reduced pressure. The residue was recrystallized from ethyl acetate.
N-(hexacyclo [9.2.1.02,7.03,5.04,8.09,13]tetradec-10-exo-yl)propionamide (1). Yield 0.25 g (92%), white crystals, m.p. 144–145 °C (from ethyl acetate). IR spectrum, (thin layer), ν, cm−1:3478, 3300, 1635, 1553. Спектр NMR 1Н (CDCl3), δ, ppm: 5.50 s (1 H, СН), 3.90 d (1 H, СН), 2.24–1.98 m (1 H, СН), 1.98–2.50 m (1 H, СН), 2.50–2.015 m (2 H, СН2), 1.97–1.96 m (1 H, СН), 1.87–1.85 m (1 H, СН), 1.73–1.70 m (2 H, СН2), 1.57–1.44 m (2 H, СН2), 1.37–1.26 m (3 H, СН, СН2), 1.21–1.19 m (2 H, СН2), 1.12–1.09 m (4 H, 2СН2), 0.98–0.91 m (1 H, СН). NMR 13С, δ, ppm: 9.95 (С18), 56.15 (С10), 46.42 (С9), 44.23 (С1), 41.61 (С2), 40.65 (С8), 37.17 (С7), 36.08 (С13), 34.82 (С12), 34.02 (С11), 32.48 (С14), 31.93 (С6), 29.78 (С17), 15.75 (С5), 15.41 (С3), 14.98 (С4), 172.79 (СО). MS, m/z (Irel, %): 255 [M]+ (20), 184 (24), 228 (49), 57 (69), 200 (100). Found, %: C, 79.40; H 8.97; N 5.50. C17H23NO. Calculated: C, 79.33; H, 9.01; N, 5.44. М 255.37.
N-(hexacyclo[9.2.1.02,7.03,5.04,8.09,13]tetradec-10-exo-yl)cyclopropanecarboxamide (2). Yield 0.26 g (94%), white crystals, m.p. 178–180 °С (from ethyl acetate). IR spectrum, (thin layer), ν, cm−1 3319.95 (NH), 1640.73 (CO). NMR 1Н (CDCl3), δ, ppm: 0.69–0.7 m (2H, CH2), 0.95–1.01 m (2H,CH2), 1.07–1.15 m (2Н, 2СН), 1.24–1.26 m (2 Н, СН2), 1.30–1.33 m (2Н, СН2), 1.39–1.41 m (1 Н, СН), 1.47–1.50 m (1 Н, СН), 1.55–1.62 m (1Н, СН), 1.74–1.82 m (2 Н, 2СН), 1.89–1.92 m (1 Н, СН), 2.01–1.99 m (1Н, СН), 2.06–2.08 m (2Н, 2СН), 2.10–2.12 m (1Н, СН), 3.95–4.13 m (1Н.СНNH), 5.73 s (1Н, NH). NMR 13С, δ, ppm: 6.83 (С18), 6.97 (С19), 10.53 (С17), 14.90 (С4), 15.35 (С3), 15.67 35 (С5), 31.89 (С6), 32.43 (С14), 33.95 (С11), 34.75 (С12), 36.06 (С13), 37.13 (С7), 40.59 (С8), 41.54 (С2), 44.19 (С1), 37.09 (С11), 46.45 (С9), 56.36 (С10), 172.55 (СО). MS, m/z (Irel, %): 271.18 (2.1) [М]+, 270.18 (19.8), 269.18 (100). Found, %: С 80.35; Н 8.79; N 5.29. С18Н23NО. Calculated, %: С 80.26; Н 8.61; N 5.20. М 271.39).
Author Contributions
Conceptualization, I.R.R. and K.S.F.; methodology, K.S.F.; software, T.P.Z.; validation, T.P.Z.; formal analysis, A.V.A.; investigation, A.V.A.; resources, I.R.R.; data curation, T.P.Z.; writing—original draft preparation, K.S.F.; writing—review and editing, I.R.R.; visualization, A.V.A.; supervision, I.R.R.; project administration, K.S.F.; funding acquisition, T.P.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Russian Science Foundation, grant number 19–73-20128. The analytical part of the study was carried out within the framework of the state assignment of the Ministry of Education and Science (No. FMRS-2022-0076).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The results were obtained on unique equipment at the “Agidel” Collective Usage Center (Ufa Federal Research Center, Russian Academy of Sciences).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baum, J.C.; Milne, J.E.; Murry, J.A.; Thiel, O.R. An Efficient and Scalable Ritter Reaction for the Synthesis of tert-Butyl Amides. J. Org. Chem. 2009, 74, 2207–2209. [Google Scholar] [CrossRef] [PubMed]
- Norell, J.R. Organic Reactions in Liquid Hydrogen Fluoride. I. Synthetic Aspects of the Ritter Reaction in Hydrogen Fluoride. J. Org. Chem. 1970, 35, 1611–1618. [Google Scholar] [CrossRef]
- Mukhopadhyay, M.; Reddy, M.M.; Maikap, G.C. Cobalt(I1)-Catalyzed Conversion of Allylic Alcohols/Acetates to Allylic Amides in the Presence of Nitriles. J. Org. Chem. 1995, 60, 2670–2676. [Google Scholar] [CrossRef]
- Zhu, N.; Wang, T.; Ge, L.; Li, Y. γ Amino Butyric Acid (GABA) Synthesis Enabled by Copper-Catalyzed Carboamination of Alkenes. Org. Lett. 2017, 19, 4718–4721. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Yan, G.; Yin, J. Fe(ClO4)3*H2O-Catalyzed Ritter Reaction: A Convenient Synthesis of Amides from Esters and Nitriles. Synlett 2018, 29, 2257–2264. [Google Scholar]
- Huang, H.; Ji, X.; Xiao, F.; Deng, G.-J. Copper(I)/Lewis Acid Triggered Ring-Opening Coupling Reaction of Cyclopropenes with Nitriles. RSC Adv. 2015, 5, 26335–26338. [Google Scholar] [CrossRef]
- Vankar, Y.D.; Kumaravel, G.R. Ritter Reaction with Cyclopropyl Ketones and Cyclopropyl Alcohols: Synthesis of N-Actyl-γ-Keto and N-Acyl Homoallyl Amines. Synth. Commun. 1989, 19, 2181–2198. [Google Scholar] [CrossRef]
- Kutateladze, T.G.; Mironova, A.A.; Mochalov, S.S.; Shabarov, Y.S. Arylcyclopropanes in the Ritter Reaction. J. Org. Chem. USSR 1990, 26, 1908–1909. [Google Scholar]
- Khusnutdinov, R.I.; Egorova, T.M.; Khalilov, L.M.; Meshcheriakova, E.S.; Dzhemilev, U.M. Direct and stereoselective iron-catalyzed amidation of binor-S with alkyl and aryl cyanides in water. Synthesis 2018, 50, 1555–1559. [Google Scholar] [CrossRef]
- Khusnutdinov, R.I.; Egorova, T.M.; Aminov, R.I.; Mayakova, Y.Y.; Mescheryakova, E.S. Amidation of deltacyclene and hexacyclic norbornadiene dimers with acetonitrile and water catalyzed by FeCl3·6H2O. Synth. Commun. 2020, 50, 564–570. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).