Abstract
Obesity affects approximately 40% of U.S. adults and is associated with increased cardiometabolic risk. While lifestyle interventions remain fundamental, pharmacologic therapies such as Semaglutide and tirzepatide have demonstrated significant weight reduction in clinical trials when titrated to maintenance doses. However, real-world adherence to recommended titration schedules remains unclear. This retrospective observational study evaluated adults prescribed Semaglutide (Wegovy®) or Tirzepatide (Zepbound®) for weight management between January 2021 and April 2025 through ICUBAcares, a pharmacist-led call center. Primary outcomes included the proportion of patients reaching the recommended maintenance dose and time required to do so. Secondary outcomes examined prescriber specialty patterns and monthly plan costs for non-optimized dosing. Among 739 medication courses, 52.9% of Semaglutide users reached the 2.4 mg dose versus 77.6% of tirzepatide users reaching 15 mg (p < 0.001). Median time to maintenance was significantly shorter for tirzepatide (32 days) than Semaglutide (143 days) (p < 0.001). Endocrinologists had the highest success rate for Tirzepatide (88.2%), while family medicine had the highest volume for both. Non-optimized dosing was associated with higher estimated monthly plan costs. These findings underscore the importance of improving adherence to titration protocols in real-world settings to maximize both clinical and economic outcomes in obesity pharmacotherapy.
1. Introduction
Obesity is a chronic, complex disorder affecting approximately 40% of the U.S. adult population as of 2023, linked to heightened risks of cardiovascular disease, type 2 diabetes, and overall mortality [1]. While lifestyle adjustment is fundamental to weight management, it often does not achieve or sustain clinically meaningful weight reduction in many people [2]. Consequently, pharmacologic treatments have become a vital component of obesity management, especially for individuals requiring additional interventions to attain their health goals [3,4].
Semaglutide and tirzepatide, two incretin-based therapies approved by the Food and Drug Administration (FDA) for sustained weight management, are among the most promising pharmaceuticals [5,6]. Semaglutide, a glucagon-like peptide-1 receptor agonist (GLP-1 RA), facilitates weight loss by enhancing glucose-dependent insulin secretion, delaying gastric emptying, and reducing appetite via neurological mechanisms [4,7]. Tirzepatide is a novel dual agonist of glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 receptors [4,7]. It is more effective than GLP-1 RA alone in regulating appetite and energy consumption, resulting in more weight reduction [8,9]. In phase III clinical trials, both agents have demonstrated substantial efficacy in weight reduction [9,10]. In the STEP 1 and STEP 4 trials, patients treated with Semaglutide 2.4 mg once weekly achieved average weight reductions of 14.9% and 15.2% of baseline body weight, respectively, over 68 weeks [10,11]. Similarly, in the SURMOUNT-1 and SURMOUNT-2 trials, patients receiving tirzepatide at the 15 mg maintenance dose experienced reductions of up to 20.9% of baseline weight in those without diabetes [9,12]. These findings support maintenance-dose achievement as a clinically meaningful surrogate for effective weight reduction.
Both medications require a gradual, stepwise dose-escalation schedule to ensure safety and adherence, to minimize side effects, particularly gastrointestinal intolerance, and to reach their full therapeutic potential among individuals [9,10]. Commence Semaglutide at a dosage of 0.25 mg weekly, thereafter increasing the dosage by 0.25 mg every four weeks until achieving the goal dose of 2.4 mg within 16 to 20 weeks [10]. Tirzepatide is titrated from 2.5 mg to a maximum of 15 mg, with increments of 2.5 mg every four weeks [9]. These titration protocols are crucial not only for achieving a clinically meaningful surrogate of effective weight reduction and substantial improvements in cardiometabolic health but also for mitigating gastrointestinal adverse effects which are among the primary reasons for discontinuing medication [9]. Insufficient titration may result in subtherapeutic exposure and worse clinical outcomes [13].
Potent incretin-based therapy shows promise for the treatment of obesity along with reduced incidence of cardiovascular events in patients with preexisting cardiovascular disease and obesity [14]. The adverse events related to Semaglutide and Tirzepatide were primarily of mild-to-moderate severity and mostly gastrointestinal, which was more frequent during the dose-titration period and leveled off during the treatment period [14]. This emphasizes that once-weekly subcutaneous Semaglutide 2.4 mg and Tirzepatide 10 or 15 mg induce large reductions in body weight and waist circumference and are generally well-tolerated [14]. Trials of the Glucagon-like Peptide-1 Receptor Agonists (GLP1RAs) found mean weight losses of 15–21%, yet real-world dose titration and persistence remain suboptimal, limiting effectiveness [13]. GLP1RA persistence and dose-titration adherence were moderate but weight loss approximated that seen in clinical trials, supporting real-world effectiveness [13].
Real-world adherence to recommended titration schedules for Semaglutide and Tirzepatide is often suboptimal, influenced by factors such as limited patient follow-up, gastrointestinal adverse effects, insurance barriers, and provider variability [13,15]. Studies reveal that many patients fail to reach target maintenance doses—2.4 mg for Semaglutide and 15 mg for Tirzepatide—which in turn reduces treatment effectiveness and increases discontinuation rates [15,16]. This inadequate titration compromises clinical and economic outcomes by limiting the likelihood of achieving at least a 5% weight loss—a threshold linked to improvements in glycemic control, blood pressure, and cardiovascular risk [17]. As prescribing of GLP-1-based therapies expands beyond specialized obesity clinics [15], evaluating how current practice aligns with clinical trial-based titration recommendations is essential. This study explores real-world titration patterns and identifies gaps to guide optimization strategies that support improved long-term outcomes in obesity pharmacotherapy.
2. Materials and Methods
2.1. Study Design
This retrospective observational study utilizing pharmacy claims data (January 2021–April 2025) aims to evaluate the alignment of current clinical practice at NSU Health, a university health clinic, with the recommended titration schedules for Semaglutide and Tirzepatide in weight management.
2.2. Setting and Population
The study was conducted at NSU’s Barry and Judy Silverman College of Pharmacy through ICUBAcares, a pharmacist-led advocacy program where pharmacists act as liaisons between providers, patients, and insurance companies. The program operates within a call center that serves over 15,000 members by assisting with prior authorizations, formulary management, adherence monitoring, medication therapy management, and high-cost medication reviews, among other services.
2.3. Data Collection
Data were extracted from the pharmacy claims system and transferred into an Excel spreadsheet. The dataset included patients prescribed Semaglutide or Tirzepatide. All data were de-identified in compliance with HIPAA regulations before analysis.
2.4. Inclusion/Exclusion Criteria
Inclusion criteria were adults aged 18 years or older with pharmacy claims for Semaglutide (Wegovy®, Novo Nordisk, Plainsboro, USA) or Tirzepatide (Zepbound®, Eli Lilly, Indianapolis, IN, USA) prescribed specifically for weight management. Patients using Semaglutide (Ozempic®, Novo Nordisk, Plainsboro, NJ, USA) or Tirzepatide (Mounjaro®, Eli Lilly, Indianapolis, IN, USA) for type 2 diabetes were excluded, as the claims system differentiates by trademark name, allowing for identification of the indication.
2.5. Outcome Measures
The primary outcome of this study was to evaluate the proportion of members who achieved the recommended maintenance doses of Semaglutide (Wegovy® 2.4 mg) and Tirzepatide (Zepbound® 15 mg), as established in pivotal STEP and SURMOUNT clinical trials. Additionally, the time to reach maintenance dose was measured within the context of an academic call-center-based clinical setting.
The secondary outcomes included the costs paid by insurance for those who did not achieve the recommended doses (i.e., non-optimized dosing), as well as prescribing patterns and maintenance dose achievement stratified by health provider specialty.
2.6. Statistical Analysis
Descriptive statistical analyses, including frequencies and relative frequencies, were performed to summarize patient characteristics such as gender, the proportion of patients who achieved the maintenance dose by provider specialty. Mean and standard deviation were used to report monthly plan costs among those who did not reach the maintenance dose. Median time to maintenance dose achievement was described based on Kaplan–Meier method. Comparisons between Semaglutide and Tirzepatide in achieving maintenance doses were assessed using the log-rank test, with statistical significance defined as a p-value < 0.05. All statistical analyses were performed by using SAS 9.4.
3. Results
A total of 662 patients were included in the study sample. These individuals were prescribed Semaglutide (46.7%), Tirzepatide (41.7), or both (11.6%) at different times, resulting in 739 total medication courses. The higher number of medication courses reflects patients who switched between medications during the study period. This 11.6% (n = 77) represents patients who received prescriptions for both semaglutide and tirzepatide during the study period. While the reasons for switching therapies could not be determined from claims data, this group may reflect patients with tolerability challenges or suboptimal response. All patients included in the study were adults aged 18 years or older, with 63% identifying as female and 37% as male (Table 1). Among patients prescribed Semaglutide (n = 386), 64% (n = 248) were female and 36% (n = 138) were male. Similarly, among those prescribed Tirzepatide (n = 353), 64% (n = 229) were female and 36% (n = 124) were male (Table 1).

Table 1.
Sex distribution of the study sample by drug.
Among patients prescribed these medications, 52.9% of those on Semaglutide reached the recommended maintenance dose, compared to 77.6% on Tirzepatide (Table 2). The median time to reach maintenance dose was significantly longer with Semaglutide (143 days; 95% CI, 119–173) than with Tirzepatide (32 days; 95% CI, 30–38), also with p < 0.001 (Table 2).

Table 2.
Maintenance dose achievement in patients using Semaglutide vs. Tirzepatide.
The estimated total monthly cost to the plan for patients who achieved the recommended maintenance dose was approximately $1059 for Tirzepatide and $1330 for Semaglutide. For patients who did not reach the maintenance dose, the monthly cost was substantially higher, totaling $83,705 for Tirzepatide and $242,133 for Semaglutide (Table 3).

Table 3.
Monthly cost paid by insurance among patients not achieving maintenance dose during the study period after using Semaglutide or Tirzepatide.
Overall, family medicine practitioners accounted for the largest proportions of prescriptions of Semaglutide (47.9%) and Tirzepatide (50.7%) (Figure 1). Figure 2 presents the proportions of patients who achieved the recommended maintenance dose stratified by prescriber specialty. Among Semaglutide users, 55.7% achieved the maintenance dose under the care of family medicine, followed by internal medicine in endocrinology (52%). Among Tirzepatide users, 88.2% achieved the maintenance dose under the care of internal medicine in endocrinology, followed by family medicine (72.6%) and primary care (66.7%). It should be noted that while all Tirzepatide users under the care of internal medicine in cardiology achieved the maintenance dose, the small sample size for this specific group suggests the results may not fully represent the broader study sample.
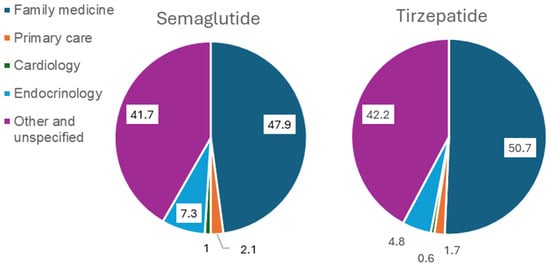
Figure 1.
Prescribing patterns of semaglutide and tirzepatide by specialty.
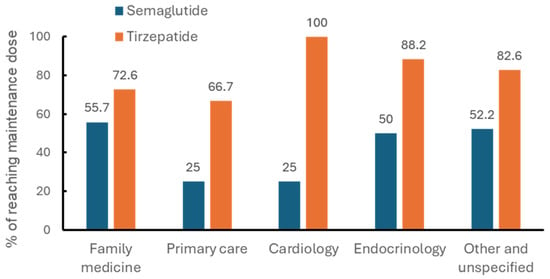
Figure 2.
Percentages of maintenance dose achievement by prescriber specialty in patients using semaglutide or tirzepatide.
4. Discussion
This study provides real-world evidence on titration patterns and maintenance dose achievement for Semaglutide (Wegovy®) and Tirzepatide (Zepbound®) within an academic call-center-based setting. A significantly higher proportion of Tirzepatide users (77.6%) achieved the guideline-recommended maintenance dose compared to Semaglutide users (52.9%), with Tirzepatide patients also reaching maintenance more quickly (median: 32 vs. 143 days; p < 0.001).
These findings align with pivotal clinical trials but also reveal important real-world adjustments. In the STEP 1 trial, Semaglutide patients reached the 2.4 mg maintenance dose by week 16 (~112 days) and achieved ~14.9% mean weight loss over 68 weeks [10], while in the SURMOUNT-1 trial, Tirzepatide patients completed a 20-week dose-escalation schedule (~140 days) to reach the 15 mg maintenance dose and achieved up to 20.9% weight loss over 72 weeks [9]. In contrast, our real-world data showed that Tirzepatide users reached the maintenance dose in a median of 32 days, whereas Semaglutide users required a median of 143 days—substantially longer than recommended. This observation aligns with findings from Rodriguez et al., who reported in a real-world comparative study that Tirzepatide was associated with significantly greater weight loss and faster treatment effect than Semaglutide, highlighting the need to investigate underlying barriers to timely titration in practice [18] (Figure 3).

Figure 3.
Time to maintenance dose achievement (days) in patients using semaglutide or tirzepatide: real-world study vs. pivotal trials.
Prescriber specialty also influenced titration outcomes. Family medicine providers accounted for most prescriptions, yet endocrinologists achieved the highest success rates for Tirzepatide, with 88.2% of patients reaching target dosing. This may highlight the benefit of specialized knowledge in optimizing titration strategies for newer anti-obesity therapies [13,14].
Economic implications were notable. While weight loss outcomes were not available in our dataset to confirm full therapeutic benefit, patients who did not reach maintenance dosing incurred substantial costs, underscoring the importance of dose optimization. Although these patients had lower per-patient medication costs, they may still have achieved health benefits that were not captured in our analysis. Future studies should examine whether suboptimal titration reduces the need for other pharmacotherapies or impacts overall healthcare utilization. Non-optimized Semaglutide therapy exceeded $240,000 per month (≈$1330 per patient), while Tirzepatide cost over $83,000 monthly (≈$1059 per patient). These findings underscore the importance 188 of adherence to structured titration protocols to maximize both clinical benefit and cost- 189 effectiveness [15,16].
Our findings support the need for structured provider education, system-level protocols, and pharmacist-led titration management—especially in telehealth or call-center-based models of care. Embedding automatic reminders, managing side effects proactively, and reducing insurance-related barriers to dose escalation may help reduce therapeutic inertia. Additionally, care coordination and patient education should be enhanced to support adherence.
Future research should examine whether consistent, timely titration correlates with improved long-term clinical outcomes such as weight loss durability, glycemic control, and medication persistence. Investigations should also assess the roles of provider training, delivery models, and patient engagement in optimizing titration strategies across diverse clinical settings.
5. Limitations
The most notable limitation of this study is the inability to assess actual weight loss outcomes, as individual patient weight data were not available. Therefore, achievement of maintenance dosing was used as a surrogate marker for treatment efficacy. While this approach is supported by clinical trial data linking maintenance dose to ≥5% weight reduction, individual variability in response must be acknowledged. Future studies should incorporate objective weight data to directly assess clinical outcomes and validate the use of dosing achievement as a reliable proxy. Finally, our study sample included patients switching between Semaglutide and Tirzepatide. The two treatment groups were not fully independent when comparing the time to the recommended maintenance dose. Additionally, while our discussion references real-world findings supporting delayed titration with Semaglutide, our study did not assess specific causes for these delays, which remains a limitation.
6. Conclusions
In this real-world analysis, Tirzepatide was associated with faster titration and higher rates of maintenance dose achievement compared to Semaglutide, translating into lower estimated costs when titration was completed. However, delays observed with Semaglutide may reflect real-world barriers such as tolerability, access, or provider practices rather than inherent drug limitations. Optimizing titration strategies for both therapies remains essential to maximize clinical benefit and economic value in obesity pharmacotherapy.
Author Contributions
Conceptualization, G.A. and L.V.; methodology, G.A., L.V. and J.W.; software, J.W.; validation, L.V., S.M. and D.P.; formal analysis, J.W.; investigation, L.V.; resources, G.A.; data curation, J.W.; writing—original draft preparation, G.A.; writing—review and editing, S.M., D.P. and J.W.; supervision, G.A.; project administration, G.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Nova Southeastern University (protocol code: [2025, 202], date of approval: [10 April 2025]).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study using de-identified data.
Data Availability Statement
The data utilized in this study were obtained through the ICUBAcares pharmacist-led advocacy program and contain sensitive health information protected under privacy regulations. Due to these ethical and confidentiality restrictions, the data are not publicly available. Access is limited to authorized personnel affiliated with ICUBA and Nova Southeastern University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Emmerich, S.; Fryar, C.; Stierman, B.; Ogden, C. Obesity and Severe Obesity Prevalence in Adults: United States, August 2021–August 2023; National Center for Health Statistics (U.S.): Hyattsville, Maryland, 2024. Available online: https://stacks.cdc.gov/view/cdc/159281 (accessed on 6 November 2025).
- Kushner, R.F.; Ryan, D.H. Assessment and Lifestyle Management of Patients with Obesity: Clinical Recommendations From Systematic Reviews. JAMA 2014, 312, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Apovian, C.M.; Aronne, L.J.; Bessesen, D.H.; McDonnell, M.E.; Murad, M.H.; Pagotto, U.; Ryan, D.H.; Still, C.D. Pharmacological Management of Obesity: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2015, 100, 342–362. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, O.; Gulati, M.; Gluckman, T.J.; Kittleson, M.M.; Rikhi, R.; Saseen, J.J.; Tchang, B.G. 2025 Concise Clinical Guidance: An ACC Expert Consensus Statement on Medical Weight Management for Optimization of Cardiovascular Health. J. Am. Coll. Cardiol. 2025, 86, 536–555. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. FDA Approves First Treatment to Reduce the Risk of Serious Heart Problems Specifically in Adults with Obesity or Overweight. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-reduce-risk-serious-heart-problems-specifically-adults-obesity-or (accessed on 6 August 2025).
- U.S. Food and Drug Administration. FDA Approves New Medication for Chronic Weight Management. 2023. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-medication-chronic-weight-management (accessed on 6 August 2025).
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Frías, J.P.; Davies, M.J.; Rosenstock, J.; Pérez Manghi, F.C.; Fernández Landó, L.; Bergman, B.K.; Liu, B.; Cui, X.; Brown, K. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Rubino, D.; Abrahamsson, N.; Davies, M.; Hesse, D.; Greenway, F.L.; Jensen, C.; Lingvay, I.; Mosenzon, O.; Rosenstock, J.; Rubio, M.A.; et al. Effect of Continued Weekly Subcutaneous Semaglutide vs. Placebo on Weight Loss Maintenance in Adults With Overweight or Obesity: The STEP 4 Randomized Clinical Trial. JAMA 2021, 325, 1414. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.T.; Frias, J.P.; Jastreboff, A.M.; Le Roux, C.W.; Sattar, N.; Aizenberg, D.; Mao, H.; Zhang, S.; Ahmad, N.N.; Bunck, M.C.; et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): A double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2023, 402, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Samuels, J.M.; Ye, F.; Irlmeier, R.; Silver, H.; Srivastava, G.; Spann, M. Real-world titration, persistence & weight loss of semaglutide and tirzepatide in an academic obesity clinic. Diabetes Obes. Metab. 2025, 27, 6200–6209. [Google Scholar] [CrossRef] [PubMed]
- Müllertz, A.L.O.; Sandsdal, R.M.; Jensen, S.B.K.; Torekov, S.S. Potent incretin-based therapy for obesity: A systematic review and meta-analysis of the efficacy of semaglutide and tirzepatide on body weight and waist circumference, and safety. Obes. Rev. 2024, 25, e13717. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, R.W.; Mailhac, A.; Løhde, J.B.; Pottegård, A. Real-world evidence on the utilization, clinical and comparative effectiveness, and adverse effects of newer GLP-1RA-based weight-loss therapies. Diabetes Obes. Metab. 2025, 27, 66–88. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.J.; Goodwin Cartwright, B.M.; Gratzl, S.; Brar, R.; Baker, C.; Gluckman, T.J.; Stucky, N.L. Comparative Effectiveness of Semaglutide and Tirzepatide for Weight Loss in Adults with Overweight and Obesity in the US: A Real-World Evidence Study. MedRxiv 2023. [Google Scholar] [CrossRef]
- Brown, J.D.; Buscemi, J.; Milsom, V.; Malcolm, R.; O’Neil, P.M. Effects on cardiovascular risk factors of weight losses limited to 5–10%. Transl. Behav. Med. 2016, 6, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.J.; Goodwin Cartwright, B.M.; Gratzl, S.; Brar, R.; Baker, C.; Gluckman, T.J.; Stucky, N.L. Semaglutide vs. Tirzepatide for Weight Loss in Adults With Overweight or Obesity. JAMA Intern. Med. 2024, 184, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).