Abstract
The fish fauna of Rio de Janeiro has been extensively studied, resulting in a comprehensive database of species collected over more than three centuries. This study aimed to provide a checklist of species, to identify patterns of diversity and the distribution of freshwater ichthyofauna, to delineate biogeographic units, and to explore changes in faunal composition among different areas. Analyzing data from ichthyological collections and the literature on original species descriptions revealed 206 freshwater fish species: 183 native and 23 allochthonous. The checklist includes updated species names. The sampling effort in Rio de Janeiro is extensive, especially in coastal lowlands. The findings indicate that inventory work is still needed in some areas, particularly within the Rio Paraíba do Sul basin. Seven bioregions of freshwater ichthyofauna were identified, including a major region of higher species richness and smaller areas with higher endemism of restricted-range species. This biogeographic assessment underscores the diverse and distinctive freshwater fish fauna in the basins of Rio de Janeiro, with well-defined biogeographic units.
1. Introduction
The state of Rio de Janeiro possesses unique characteristics of the Atlantic Forest biome, comprising a diverse array of ecological niches shaped by proximity to the coast, varied relief, soil types, and rainfall regimes. In the mountainous areas, the Rio Paraíba do Sul stands out to the west in its middle courses, embedded in the Serra da Mantiqueira, and in its lower course towards the coast. To the east, smaller coastal rivers descend the slopes of the Serra do Mar. The coastal region is distinguished by sandbanks, dunes, mangroves, swampy forests, ponds, and swamps. In the central and southern portions of Rio de Janeiro, bays occupy the coastal lowlands, notably Guanabara Bay, Sepetiba Bay, and Ilha Grande Bay. The remaining rivers and streams regulate water flow, ensure soil fertility, control the climate, and protect escarpments and mountain slopes. In the eastern and northern coastal regions, lake systems dominate the landscape, with many lagoons, such as Maricá, Saquarema, Araruama, and Feia [1]. The significant variety of environments, combined with the complex geomorphological history of this region, have driven the evolution of a rich and diverse biota, encompassing both forest and adjacent aquatic systems.
The fish fauna of Rio de Janeiro has been extensively studied, initially by foreign naturalists, such as Johann von Spix, Johann Natterer, Carl von Martius, Louis Agassiz, Francis de Castelnau, Jean René Quoy, Joseph Gaimard, Charles Hartt, Edward Copeland, John Hasemann, and George Myers, and further in the studies of the Brazilian naturalists Alípio de Miranda Ribeiro, Paulo de Miranda Ribeiro, and Haroldo Travassos up until the mid-20th century [1]. With the establishment of the Museu Nacional as a referential research center, holding one of the largest ichthyological collections in Latin America, along with other institutions established later, ichthyological research in the state of Rio de Janeiro increased considerably. Numerous studies have been conducted, including taxonomic papers (e.g., [2,3,4,5,6,7,8,9,10,11,12,13]), inventories (e.g., [1,14,15,16,17,18,19,20,21]), and studies in ecology and other fields (e.g., [22,23,24,25,26,27,28,29,30]). The collective effort of many researchers over three centuries resulted in the creation of a robust database of species records and environmental data in museums and university collections.
However, there is a gap of comprehensive and cohesive information. Investigating the fish species inhabiting each region, consolidating scattered information, and making it easily accessible is now more indispensable than ever, particularly for disseminating and valuing freshwater ecosystems in the face of rapid climate change. Regional species lists are important to identify priority areas for future studies, to point out taxonomic gaps and putatively undescribed species, and to generate estimates on the number of existing species and their geographic distributions [31]. In this context, this study highlights the rivers of Rio de Janeiro, identifies the respective basins and fishes in a territorial context, and provides the first comprehensive framework of spatial biodiversity patterns for the freshwater fish fauna of the state.
2. Materials and Methods
2.1. Study Area
The state of Rio de Janeiro covers an area of approximately 43,750 km2, inserted in the Atlantic Forest biome and presenting a great diversity of phytophysiognomies, climates, and relief, in addition to being home to the second highest population density in the country [32]. The relief of the state is intersected by mountain ranges that generate and divide the hydrographic basins of this region (Figure 1). The state is bordered in its northern and western regions by the Paraíba do Sul River and in part by the Serra da Mantiqueira, which separates the middle and lower stretches of the Paraíba do Sul River basin from the Upper Rio Paraná basin, in São Paulo, and the Rio Doce basin, in Minas Gerais. The Rio Paraíba do Sul basin is separated from the other coastal basins of Rio de Janeiro by the Serra do Mar, a crystalline formation from which several Atlantic drainages emerge that cut through the state, flowing into lowland areas [33].
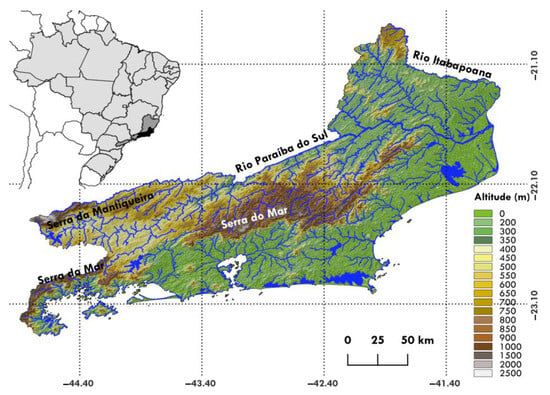
Figure 1.
Hypsometric map of the state of Rio de Janeiro, illustrating its main mountain formations and its hydrographic network. In detail, the location of the state (black) in the Southeast Atlantic Hydrographic Region (dark gray) and in Brazil (light gray).
Rio de Janeiro is part of the Southeast Atlantic Hydrographic Region, comprising river basins flowing into the Atlantic Ocean on southeastern portion of the country. The hydrographic area extends from the Rio Doce basin, in the states of Espírito Santo and Minas Gerais, to the Rio Ribeira do Iguape basin, in the state of Paraná. The hydrographic region is bordered to the west by the hydrographic regions of the São Francisco and Paraná. Each river basin in Rio de Janeiro is identified by a number between 20 and 39.
The state of Rio de Janeiro is divided into nine hydrographic regions—RH-1 to RH-9—representing a political division for territorial governance (Figure 2A). The river basins within Rio de Janeiro are categorized into 24 groups (Figure 2B). Of these, 5 belong to the Rio Paraíba do Sul basin, while 19 are coastal basins and micro basins located on the eastern slope of the Serra do Mar and within coastal lagoon systems (Figure 2B).
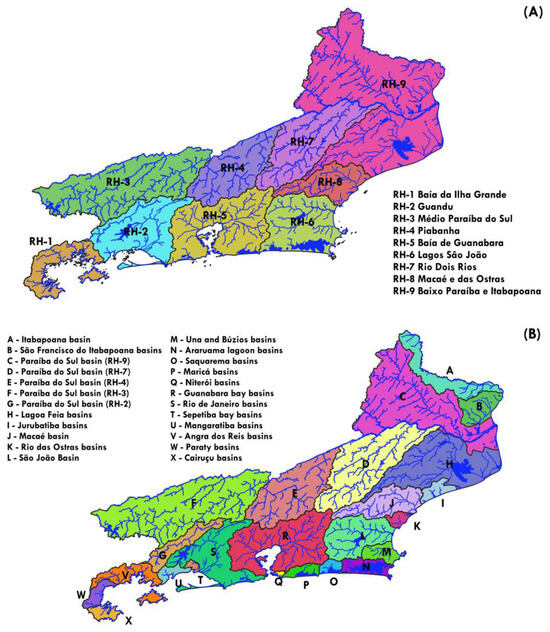
Figure 2.
The 9 hydrographic regions (A) and the 24 river basin groups (B) in the state of Rio de Janeiro.
2.2. Species Data
Available records from fish collections in Rio de Janeiro were reviewed and their identifications confirmed. The coordinates for each sampling point were estimated from the locations provided in the records. Initially, a curatorial survey of data was conducted using the fish collections at the Museu Nacional (MNRJ) and the Museu de Biologia Mello Leitão (MBML). Additionally, records from the SpeciesLink database at the Centro de Referência em Informação Ambiental (CRIA) were consulted, along with the literature on species descriptions. Data were compiled from the ichthyological collections of AMNH, ANSP, BMHN, BMNH, CAGC, CAS (including CAS-SU), CICCAA, DZSJRP, FMNH, INPA, MCP, MCZ, MHNG, MTD, MZUEL, MZUSP, NPM, NRM, UF, UFMT, UFRGS, UFRJ, UFRN, UMMZ, UMZC, UNT, USNM, ZFMK, ZMH, and ZUEC (Supplementary File—Table S1). Institutional acronyms are detailed in [34]. The records were georeferenced and plotted on a map of the state of Rio de Janeiro to ensure their accuracy. A total of 13,585 lots were inventoried (Supplementary File—Table S2). Taxonomic classification follows [35].
Some species discussed here have complex taxonomy with unresolved phylogenies, and possibly represent species complexes. For analytical and inventory purposes, these species are considered as single taxa. Rhamdia quelen has recently been redescribed, and its distribution is now restricted to coastal basins from Rio de Janeiro south to the Rio Tubarão basin in the state of Santa Catarina [36]. Ref. [37] describes seven subspecies of Gymnotus carapo for South American basins and suggests that the species appears to be absent from the coastal drainages of Northeastern and Southeastern Brazil, despite several records tentatively identified as G. carapo, G. aff. carapo, or G. cf. carapo in museum collections and databases. Hoplias malabaricus and Eigenmannia virescens likely represent species complexes that require revision [38,39,40]. Additionally, we adopt the name Synbranchus sp. as recommended by group specialists ([41], T. Roberts, pers. comm.).
2.3. Geographic Data
In the process of georeferencing and verifying the accuracy of coordinates for each sampling point, the originally provided values were initially used. When a small discrepancy was found between the reported value and the indicated locality data, the value was adjusted according to the locality. In cases where there was a significant discrepancy between the indicated coordinates and the locality, or when the coordinates were unavailable, the coordinates were estimated based on the available information.
The hydrographic maps were adapted for use in TrackMaker software version 5.1 [42], starting with the 1:25,000 resolution provided by the Instituto Brasileiro de Geografia e Estatística (IBGE) [43]. For suggested locality data (in the absence or discrepancy of provided coordinates), information was used according to the river names in this version, supplemented by names from the IBGE 1:50,000 topographic maps. Municipality areas were calculated using IBGE data [44].
2.4. Sampling Coverage Assessment
The sampling coverage in the state was assessed by calculating the total lots index (il) and the sampling points index (ip) per 100 km2. These indices were computed for each municipality, hydrographic region, and group of basins, and the results were compared with the index for the entire state [45]. Sampling quality was considered average when within about 30%, poor when significantly below 30%, and good when above 30% (for both il and ip). We assessed the sampling quality for the nine hydrographic regions, twenty-nine river basin divisions, and by municipality.
2.5. Biogeographic and Diversity Patterns
We applied the constancy index [46] to determine which species are constant over time, calculated as C = n/N × 100, where n is the number of collection points where the species was captured and N = total number of collection points. Based on the results obtained, each species was classified as follows: constant if C > 50%; accessory if C ranges between 25% and 50%; and accidental if C < 25%.
The diversity patterns of each hydrographic region (alpha-diversity) were assessed by calculating the indices of absolute richness, Shannon diversity, equitability, and dominance, using species abundance data for each region [47]. To evaluate differences between communities (beta-diversity or species turnover) among hydrographic regions, we applied non-metric multidimensional scaling (NMDS) using a binary matrix and the Jaccard dissimilarity index, with 100 trials. These analyses were conducted using PAST software version 4.17 [48].
Since determining the total number of species in an area is virtually impossible—especially in regions with high species richness—estimators are useful for extrapolating observed richness and estimating total richness from an incomplete sample of a biological community [49]. Consequently, we employed several diversity estimators to assess the completeness of species sampling for the hydrographic regions, as follows: the Chao 1 index: A simple estimator of the absolute number of species in a community, based on the number of rare species within a sample [50,51]; the iChao index: An improved version of the Chao 1 index, offering greater precision in the evaluation of results [52]; the AC estimator (abundance-based coverage estimator): This method uses the abundance of rare species (i.e., species with low abundance) [53,54]. Unlike the previous estimators, the AC estimator allows for setting thresholds to define what constitutes a rare species. Generally, species with an abundance of 1 to 10 individuals are considered rare, and the estimated richness can vary depending on the abundance threshold chosen. Unfortunately, there are no defined biological criteria for selecting the optimal range. Additionally, we used the squares index, a richness estimator designed to be more accurate than Chao 1 when abundance distributions are uniform [55].
Only records of native freshwater species were considered for the following spatial assessments. We executed a data cleaning routine using the Coordinate Cleaner package [56] in the R software v. 4.3 [57], removing duplicated coordinates for each species, to avoid pseudoreplication, which can produce distorted results. A bioregionalization analysis was performed using the Infomap Bioregions 2 algorithm [58], to subdivide the state of Rio de Janeiro into smaller biogeographical units that reflect the distribution patterns of species. This algorithm uses species distribution data, even in cases of inconsistent sampling efforts. It employs an adaptive resolution method that generates a bipartite network of species and grids, followed by a clustering analysis to create bioregions based on the presence of specific taxa. A wide range of scaling parameters were tested, and the most consistent results were obtained with the following scenario: cell size ranging from 1/16° to 1/2°, cell capacity ranging from 1 to 300 samples, and 100 trials. The remaining settings followed the program defaults. A cluster graph using the binary matrix resulting from the bioregionalization algorithm (inspected to correct possible inconsistencies in the distribution of species) was produced to verify the similarity in faunal compositions among the bioregions generated, employing the UPGMA algorithm and Jaccard’s similarity coefficient.
A species richness interpolation (SRI) analysis was performed to map diversity patterns using the spline interpolation method, which smooths out potential sampling gaps by creating a continuous surface of data values, in the BioDinamica model [59] of the Dinamica-EGO software v. 7.8 [60]. The following parameters were used: kriging interpolation model = Gaussian, raster grid size = 0.02, hexagon size = 0.2 (20 km), and a minimum of one sample per hexagon.
We applied the weight endemism index (WEI), which combines endemism and species richness to identify areas with high endemicity of restricted-range species [61,62]. The following parameters were used: kriging interpolation model = Gaussian, raster grid size = 0.02, hexagon size = 0.2 (20 km), and a minimum of one sample per hexagon, in the BioDinamica model [59].
3. Results
The freshwater environments of Rio de Janeiro encompass 206 species (183 native and 23 allochthonous) (Table A2), distributed across 10 orders, 33 families, 42 subfamilies, and 102 genera.
3.1. Rio de Janeiro According to the Collections—Diversity in Numbers
Lots sampled. A total of 19,770 lots from the collections were recorded. Among these, 4466 (22.59%) were from the oceanic area. Additionally, 379 fish records (1.92%) were not identified at the species level. The 4466 marine lots, 1340 estuarine lots, and 379 lots of unidentified material were not evaluated in the present study but are available in the Supplementary Material. The remaining fish lots were considered for analysis (Table 1).

Table 1.
Lots sampled in the state of Rio de Janeiro.
Of the 13,585 lots sampled from continental waters of the state of Rio de Janeiro, 533 (2.7%) are from allochthonous species and 13,052 (66.02%) are from native species. The available species names were updated to include only valid names. A detailed revisionary study of fish species in the territory is beyond the scope of this research.
Regarding the geographical accuracy of the 13,585 freshwater records, 4012 had their coordinates provided correctly, 5527 coordinates were adjusted, and 4046 coordinates were estimated based on locality data. This estimation considered factors, such as historical pathways, available roads, and access to sampling sites, particularly for older records deposited in collections before the advent of georeferencing systems.
Sampling index. The average sampling index for deposited lots of freshwater fish in the state of Rio de Janeiro was 31.0 per 100 km2. The average index of sampling points on the continent was 4.2 per 100 km2
Three hydrographic regions were adequately sampled, considering both indices (Table 2). In contrast, two regions exhibit poor sampling quality. Four other regions have sampling quality that ranges from good to poor, depending on the index considered. Overall, the regions with lower sampling quality mainly correspond to the Paraíba do Sul valley, especially in its lower portion. See discussion for more detail.

Table 2.
Quality of sampling per hydrographic region.
Among the 92 municipalities in Rio, 27 have a good number of lots (Table A1), 11 are within the state average, and 54 have a poor number of lots. Regarding sampling points, 27 municipalities have a good number of points, 12 are within the state average, and 53 have a poor number of sites (Figure 3B). Nine municipalities (Areal, Arraial do Cabo, Belford Roxo, Macuco, Miracema, Nilópolis, Porciúncula, São João de Meriti, and Varre-Sai) do not have any records of freshwater species in the ichthyological collections. Arraial do Cabo only has records of marine species.
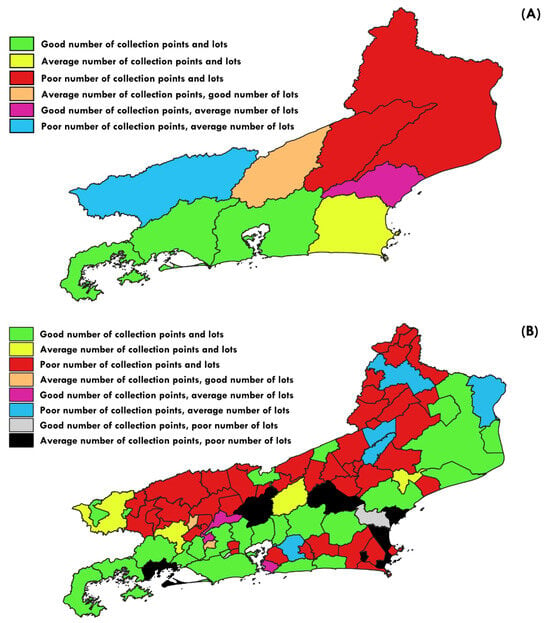
Figure 3.
Quality of sampling per hydrographic regions (A) and municipalities (B). Green = good quality of both indices; yellow = average quality; red = poor quality.
Eleven basin groups have a good number of deposited lots, two are within the state average, and another eleven ones show poor quality in this index (Table 3). Twelve basins have a good number of sampling points, four are within the state average, and eight have poor coverage in this aspect.

Table 3.
Sample quality per basin group.
Temporal variation. The earliest records deposited in ichthyological collections in this assessment date back to 1832. From 1980 onwards, the collections experienced significantly higher growth compared to the entire preceding period, with continuous increases since 2000, especially in the 2010s (Figure 4). The historical reasons behind the rise in deposition numbers within the territory are beyond the scope of this contribution.
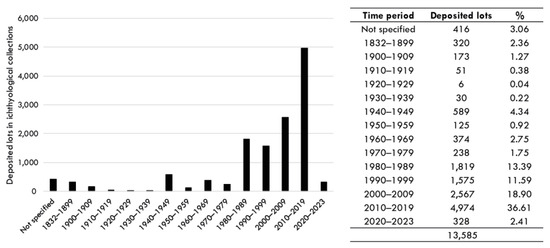
Figure 4.
Temporal variation in the cataloging of fish records in museum collections.
Taxonomic Diversity
Native species. Siluriformes (94 spp., 51.4%), Characiformes (43 spp. 23.5%), and Cyprinodontiformes (31 spp., 16.9%) are the most species-rich orders among the native species recorded in Rio de Janeiro (Figure 5A). The most representative families of the total (Figure 5B) are Loricariidae (36 spp., 19.7%), Trichomycteridae (36 spp., 19.7%), Characidae (24 spp., 13.1%) and Rivulidae (23 spp., 12.6%). Trichomycterus (24 species), Characidium, and Deuterodon (7 species) are the richest genera. A special mention is given to two species in the metropolitan area of Rio de Janeiro that were not included in our assessment. Astyanax scabripinnis (Jenyns 1842) is a species restricted to the state of Rio de Janeiro. It was first described based on a single specimen collected by Charles Darwin during his stay in the city of Rio de Janeiro from April to July 1832, according to Jenyns (1842) (holotype BMNH 1917.7.14.15, Rio de Janeiro). The region explored by Darwin included the small drainages flowing into Guanabara Bay. Two centuries later, ref. [63] revised the Astyanax species from the Serra dos Órgãos, a region of rivers draining towards Guanabara Bay, but did not find any populations that could be clearly identified as A. scabripinnis, raising the possibility of species extinction. Although Astyanax scabripinnis is currently considered part of a species complex, the nominal species Astyanax scabripinnis is possibly extinct [63,64]. Another peculiar situation involves Harttia rhombocephala, known from a single specimen collected in the Rio Farias, a small creek that is a tributary of Guanabara Bay (holotype MNRJ 712). This river is a tributary of the Rio Jacaré, within the Guanabara Bay hydrographic region [65]. Currently, these rivers suffer from severe anthropogenic impacts, as they are located in the densely populated metropolitan area of Rio de Janeiro.
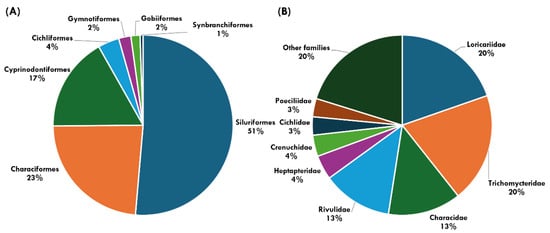
Figure 5.
Taxonomic representativeness of freshwater fish orders (A) and families (B) of Rio de Janeiro state.
Allochthonous species. Characiformes and Cichliformes account for more than 70% of non-native species resulting from intentional or accidental introductions due to aquarism, biological control, and fish farming. Poecilia reticulata, native to northern South America and the Caribbean, is the most abundant non-native species in the rivers of the state, widely distributed across various basins, followed by Coptodon rendalli, originating from Southern–Central Africa, and Hyphessobrycon eques, native to the Amazon Basin and the Rio Paraguay [35]. Rio de Janeiro has a long history in the aquarium trade in Brazil [66]. Since the 1940s, aquarium keepers have engaged in commerce with European markets. Some of these freshwater aquarium fish have been deposited in ichthyological collections [67]. The early aquarist teams contributed significantly to the knowledge of natural biodiversity [66] and left behind specimens of other common aquarium fish in museum collections, most of which are alien to the native fauna of Rio de Janeiro. More recently, collections have received records of fish introduced for commercial purposes and those documented during environmental impact assessments conducted by various enterprises.
Constancy index. A total of 1854 unique sampling points were identified in the state of Rio de Janeiro. No species was classified as constant, and only Geophagus brasiliensis appeared as an accessory species, being recorded in 555 points (29.9%). The others were all accidental, such as Poecilia vivipara in 319 sites (17.2%) and Phalloceros harpagos in 208 sites (11.2%).
Non-parametric estimation and diversity indices. The largest variations in species richness indicated by the non-parametric estimators (Table 4) are related to the Médio Paraíba do Sul hydrographic region (RH-3) (ranging from 6.5% to 19.5%), followed by the Rio Dois Rios hydrographic region (RH-7) (1.8% to 12.5%) and the Lower Paraíba do Sul and Itabapoana hydrographic region (RH-9) (5.8% to 11.1%). All regions with higher variations in species richness estimators are associated with the Paraíba do Sul River basin.

Table 4.
Parametric and non-parametric diversity estimation indices per hydrographic region in the state of Rio de Janeiro.
The Guandu hydrographic region (RH-2) exhibited the highest species richness within the state, with 110 species, closely followed by the Guanabara Bay hydrographic region (RH-5), with 109 species. The lowest richness was observed in the Baía da Ilha Grande hydrographic region (RH-1), with 46 species. The RH-3 and Macaé and das Ostras hydrographic region (RH-8) each had 82 species, the Piabanha hydrographic region (RH-4) had 81 species, and RH-7 had 80 species, all showing similar levels of richness. The Shannon index indicates the greatest diversity in RH-7 (H = 3.60), followed by RH-5 (H = 3.57). The lowest diversity was recorded in RH-8 (H = 2.62).
The greatest species richness in RH-2 (110 spp.) is linked to the fact that this hydrographic region, encompassing coastal rivers, is connected to the Rio Paraíba do Sul through a river transfer system with the Rio Guandu basin, which supplies water to the city of Rio de Janeiro. In contrast, the lowest species richness in RH-1 (46 spp.) is related to the geographic characteristics of this region, where the Serra do Mar is very close to the coast. The habitats in RH-1 are characterized either by steep rapids or estuarine plains.
The beta-diversity NMDS plot (stress = 0.078, Figure 6) was able to consistently discriminate three clusters of hydrographic regions in the state: Lower Paraíba do Sul basin (RH-4, 7, and 9), coastal drainages in the oceanic slope of Serra do Mar (RH-2, 5), and basins of the Lagos region (RH-6 and 8). The Ilha Grande (RH-1) and the Middle Paraíba do Sul (RH-3) regions stood out as outliers.
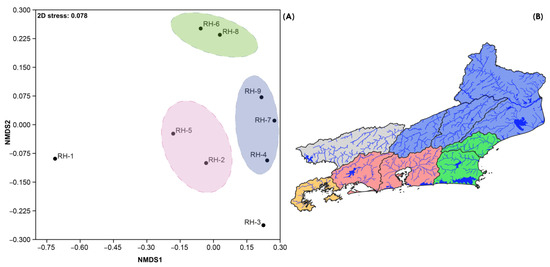
Figure 6.
Beta-diversity NMDS ordination plot (A) and the clusters of hydrographic regions discriminated by the analysis (B).
Although Rio de Janeiro is well-sampled within the Atlantic Forest, the sampling is not uniform. Quality indices for the sampled lots are good for areas ranging between 20% and 32% of the state (depending on the division by municipalities, basins, or hydrographic regions). In contrast, between 11% and 18% of the state area has average sampling quality, while between 55% and 60% of the state presents poor sampling quality.
3.2. Spatial Patterns of Distribution
Seven bioregions have been delineated based on patterns of distribution of the freshwater fish fauna (Figure 7A). The delineated bioregions bear similarities to the configuration generated by the beta-diversity analysis. These areas are herein referred to as Lower Rio Paraíba do Sul bioregion, Middle Rio Paraíba do Sul I and II bioregions, Lagos-São João bioregion, Lagos-Saquarema bioregion, Guanabara-Sepetiba bioregion, and Costa Verde bioregion.
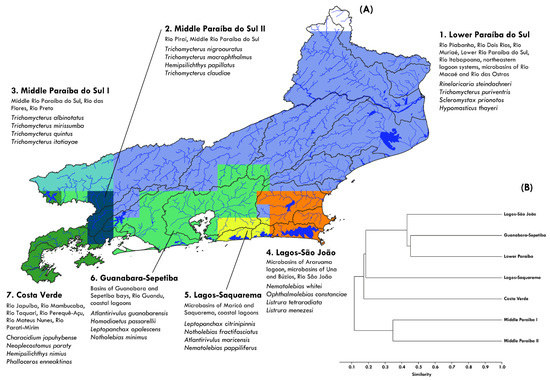
Figure 7.
Bioregions of the freshwater icthyofauna of Rio de Janeiro (A) and Jaccard similarity among these areas (B).
- Lower Rio Paraíba do Sul bioregion: The largest area in terms of territorial extension, this biogeographic unit consists of Rio Piabanha, Rio Dois Rios, lower Rio Paraíba do Sul basin and its tributaries, such as the Rio Muriaé, Rio Dois Rios and Rio Pomba, as well as independent coastal basins, such as the Rio Itabapoana (the geographical divide with the state of Espírito Santo), Rio Macaé, and coastal lagoon systems. Taxa that delimit this bioregion are shared with other basins in southern Espírito Santo, such as Rineloricaria steindachneri, Hypomasticus thayeri, and Loricariichthys melanurus, as well as Scleromystax prionotos, Trichomycterus puriventris and T. fuliginosus. It is the most speciose bioregion, presenting 120 species, of which 23 occur only in this area (e.g., Astyanax jenynsii, A. jurubatibensis, Delturus parahybae, Homodiaetus banguela, Ituglanis parahybae, Listrura macaensis, Parotocinclus muriaensis, Scleromystax prionotos, Trichomycterus caipora, T. fuliginosus, T. paquequerense, and T. vitalbrazili). Covering a large part of the basins of the state of Rio de Janeiro, many of the species present in these drainages are common to several other drainages of the Atlantic Forest, such as fish species within the genera Deuterodon, Hypostomus, and Trichomycterus.
- Middle Paraíba do Sul I bioregion: The bioregion consists mainly of a middle section of the Rio Paraíba do Sul basin and its tributaries, such as the Rio das Flores and Rio Preto, under the influence of the Serra da Mantiqueira. This area has a particularly high diversity of species of the Trichomycterus genus, including T. albinotatus, T. mirissumba, T. quintus, and T. itatiayae, which support the delimitation of this region. Fish occurring in this bioregion are commonly present in other reaches of the upper and middle Rio Paraíba do Sul in the states of São Paulo and Minas Gerais.
- Middle Rio Paraíba do Sul II bioregion: This area of the middle Rio Paraíba do Sul consists primarily of the Rio Piraí basin. This region also presents considerable diversity of species of the Trichomycterus genus, such as T. claudiae, T. macrophtalmus, and T. nigroauratus, which outline the area.
- Lagos-São João bioregion: The Rio São João basin and the coastal systems of Búzios, Una and Araruama Lagoon constitute this region, delimited by species, such as Nematolebias whitei, Listrura menezesi, Listrura tetraradiata, Parotocinclus fluminense, and Homodiaetus banguela.
- Lagos-Saquarema bioregion: This bioregion consists of the coastal basins of Maricá and Saquarema and adjacent lagoon systems. This delineation is mainly supported by rivulid species, such as Atlantirivulus maricensis, Leptopanchax citrinipinnis, Nematolebias catimbau, Nematolebias papilliferus, Notholebias fractifasciatus, and Notholebias vermiculatus, all of which are endemic to this area.
- Guanabara-Sepetiba bioregion: This bioregion consists of rivers that mostly originate on the oceanic slopes of the Serra do Mar and flow into the Guanabara and Sepetiba bays, as well as coastal lagoon systems in the lowland areas, such as Jacarepaguá. This unit includes relevant basins for the metropolitan region of the state, such as the Rio Caceribu, Rio Guapimirim, Rio Macacu, Rio Roncador, Rio Suruí, Rio Iguaçu, and Rio Guandu. The second most diverse bioregion in the state, it presents 105 species, 17 of which are endemic, such as several rivulids and trichomicterids that support this delimitation (e.g., Atlantirivulus guanabarensis, Kryptolebias caudomarginatus, Leptolebias marmoratus, Leptopanchax opalescens, Leptopanchax sanguineus, Leptopanchax splendens, Notholebias minimus, Homodiaetus passarellii, and Listrura nematopteryx).
- Costa Verde bioregion: This biogeographic unit comprises small basins that flow into Ilha Grande Bay, including the drainages of the Rio Mambucaba, Rio Perequê-Açu, Rio Taquari, and Rio Parati-Mirim, among others. This region is supported by the presence of endemic species, such as Atlantirivulus lazzarotoi, A. simplicis, Listrura costai, Phalloceros enneaktinos, Characidium japuhybense, Hemipsilichthys nimius, and Neoplecostomus paraty.
The cluster analysis (cophenetic correlation = 0.9) indicates that the Lower Paraíba do Sul and Guanabara-Sepetiba bioregions exhibit approximately 47% ichthyofaunistic similarity (Figure 7B). This grouping shows approximately 43% similarity with the Lagos-São João bioregion. Consistent with the beta-diversity analysis, the Costa Verde and Middle Paraíba I and II regions diverge from the other basins in the state, showing less than 20% ichthyofaunistic similarity.
The species richness interpolation results suggests that the greatest diversity in the state is located in a large area corresponding to the drainages around Guanabara Bay, extending to the east and west and encompassing various basins originating from the Serra do Mar (e.g., Rio Macacu, Rio Suruí, Rio Guapimirim), as well as on the opposite slope of this mountain range, covering tributaries of the Rio Paraíba do Sul, such as the headwaters of the Rio Piabanha and Rio Dois Rios (Figure 8).
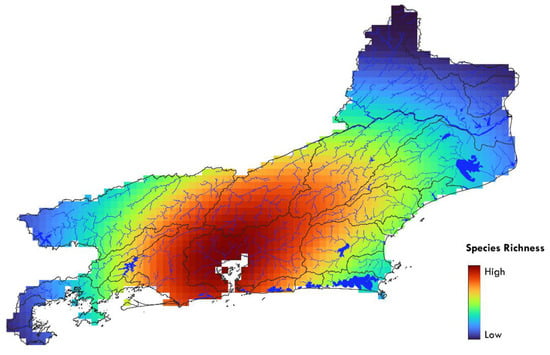
Figure 8.
Species richness interpolation within the state of Rio de Janeiro.
The weight endemism index was higher in several areas of the state (Figure 9): in the headwaters to the east of Guanabara Bay, such as the Rio Macacu and Rio Caceribu; in the systems to the north and west of Guanabara Bay, such as the Rio Iguaçu, Rio Estrela, Rio Suruí, and Rio Roncador; in the headwaters of Rio São João; in coastal areas of Maricá and Saquarema; in the headwaters of the Rio Piraí; in the reach of the middle Rio Paraíba do Sul in the Serra da Mantiqueira; and in the basins of the Costa Verde region. Areas of low endemism mainly include stretches of the middle and lower Rio Paraíba do Sul and other lowland areas in the east and northeast of the state.
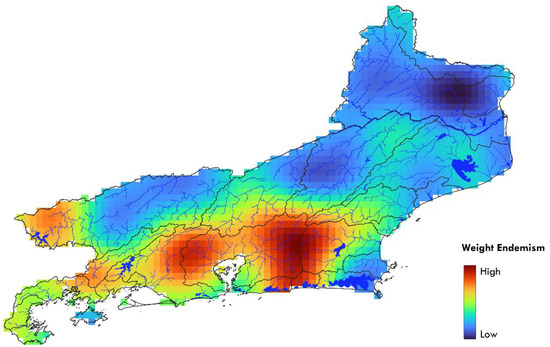
Figure 9.
Weight endemism interpolation within the state of Rio de Janeiro.
4. Discussion
4.1. Taxonomic Diversity and Sampling Coverage
The orders Siluriformes and Characiformes were predominant in terms of the number of species, repeating a pattern commonly found in drainages of the Neotropical region and the Atlantic Forest biome [68,69], followed by Cyprinodontiformes. The predominance of these three orders is the result of characteristics that facilitate the occupation of species in different habitats and the great heterogeneity of environments available in the drainages of the Atlantic Forest. This result is in line with several regional studies for the Rio de Janeiro basins (e.g., [15,16,17,18,19,20,21]). There has been a substantial increase in the number of species described and reported for Rio de Janeiro since the last comprehensive assessment [1], from 149 freshwater species to 205 (an increase of nearly 40%), including non-native and exotic species. Closing these taxonomic and distributional gaps is largely attributed to intensive research on taxa with complex or unclear taxonomy (e.g., species complexes) and the exploration of undersampled areas, which has led to the discovery of new fish species in the state.
However, there are still many areas with low sampling coverage in Rio de Janeiro, particularly in the Rio Paraíba do Sul basin. The results indicate that most municipalities and hydrographic regions within the basin have an average to poor representativity of sampling points and specimens deposited in collections. These areas, especially in the lower section, are traditional areas of coffee cultivation and are now largely deforested, with river siltation and pollution. In contrast, the more sampled and diversified freshwater area—the vicinities of the Guanabara Bay—may be due to the variety of environments in an area where rivers come from a slope mountainous area, the Serra dos Órgãos, with fast-flowing, clear water rivers and pebbles substrate, as well as swamps, slow flowing creeks, and transitional environments, such as mangroves, in the coastal areas. Additionally, the proximity to the metropolitan area of Rio de Janeiro and its research centers has historically facilitated access. While the state has good overall sampling coverage, further targeted investigations are still necessary in certain regions.
4.2. Biogeographic Patterns
The perception that the rivers of the Atlantic Forest in Rio de Janeiro comprise sets of heterogenous, distinct areas of endemism for fish has been previously detailed by authors who have analyzed the biome as a whole [68,70]. Subsequently, ref. [71], in a global analysis of freshwater ecoregions, recognized 3 distinct ecoregions that include, in part, the territory of Rio de Janeiro, 329, Paraíba do Sul, 330, Ribeira de Iguape, and 352, Fluminense (see Figure 1 in [71]). The division of Rio de Janeiro into bioregions of ichthyofaunal endemism recognized here partially matches the findings of [71]. The bioregions Lower Rio Paraíba do Sul and Middle Rio Paraíba do Sul I and II loosely adjust to ecoregion 329. The bioregion Costa Verde corresponds to part of the Ribeira do Iguape ecoregion. Additionally, the bioregions Guanabara-Sepetiba, Lagos-Saquarema, and Lagos-São-João almost corroborate the delineation of the Fluminense ecoregion, as they are also in a similar configuration to the results of the beta-diversity analysis.
However, it is possible that the delimitation of ecoregions proposed by [71] may not fully align with faunal distributions when considering (a) the geomorphological histories of the basins, reflected in the faunal particularities of each area, and (b) the total species composition of the regions, as opposed to the three groups chosen by [71] to define the eastern coastal ecoregions (the extinct subfamily Neoplecostominae and the families Trichomycteridae and Rivulidae) [72,73,74,75]. Nonetheless, dividing areas into biogeographic units at different levels of detail is valuable not only for the biogeographic and evolutionary insights they provide but also because they offer a more focused framework for applying conservation efforts.
The bioregions delineated, as well as the species turnovers, appear to be influenced and partially divided by the Serra do Mar, despite plausible hypotheses of past headwater captures between adjacent basins on opposite slopes. Its continental slope is dominated by the many tributaries of the Rio Paraíba do Sul basin. The hydrographic region of the Middle Paraíba do Sul (and the bioregions Middle Paraíba do Sul I and II) stands out from other middle-lower sections of the basin due to its greater faunal similarity with the upper Paraíba do Sul, located in the state of São Paulo [27,33]. On the oceanic slope of the Serra do Mar, short and independent coastal rivers, mostly originating from its escarpments, drain the entire territory up to the lower Paraíba do Sul region. This area corresponds to RH-1, 2, 5, 6, and 8 and the bioregions Costa Verde, Guanabara-Sepetiba, Lagos-Saquarema, and Lagos-São João, which share more similarities with each other than with the basins of the Middle Rio Paraíba do Sul.
Interestingly, there is nearly 50% faunal similarity between the Guanabara-Sepetiba and Lower Paraíba do Sul bioregions according to the cluster analysis (the lower Paraíba do Sul region is located on both slopes of the Serra do Mar). These areas share several species common to various rivers in the state, such as Cyphocharax gilbert, Deuterodon hastatus, Hyphessobrycon reticulatus, Hypostomus punctatus, Loricariichthys castaneus, Mimagoniates microlepis, Parotocinclus maculicauda, and Prochilodus lineatus, among others. However, congruent species distributions do not necessarily indicate a single biogeographic history between areas [75]. Despite the similarities, these bioregions also present unique species assemblages, which may explain their segregation by this method.
It is speculated that the uplift of the Serra do Mar and Serra da Mantiqueira during the Early Miocene (23–16 Ma) altered the flow of the Upper Paraná River basin, isolating tributaries that then began to run into the Atlantic Ocean, potentially leading to various subsequent vicariance and geodispersal events [76,77]. This later diversification, accompanied by numerous geographic isolation events, possibly driven by tectonic reactivations and erosive processes on the Serra do Mar escarpments, may be responsible for the icthyofaunistic differences found on both sides of this mountain range. However, as previously mentioned, some hypotheses suggest possible later headwater capture events that dispersed some species between adjacent basins on both slopes, such as Phalloceros leptokeras, which geodispersed from the Rio Macacu basin to the Rio Piabanha, a tributary of the Rio Paraíba do Sul [78], and Astyanax viridis, also found in the Rio Macacu basin, Rio Piabanha, and other drainages in the state [79].
These geological developments also help to explain patterns of endemism in highly restricted species found in basins draining the Serra do Mar. In particular, there is significant endemism among rivulid and catfish species in areas delineated by the weight endemism analysis, such as the headwaters of the Rio Macacu and Rio Caceribu (Listrura macacuensis, Microcambeva bendego, and Microglanis nigripinnis); Rio São João (Homodiaetus banguela, Listrura menezesi, and Parotocinclus fluminense); in the systems to the north and west of Guanabara Bay, such as the Rio Iguaçu, Rio Estrela, Rio Suruí, and Rio Roncador (Leptolebias marmoratus, Leptopanchax opalescens, Leptopanchax sanguineus, Leptopanchax splendens, and Listrura nematopteryx); in the coastal areas of Maricá and Saquarema (Atlantirivulus maricensis, Leptopanchax citrinipinnis, Listrura tetraradiata, Nematolebias catimbau, Nematolebias papilliferus, Notholebias fractifasciatus, Notholebias vermiculatus, and Trichomycterus saquarema); in the headwaters of the Rio Piraí (Trichomycterus macrophthalmus and T. nigroauratus); in the reach of the Middle Rio Paraíba do Sul in the Serra da Mantiqueira (Pareiorhina brachyrhyncha, Trichomycterus albinotatus, T. auroguttatus, T. itatiayae, T. mirissumba, and T. quintus, also found in sections of the Paraíba do Sul basin in other states); and in the basins of the Costa Verde region, discussed in detail further below. Apart from the Middle Paraíba do Sul section, these areas represent distributions of endemic species in Rio de Janeiro with a narrow geographic range (in contrast to state-endemic species distributed across multiple basins, such as Astyanax viridis, Atlantirivulus guanabarensis, and Characidium grajahuense, among others).
In the set of coastal lowlands, it is worth highlighting the special interest and diversity of freshwater species on the small river drainages at the Costa Verde bioregion. Despite corresponding to a small area of territory, the Costa Verde has a pronounced endemism of species, with a unique set of stream fishes, such as Atlantirivulus lazzarotoi, A. simplicis, Characidium japuhybense, Hemipsilichthys nimius, Listrura costai, Neoplecostomus paraty, and Phalloceros enneaktinos [21]. Possible reasons for this include the presence of numerous coastal drainages flowing from the Serra do Mar, with some of them presenting coastal floodplain areas with bays and lagoon systems. These diverse environments support a significant diversity of small-sized fish species, with a standard length of 15 cm or less. Although these small-sized species represent about 70% of the total diversity in the Neotropical region, their existence is often almost neglected [80]. Being mostly fish of the primary division, that is, intolerant of survival in brackish or salty waters, the process of isolation, dispersion, and occupation of these species in the numerous small coastal streams has always been intriguing. The coastal drainages of the Costa Verde have particularities not observed in other areas, such as rivers flowing abruptly towards the coast, some of which are even devoid of coastal plain areas. This topographic configuration has significant implications for aquatic biota, as isolated basins often present similar fish fauna [76]. The coastal basins of the Atlantic Forest, where the Serra do Mar is very close to the coast, such as in the Costa Verde area, were influenced by climate change that caused sea level oscillations during the Pleistocene. These transgressions and regressions led to the isolation and reconnection of rivers [81]. Past connections among these coastal basins are hypothesized by [82] based on molecular evidence and paleodrainage reconstruction.
The particularities of the relief and the configuration of the hydrographic basins in the Atlantic Forest biome within Rio de Janeiro include the Paraíba valley—depressed tectonic valley corridors along faults [83]—with rivers flowing between the Serra do Mar and Serra da Mantiqueira mountain ranges. Although the Rio Paraíba do Sul is the largest river system partially crossing the Rio de Janeiro territory, it was not identified as the most diverse area for freshwater fishes in its entirety. However, its diversity remains significant, considering that the basin is located among the largest urban–industrial centers of the state. Nevertheless, for this reason, the ichthyofauna faces various threats. Major impacts include environmental degradation, dam construction, the destruction of riparian forests, untreated discharge of domestic and industrial sewage, and mining. On the other hand, approximately ten endangered freshwater fish species inhabit the Rio Paraíba do Sul Valley and are the focus of conservation efforts [84]. The mountainous terrain of this region, characterized by vertical escarpments and difficult access, which helped keep the mountain barrier impassable during the early centuries of colonization [85], has acted as a geographical divide, isolating the Rio Paraíba do Sul from the coastal rivers.
5. Conclusions
This is the first comprehensive evaluation of freshwater fishes in the Rio de Janeiro region with precise geographic data. We hope that our findings will advance future research and inform conservation management strategies for this complex and diverse area, serving as a foundational step toward further assessments. The distribution patterns of fish species in the region support earlier studies of the Atlantic Forest and suggest that the identified bioregions align partially with previously established biogeographic units.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ecologies5040033/s1, Table S1: Ichthyological collections with inventoried records from the state of Rio de Janeiro; Table S2: Records of fish species from the state of Rio de Janeiro listed for this study; Table S3: Inventory of all species recorded for the state of Rio de Janeiro.
Author Contributions
All authors designed the study; R.F.M.-P. built the dataset and checked species distribution ranges. L.M.S.-S. and F.V.-G. performed data curation and taxonomic validation. Biogeographical analysis was performed by F.V.-G. Statistical analysis was performed by RFMP and F.V.-G. All authors wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
F.V.-G. was funded by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Finance Code 001, process no. 88887.512702/2020-00.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available in Supplementary Material Table S2.
Acknowledgments
We extend our gratitude to M. Gianeti, O. Oyakawa (MZUSP), M. R. Britto, and P.A. Buckup (MNRJ) for providing collection records. We are grateful to M.R. Britto, P.A. Buckup (MNRJ), and J. P. da Silva (MBML) for the courtesy extended during the visit to their institutions. Thanks to T. Roberts for taxonomic suggestions. We acknowledge A.M. Katz, F.C.P. Dagosta, L.S. Medeiros, and anonymous reviewers for suggestions to improve the manuscript. F.V.-G. is grateful to CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Finance Code 001, process no. 88887.512702/2020-00) for his Ph.D. scholarship. This work received laboratory support from the Instituto Nossos Riachos (INR).
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Quality of sampling per municipalities in the state of Rio de Janeiro.
Table A1.
Quality of sampling per municipalities in the state of Rio de Janeiro.
| Municipality | Area (km2) | Lots | Points | Il | Ilq | Ip | Ipq |
|---|---|---|---|---|---|---|---|
| Angra dos Reis | 813.21 | 532 | 57 | 65.4 | Good | 7.0 | Good |
| Aperibé | 94.54 | 2 | 2 | 2.1 | Poor | 2.1 | Poor |
| Araruama | 638.15 | 23 | 4 | 3.6 | Poor | 0.6 | Poor |
| Areal | 110.72 | 0 | 0 | 0.0 | Poor | 0.0 | Poor |
| Armação dos Búzios | 70.98 | 2 | 1 | 2.8 | Poor | 1.4 | Poor |
| Arraial do Cabo | 152.11 | 0 | 0 | 0.0 | Poor | 0.0 | Poor |
| Barra do Piraí | 584.61 | 53 | 11 | 9.1 | Poor | 1.9 | Poor |
| Barra Mansa | 547.13 | 40 | 7 | 7.3 | Poor | 1.3 | Poor |
| Belford Roxo | 78.99 | 0 | 0 | 0.0 | Poor | 0.0 | Poor |
| Bom Jardim | 382.43 | 19 | 5 | 5.0 | Poor | 1.3 | Poor |
| Bom Jesus do Itabapoana | 596.66 | 32 | 8 | 5.4 | Poor | 1.3 | Poor |
| Cabo Frio | 413.58 | 70 | 20 | 16.9 | Poor | 4.8 | Average |
| Cachoeiras de Macacu | 954.75 | 944 | 93 | 98.9 | Good | 9.7 | Good |
| Cambuci | 558.28 | 6 | 2 | 1.1 | Poor | 0.4 | Poor |
| Campos dos Goytacazes | 4032.49 | 761 | 93 | 18.9 | Poor | 2.3 | Poor |
| Cantagalo | 747.21 | 56 | 7 | 7.5 | Poor | 0.9 | Poor |
| Carapebus | 304.89 | 261 | 25 | 85.6 | Good | 8.2 | Good |
| Cardoso Moreira | 522.6 | 24 | 3 | 4.6 | Poor | 0.6 | Poor |
| Carmo | 305.75 | 256 | 24 | 83.7 | Good | 7.8 | Good |
| Casimiro de Abreu | 462.92 | 355 | 44 | 76.7 | Good | 9.5 | Good |
| Comendador Levy Gasparian | 108.64 | 1 | 1 | 0.9 | Poor | 0.9 | Poor |
| Conceição de Macabu | 338.26 | 114 | 16 | 33.7 | Average | 4.7 | Average |
| Cordeiro | 113.05 | 8 | 1 | 7.1 | Poor | 0.9 | Poor |
| Duas Barras | 379.62 | 3 | 1 | 0.8 | Poor | 0.3 | Poor |
| Duque de Caxias | 467.32 | 269 | 42 | 57.6 | Good | 9.0 | Good |
| Engenheiro Paulo de Frontin | 139.38 | 30 | 4 | 21.5 | Poor | 2.9 | Poor |
| Guapimirim | 358.44 | 294 | 30 | 82.0 | Good | 8.4 | Good |
| Iguaba Grande | 50.98 | 6 | 2 | 11.8 | Poor | 3.9 | Average |
| Itaboraí | 429.96 | 132 | 11 | 30.7 | Average | 2.6 | Poor |
| Itaguaí | 282.61 | 150 | 23 | 53.1 | Good | 8.1 | Good |
| Italva | 291.19 | 17 | 2 | 5.8 | Poor | 0.7 | Poor |
| Itaocara | 433.18 | 120 | 9 | 27.7 | Average | 2.1 | Poor |
| Itaperuna | 1106.69 | 313 | 17 | 28.3 | Average | 1.5 | Poor |
| Itatiaia | 241.04 | 231 | 41 | 95.8 | Good | 17.0 | Good |
| Japeri | 81.7 | 59 | 3 | 72.2 | Good | 3.7 | Average |
| Laje do Muriaé | 253.53 | 7 | 1 | 2.8 | Poor | 0.4 | Poor |
| Macaé | 1216.99 | 827 | 102 | 68.0 | Good | 8.4 | Good |
| Macuco | 78.36 | 0 | 0 | 0.0 | Poor | 0.0 | Poor |
| Magé | 390.78 | 335 | 46 | 85.7 | Good | 11.8 | Good |
| Mangaratiba | 367.82 | 61 | 11 | 16.6 | Poor | 3.0 | Average |
| Maricá | 361.57 | 233 | 63 | 64.4 | Good | 17.4 | Good |
| Mendes | 95.32 | 46 | 3 | 48.3 | Good | 3.1 | Average |
| Mesquita | 41.17 | 37 | 4 | 89.9 | Good | 9.7 | Good |
| Miguel Pereira | 287.93 | 98 | 16 | 34.0 | Average | 5.6 | Good |
| Miracema | 303.27 | 0 | 0 | 0.0 | Poor | 0.0 | Poor |
| Natividade | 387.07 | 8 | 2 | 2.1 | Poor | 0.5 | Poor |
| Nilópolis | 19.39 | 0 | 0 | 0.0 | Poor | 0.0 | Good |
| Niterói | 133.76 | 22 | 10 | 16.4 | Average | 7.5 | Good |
| Nova Friburgo | 935.43 | 190 | 35 | 20.3 | Poor | 3.7 | Average |
| Nova Iguaçu | 520.58 | 488 | 64 | 93.7 | Good | 12.3 | Good |
| Paracambi | 190.95 | 24 | 3 | 12.6 | Poor | 1.6 | Poor |
| Paraíba do Sul | 571.12 | 47 | 6 | 8.2 | Poor | 1.1 | Poor |
| Paraty | 924.3 | 592 | 88 | 64.0 | Good | 9.5 | Good |
| Paty do Alferes | 314.34 | 8 | 2 | 2.5 | Poor | 0.6 | Poor |
| Petrópolis | 791.14 | 146 | 26 | 18.5 | Poor | 3.3 | Average |
| Pinheiral | 82.25 | 2 | 1 | 2.4 | Poor | 1.2 | Poor |
| Piraí | 490.26 | 115 | 17 | 23.5 | Average | 3.5 | Average |
| Porciúncula | 291.85 | 0 | 0 | 0.0 | Poor | 0.0 | Poor |
| Porto Real | 50.89 | 51 | 5 | 100.2 | Good | 9.8 | Good |
| Quatis | 284.83 | 51 | 8 | 17.9 | Poor | 2.8 | Poor |
| Queimados | 75.93 | 33 | 2 | 43.5 | Good | 2.6 | Poor |
| Quissamã | 719.64 | 339 | 56 | 47.1 | Good | 7.8 | Good |
| Resende | 1099.34 | 290 | 47 | 26.4 | Average | 4.3 | Average |
| Rio Bonito | 459.46 | 32 | 5 | 7.0 | Poor | 1.1 | Poor |
| Rio Claro | 846.8 | 721 | 75 | 85.1 | Good | 8.9 | Good |
| Rio das Flores | 478.78 | 10 | 2 | 2.1 | Poor | 0.4 | Poor |
| Rio das Ostras | 228.04 | 40 | 8 | 17.5 | Poor | 3.5 | Average |
| Rio de Janeiro | 1200.33 | 904 | 194 | 75.3 | Good | 16.2 | Good |
| Santa Maria Madalena | 810.96 | 84 | 20 | 10.4 | Poor | 2.5 | Poor |
| Santo Antônio de Pádua | 603.63 | 44 | 3 | 7.3 | Poor | 0.5 | Poor |
| São Fidélis | 1034.83 | 257 | 16 | 24.8 | Average | 1.5 | Poor |
| São Francisco de Itabapoana | 1118.04 | 111 | 19 | 9.9 | Poor | 1.7 | Poor |
| São Gonçalo | 248.16 | 3 | 2 | 1.2 | Poor | 0.8 | Poor |
| São João da Barra | 452.4 | 239 | 26 | 52.8 | Good | 5.7 | Good |
| São João de Meriti | 35.22 | 0 | 0 | 0.0 | Poor | 0.0 | Poor |
| São José de Ubá | 249.69 | 29 | 3 | 11.6 | Poor | 1.2 | Poor |
| São José do Vale do Rio Preto | 220.18 | 12 | 5 | 5.5 | Poor | 2.3 | Poor |
| São Pedro da Aldeia | 332.49 | 28 | 6 | 8.4 | Poor | 1.8 | Poor |
| São Sebastião do Alto | 397.21 | 107 | 11 | 26.9 | Average | 2.8 | Poor |
| Sapucaia | 540.67 | 95 | 14 | 17.6 | Poor | 2.6 | Poor |
| Saquarema | 352.13 | 220 | 45 | 62.5 | Good | 12.8 | Good |
| Seropédica | 265.19 | 135 | 29 | 50.9 | Good | 10.9 | Good |
| Silva Jardim | 937.76 | 692 | 57 | 73.8 | Good | 6.1 | Good |
| Sumidouro | 413.41 | 18 | 3 | 4.4 | Poor | 0.7 | Poor |
| Tanguá | 143.01 | 8 | 2 | 5.6 | Poor | 1.4 | Poor |
| Teresópolis | 773.34 | 228 | 42 | 29.5 | Average | 5.4 | Average |
| Trajano de Moraes | 591.15 | 30 | 6 | 5.1 | Poor | 1.0 | Poor |
| Três Rios | 322.84 | 196 | 19 | 60.7 | Good | 5.9 | Good |
| Valença | 1300.77 | 8 | 5 | 0.6 | Poor | 0.1 | Poor |
| Varre-Sai | 201.94 | 0 | 0 | 0.0 | Poor | 0.0 | Poor |
| Vassouras | 536.07 | 87 | 6 | 16.2 | Poor | 1.1 | Poor |
| Volta Redonda | 182.11 | 14 | 4 | 7.7 | Poor | 2.2 | Poor |
| Rio de Janeiro State | 43,750 | 13,585 | 1854 | 31.1 | 4.2 |

Table A2.
Inventory of native freshwater species recorded for the state of Rio de Janeiro. Taxonomic nomenclature of fishes by family follows [35]). Allochthonous and exotic species indicated by an asterisk *. Species in bold are endemic to Rio de Janeiro state.
Table A2.
Inventory of native freshwater species recorded for the state of Rio de Janeiro. Taxonomic nomenclature of fishes by family follows [35]). Allochthonous and exotic species indicated by an asterisk *. Species in bold are endemic to Rio de Janeiro state.
| Taxon | Author | Record in RJ |
|---|---|---|
| CHARACIFORMES | ||
| Crenuchidae | ||
| Characidiinae | ||
| Characidium alipioi | Travassos 1955 | [1,70] |
| Characidium grajahuense | Travassos 1944 | [1,21] |
| Characidium interruptum | Pellegrin 1909 | [14,25,29] |
| Characidium japuhybense | Travassos 1949 | [21] |
| Characidium lauroi | Travassos 1949 | [1,17,19] |
| Characidium litorale | Leitão & Buckup 2014 | [9] |
| Characidium vidali | Travassos 1967 | [1,18,19,29] |
| Erythrinidae | ||
| Hoplerythrinus unitaeniatus | (Spix & Agassiz 1829) | [1,14,16] |
| Hoplias lacerdae * | Miranda Ribeiro 1908 | [1] |
| Hoplias malabaricus | (Bloch 1794) | [1,14,16,21] |
| Parodontidae | ||
| Apareiodon piracicabae | (Eigenmann 1907) | MNRJ 45942 |
| Serrasalmidae | ||
| Colossomatinae | ||
| Colossoma macropomum * | (Cuvier 1816) | [1] |
| Serrasalminae | ||
| Metynnis lippincottianus * | (Cope 1870) | MNRJ 49310 |
| Metynnis maculatus * | (Kner 1858) | [16,27,86] |
| Anostomidae | ||
| Hypomasticus copelandii | (Steindachner 1875) | [27,86] as Leporinus copelandii |
| Hypomasticus mormyrops | (Steindachner 1875) | [19,27,86] as Leporinus mormyrops |
| Hypomasticus thayeri | (Borodin 1929) | [33] as Leporinus cf. thayeri |
| Megaleporinus conirostris | (Steindachner 1875) | [1,27,86] as Leporinus conirostris |
| Curimatidae | ||
| Cyphocharax gilbert | (Quoy & Gaimard 1824) | [1,16,27] |
| Prochilodontidae | ||
| Prochilodus lineatus | (Valenciennes 1837) | [1,27] |
| Prochilodus vimboides | Kner 1859 | [1,27] |
| Lebiasinidae | ||
| Pyrrhulininae | ||
| Nannostomus beckfordi * | Günther 1872 | [1] as N. brechforti |
| Pyrrhulina australis * | Eigenmann & Kennedy 1903 | MNRJ 50781 |
| Pyrrhulina filamentosa * | Valenciennes 1847 | MNRJ 14996 |
| Bryconidae | ||
| Bryconinae | ||
| Brycon insignis | Steindachner 1877 | [27,86] |
| Brycon opalinus | (Cuvier 1819) | [19,30] |
| Salmininae | ||
| Salminus brasiliensis * | (Cuvier 1816) | [27] |
| Characidae | ||
| Stethaprioninae | ||
| Astyanax jenynsii | (Steindachner 1877) | [67,87] |
| Astyanax keronolepis | Silva, Malabarba & Malabarba 2019 | [10] |
| Astyanax lacustris | (Lütken 1875) | [21] |
| Astyanax viridis | Salgado 2021 | [79] |
| Deuterodon giton | (Eigenmann 1908) | [1,14,18,29] as Astyanax giton |
| Deuterodon hastatus | (Myers 1928) | [20,21,25,29] as Astyanax hastatus |
| Deuterodon heterostomus | (Eigenmann 1911) | [27,86] as Probolodus heterostomus |
| Deuterodon intermedius | (Eigenmann 1908) | [1,17] as Astyanax intermedius, [20,21] |
| Deuterodon janeiroensis | (Eigenmann 1908) | [1,15,25,29] as Astyanax janeiroensis, [20,21] |
| Deuterodon luetkenii | (Boulenger 1887) | [16] as Hyphessobrycon luetkenii |
| Deuterodon taeniatus | (Jenyns 1842) | [1,14,15,29] as Astyanax taeniatus |
| Hollandichthys multifasciatus | (Eigenmann & Norris 1900) | [20,21] |
| Hyphessobrycon bifasciatus | Ellis 1911 | [15,16,25] |
| Hyphessobrycon eques * | (Steindachner 1882) | [27] |
| Hyphessobrycon flammeus | Myers 1924 | [1,70,88] |
| Hyphessobrycon reticulatus | Ellis 1911 | [14,16,18,25] |
| Oligosarcus acutirostris | Menezes 1990 | MCZ 20605 |
| Oligosarcus hepsetus | (Cuvier 1829) | [16,19,21,27] |
| Psalidodon parahybae | (Eigenmann 1908) | [27,67,86] as Astyanax parahybae |
| Spintherobolinae | ||
| Spintherobolus broccae | Myers 1925 | [1,70] |
| Aphyocharacinae | ||
| Aphyocharax anisitsi * | Eigenmann & Kennedy 1903 | MNRJ 5585 |
| Stevardiinae | ||
| Bryconamericus microcephalus | (Miranda Ribeiro 1908) | [1,29] |
| Bryconamericus ornaticeps | Bizerril & Perez-Neto 1995 | [20,21,29] |
| Bryconamericus tenuis | Bizerril & Auraujo 1992 | [1,29] |
| Knodus moenkhausii * | (Eigenmann & Kennedy 1903) | MNRJ 43351 |
| Mimagoniates microlepis | (Steindachner 1877) | [14,25,29] |
| Piabina argentea | Reinhardt 1867 | [89] |
| Pseudocorynopoma doriae * | Perugia 1891 | USNM 129920 |
| SILURIFORMES | ||
| Trichomycteridae | ||
| Trichogeninae | ||
| Trichogenes longipinnis | Britski & Ortega 1983 | [20,21,90,91] |
| Trichomycterinae | ||
| Ituglanis parahybae | (Eigenmann 1918) | [1,92] |
| Trichomycterus albinotatus | Costa 1992 | [1,70] |
| Trichomycterus auroguttatus | Costa 1992 | [1,70] |
| Trichomycterus caipora | Lima, Lazzarotto & Costa 2008 | [93,94] |
| Trichomycterus claudiae | Barbosa & Costa 2010 | [7] |
| Trichomycterus florensis | (Miranda-Ribeiro 1943) | [1,94] |
| Trichomycterus fuliginosus | Barbosa & Costa 2010 | [7] |
| Trichomycterus giganteus | Lima & Costa 2004 | [95] |
| Trichomycterus goeldii | Boulenger 1896 | [1] |
| Trichomycterus itatiayae | Miranda Ribeiro 1906 | [1,96] |
| Trichomycterus jacupiranga | Wosiacki & Oyakawa 2005 | [21] |
| Trichomycterus largoperculatus | Costa & Katz 2022 | [97] |
| Trichomycterus macrophthalmus | Barbosa & Costa 2012 | [17,19,98] |
| Trichomycterus mariamole | Barbosa & Costa 2010 | [7,17,19] |
| Trichomycterus mirissumba | Costa 1992 | [1,7] |
| Trichomycterus nigricans | Valenciennes 1832 | [1,94] |
| Trichomycterus nigroauratus | Barbosa & Costa 2008 | [17,19,96] |
| Trichomycterus paquequerensis | (Miranda Ribeiro 1943) | [1,29,94] |
| Trichomycterus potschi | Barbosa & Costa 2003 | [21,99] |
| Trichomycterus puriventris | Barbosa & Costa 2012 | [100] |
| Trichomycterus quintus | Costa 2020 | [101] |
| Trichomycterus santaeritae | (Eigenmann 1918) | [94] |
| Trichomycterus saquarema | Costa, Katz, Vilardo & Amorim 2022 | [102] |
| Trichomycterus travassosi | (Miranda Ribeiro 1949) | [1,70] |
| Trichomycterus vitalbrazili | Vilardo, Katz & Costa 2020 | [103] |
| Microcambevinae | ||
| Listrura costai | Villa-Verde, Lazzarotto & Lima 2012 | [21,104,105] |
| Listrura macacuensis | Costa & Katz 2021 | [104,105] |
| Listrura macaensis | Costa & Katz 2021 | [104,105] |
| Listrura menezesi | Villa-Verde, de Pinna, Reis & Oyakawa 2022 | [105,106] |
| Listrura nematopteryx | de Pinna 1988 | [29,104,105] |
| Listrura tetraradiata | Landim & Costa 2002 | [104,105] |
| Microcambeva barbata | Costa & Bockmann 1994 | [11,107] |
| Microcambeva bendego | Medeiros, Moreira, de Pinna & Lima 2020 | [11] |
| Stegophilinae | ||
| Homodiaetus banguela | Koch 2002 | [108] |
| Homodiaetus passarellii | (Miranda Ribeiro 1944) | [29,70,108] |
| Callichthyidae | ||
| Callichthyinae | ||
| Callichthys callichthys | (Linnaeus 1758) | [1,14,15,16,25] |
| Hoplosternum littorale | (Hancock 1828) | [1,15,16,29] |
| Corydoradinae | ||
| Hoplisoma nattereri | (Steindachner 1876) | [1,18,29] as Corydoras nattereri |
| Scleromystax barbatus | (Quoy & Gaimard 1824) | [14,18,21,29] |
| Scleromystax prionotos | (Nijssen & Isbrücker 1980) | [70,109,110] |
| Loricariidae | ||
| Delturinae | ||
| Delturus parahybae | Eigenmann & Eigenmann 1889 | [1,70] |
| Hemipsilichthys gobio | (Lütken 1874) | [4,19,29] |
| Hemipsilichthys nimius | Pereira, Reis, Souza & Lazzarotto 2003 | [4,20,21] |
| Hemipsilichthys papillatus | Pereira, Oliveira & Oyakawa 2000 | [4,17,19] |
| Rhinelepinae | ||
| Pogonopoma parahybae | (Steindachner 1877) | [27,70] as Pogonopomoides parahybae |
| Loricariinae | ||
| Harttia carvalhoi | Miranda Ribeiro 1939 | [17,19] |
| Harttia loricariformis | Steindachner 1877 | [17,19] |
| Loricariichthys castaneus | (Castelnau 1855) | [27,29,111] |
| Loricariichthys melanurus | Reis, Vieira & Pereira 2021 | [111] |
| Rineloricaria nigricauda | (Regan 1904) | [70,112] |
| Rineloricaria nudipectoris | Mejia, Ferraro & Buckup 2023 | [112] |
| Rineloricaria paraibensis | Mejia & Buckup 2024 | [112] |
| Rineloricaria steindachneri | (Regan 1904) | [27,70,112] |
| Rineloricaria zawadzkii | Silva, Costa & Oliveira 2022 | [112] |
| Hypoptopomatinae | ||
| Hisonotus notatus | Eigenmann & Eigenmann 1889 | [29,113] |
| Hisonotus thayeri | Martins & Langeani 2016 | [113] |
| Kronichthys heylandi | (Boulenger 1900) | [20,21,29,114] |
| Neoplecostomus microps | (Steindachner 1877) | [17,21,29,115] |
| Neoplecostomus paraty | Cherobim, Lazzarotto & Langeani 2017 | [20,21,115] |
| Neoplecostomus variipictus | Bizerril 1995 | [115] |
| Otocinclus affinis | Steindachner 1877 | [1,70,110] |
| Otothyris lophophanes | (Eigenmann & Eigenmann 1889) | [1,70,110] |
| Pareiorhaphis garbei | (Ihering 1911) | [18,116] |
| Pareiorhina brachyrhyncha | Chamon, Aranda & Buckup 2005 | [117] |
| Pareiorhina rudolphi | (Miranda Ribeiro 1911) | [19,20,21,117] |
| Parotocinclus bidentatus | Gauger & Buckup 2005 | [5] |
| Parotocinclus fluminense | Roxo, Melo, Silva & Oliveira 2017 | [118] |
| Parotocinclus maculicauda | (Steindachner 1877) | [1,18,29,114] |
| Parotocinclus muriaensis | Gauger & Buckup 2005 | [5] |
| Pseudotothyris janeirensis | Britski & Garavello 1984 | [1] |
| Schizolecis guentheri | (Miranda Ribeiro 1918) | [14,20,21,29,91] |
| Hypostominae | ||
| Ancistrus multispinis | (Regan 1912) | [18,20,21,29] |
| Hypostomus affinis | (Steindachner 1877) | [19,29,86] |
| Hypostomus auroguttatus | Kner 1854 | [27,86] |
| Hypostomus luetkeni | (Steindachner 1877) | [1,16,19] |
| Hypostomus punctatus | Valenciennes 1840 | [14,114] |
| Aspredinidae | ||
| Pseudobunocephalinae | ||
| Pseudobunocephalus iheringii | (Boulenger 1891) | [1] as Dysichthys iheringii |
| Auchenipteridae | ||
| Centromochlinae | ||
| Glanidium melanopterum | Miranda Ribeiro 1918 | [1,110] |
| Auchenipterinae | ||
| Trachelyopterus striatulus | (Steindachner 1877) | [1] as Parauchenipterus striatulus, [16] |
| Heptapteridae | ||
| Rhamdiinae | ||
| Pimelodella lateristriga | (Lichtenstein 1823) | [14,19,20,21,29] |
| Rhamdia quelen | (Quoy & Gaimard 1824) | [14,21,29] |
| Heptapterinae | ||
| Acentronichthys leptos | Eigenmann & Eigenmann 1889 | [16,18,20,21] |
| Imparfinis minutus | (Lütken 1874) | [17,19] |
| Imparfinis piperatus | Eigenmann & Norris 1900 | [110,114] |
| Rhamdioglanis frenatus | Ihering 1907 | [1,21] |
| Rhamdioglanis transfasciatus | Miranda Ribeiro 1908 | [18,20,29] |
| Taunayia bifasciata | (Eigenmann & Norris 1900) | [21,119] |
| Pimelodidae | ||
| Pimelodus maculatus | Lacepède 1803 | [27,86] |
| Steindachneridion parahybae | (Steindachner 1877) | [1] |
| Pseudopimelodidae | ||
| Batrochoglaninae | ||
| Microglanis nigripinnis | Bizerril & Perez-Neto 1992 | [110,120] |
| Microglanis parahybae | (Steindachner 1880) | [110,120] |
| Microglanis pleriqueater | Mattos, Ottoni & Barbosa 2013 | [120] |
| Clariidae | ||
| Clarias gariepinus * | (Burchell 1822) | [1] |
| Ariidae | ||
| Ariinae | ||
| Paragenidens grandoculis | (Steindachner 1877) | [121] |
| GYMNOTIFORMES | ||
| Sternopygidae | ||
| Eigenmanniinae | ||
| Eigenmannia virescens | (Valenciennes 1836) | [1,27,86,110] |
| Gymnotidae | ||
| Gymnotinae | ||
| Gymnotus carapo | Linnaeus 1758 | [1,18,27,114] |
| Gymnotus pantherinus | (Steindachner 1908) | [1,18,19,20,29] |
| Hypopomidae | ||
| Brachyhypopomus janeiroensis | (Costa & Campos-da-Paz 1992) | [3,16,110] |
| SALMONIFORMES | ||
| Salmonidae | ||
| Salmoninae | ||
| Oncorhynchus mykiss * | (Walbaum 1792) | [1,21] |
| GOBIIFORMES | ||
| Eleotridae | ||
| Eleotrinae | ||
| Dormitator maculatus | (Bloch 1792) | [14,21] |
| Eleotris pisonis | (Gmelin 1789) | [14,16,20] |
| Oxudercidae | ||
| Gobionellinae | ||
| Awaous tajasica | (Lichtenstein 1822) | [14,16,20,25,29] |
| CICHLIFORMES | ||
| Cichlidae | ||
| Pseudocrenilabrinae | ||
| Coptodon rendalli * | (Boulenger 1897) | [1,14,16,27] as Tilapia rendalli |
| Oreochromis niloticus * | (Linnaeus 1758) | [1,14,19,27,29] |
| Cichlinae | ||
| Apistogramma sp. * | [1] | |
| Astronotus ocellatus | (Agassiz 1831) | [1] |
| Australoheros ipatinguensis | Ottoni & Costa 2008 | [122] |
| Australoheros oblongus | (Castelnau 1855) | [122] |
| Cichla kelberi * | Kullander & Ferreira 2006 | [29,86,123] |
| Crenicichla lacustris | (Castelnau 1855) | [18,27,29,86] |
| Crenicichla sp. | MNRJ 48620 | |
| Geophagus brasiliensis | (Quoy & Gaimard 1824) | [1,14,16,21,25,29] |
| Parachromis managuensis * | (Günther 1867) | NPM 1796 |
| ACANTHURIFORMES | ||
| Sciaenidae | ||
| Pachyurus adspersus | Steindachner 1879 | [27,86,124] |
| CYPRINODONTIFORMES | ||
| Rivulidae | ||
| Rivulinae | ||
| Atlantirivulus guanabarensis | Costa 2014 | [8] |
| Atlantirivulus janeiroensis | (Costa 1991) | [8], [14,25] as Rivulus janeiroensis |
| Atlantirivulus jurubatibensis | (Costa 2008) | [8,16] |
| Atlantirivulus lazzarotoi | (Costa 2007) | [8,21] |
| Atlantirivulus maricensis | Costa 2014 | [8] |
| Atlantirivulus simplicis | (Costa 2004) | [8,21] |
| Kryptolebiatinae | ||
| Kryptolebias brasiliensis | (Valenciennes 1821) | [14,18,21,29] |
| Kryptolebias caudomarginatus | (Seegers 1984) | [14,125] |
| Kryptolebias gracilis | Costa 2007 | [126] |
| Kryptolebias ocellatus | (Hensel 1868) | [14,21,125] |
| Cynolebiinae | ||
| Leptolebias marmoratus | (Ladiges 1934) | [127] |
| Leptopanchax citrinipinnis | (Costa, Lacerda & Tanizaki 1988) | [127,128] |
| Leptopanchax opalescens | (Myers 1942) | [127] |
| Leptopanchax sanguineus | Costa 2019 | [127] |
| Leptopanchax splendens | (Myers 1942) | [127] |
| Nematolebias catimbau | Costa, Amorim & Aranha 2014 | [129] |
| Nematolebias papilliferus | Costa 2002 | [129] |
| Nematolebias whitei | (Myers 1942) | [129] |
| Notholebias cruzi | (Costa 1988) | [128] |
| Notholebias fractifasciatus | (Costa 1988) | [128] |
| Notholebias minimus | (Myers 1942) | [14] as Leptolebias minimus, [128] |
| Notholebias vermiculatus | Costa & Amorim 2013 | [128] |
| Ophthalmolebias constanciae | (Myers 1942) | [130] |
| Poeciliidae | ||
| Poeciliinae | ||
| Phalloceros anisophallos | Lucinda 2008 | [6,21] |
| Phalloceros enneaktinos | Lucinda 2008 | [6,21] |
| Phalloceros harpagos | Lucinda 2008 | [6,16,21,29] |
| Phalloceros leptokeras | Lucinda 2008 | [6,21] |
| Phalloptychus januarius | (Hensel 1868) | [14,16,131] |
| Poecilia reticulata * | Peters 1859 | [1,18,21,29] |
| Poecilia vivipara | Bloch & Schneider 1801 | [1,18,21,29] |
| Xiphophorus helleri * | Heckel 1848 | [1,18,29] as Xiphophorus sp. |
| Anablepidae | ||
| Anablepinae | ||
| Jenynsia darwini | Amorim 2018 | [132] |
| Jenynsia lineata | (Jenyns 1842) | [14,16,131] as Jenynsia multidentata |
| SYNBRANCHIFORMES | ||
| Synbranchidae | ||
| Synbranchus sp. | [14,18,21,25,29,114] as S. marmoratus | |
| ANABANTIFORMES | ||
| Osphronemidae | ||
| Trichogastrinae | ||
| Trichopodus trichopterus * | (Pallas 1770) | [1] as Trichogaster trichopterus |
| Macropodusinae | ||
| Trichopsis vittata * | (Cuvier 1831) | MNRJ 40423 |

Table A3.
Abundance of freshwater native fish species per hydrographic region in the state of Rio de Janeiro.
Table A3.
Abundance of freshwater native fish species per hydrographic region in the state of Rio de Janeiro.
| Espécie | RH-1 | RH-2 | RH-3 | RH-4 | RH-5 | RH-6 | RH-7 | RH-8 | RH-9 |
|---|---|---|---|---|---|---|---|---|---|
| Acentronichthys leptos | 109 | 18 | 2 | 2 | 175 | 17 | 36 | 7 | 9 |
| Ancistrus multispinis | 145 | 119 | 0 | 0 | 503 | 35 | 0 | 6 | 0 |
| Apareiodon piracicabae | 0 | 3 | 1 | 0 | 0 | 0 | 30 | 0 | 0 |
| Aphyocharax anisitsi | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 3 | 0 |
| Apistogramma sp. | 0 | 0 | 0 | 0 | 12 | 0 | 0 | 0 | 0 |
| Astronotus ocellatus | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Astyanax jenynsii | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Astyanax keronolepis | 1091 | 97 | 0 | 0 | 547 | 0 | 0 | 0 | 0 |
| Astyanax lacustris | 2 | 518 | 90 | 83 | 123 | 202 | 173 | 2022 | 1724 |
| Astyanax viridis | 0 | 0 | 0 | 6 | 234 | 0 | 0 | 0 | 1 |
| Atlantirivulus guanabarensis | 0 | 0 | 0 | 0 | 224 | 0 | 0 | 0 | 0 |
| Atlantirivulus janeiroensis | 0 | 0 | 0 | 0 | 0 | 11 | 0 | 11 | 0 |
| Atlantirivulus jurubatibensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 56 |
| Atlantirivulus lazzarotoi | 44 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Atlantirivulus maricensis | 0 | 0 | 0 | 0 | 52 | 0 | 0 | 0 | 0 |
| Atlantirivulus simplicis | 43 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Australoheros ipatinguensis | 0 | 0 | 0 | 93 | 3 | 78 | 32 | 161 | 329 |
| Australoheros oblongus | 0 | 26 | 116 | 6 | 118 | 0 | 0 | 0 | 2 |
| Awaous tajasica | 115 | 8 | 0 | 3 | 81 | 19 | 35 | 116 | 24 |
| Brachyhypopomus janeiroensis | 0 | 0 | 0 | 1 | 127 | 82 | 12 | 13 | 50 |
| Brycon insignis | 0 | 5 | 1 | 4 | 1 | 10 | 0 | 7 | 0 |
| Brycon opalinus | 0 | 31 | 20 | 10 | 0 | 0 | 14 | 0 | 25 |
| Bryconamericus microcephalus | 919 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bryconamericus ornaticeps | 0 | 287 | 0 | 0 | 1900 | 0 | 0 | 0 | 0 |
| Bryconamericus tenuis | 0 | 0 | 0 | 20 | 0 | 128 | 88 | 287 | 40 |
| Callichthys callichthys | 0 | 59 | 27 | 3 | 207 | 21 | 11 | 57 | 168 |
| Characidium alipioi | 0 | 0 | 0 | 21 | 0 | 119 | 45 | 18 | 89 |
| Characidium grajahuense | 0 | 134 | 0 | 0 | 333 | 0 | 0 | 0 | 0 |
| Characidium interruptum | 0 | 15 | 0 | 0 | 250 | 236 | 0 | 43 | 152 |
| Characidium japuhybense | 869 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Characidium lauroi | 0 | 663 | 134 | 411 | 0 | 0 | 0 | 0 | 0 |
| Characidium litorale | 0 | 0 | 0 | 0 | 0 | 595 | 0 | 172 | 33 |
| Characidium vidali | 0 | 146 | 0 | 124 | 932 | 0 | 10 | 346 | 0 |
| Cichla kelberi | 0 | 13 | 19 | 3 | 2 | 81 | 0 | 1 | 3 |
| Clarias gariepinus | 0 | 0 | 0 | 0 | 4 | 13 | 0 | 9 | 2 |
| Colossoma macropomum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Coptodon rendalli | 1 | 48 | 2 | 2 | 352 | 0 | 5 | 22 | 475 |
| Crenicichla lacustris | 0 | 25 | 40 | 11 | 4 | 44 | 60 | 74 | 604 |
| Crenicichla sp. | 0 | 47 | 0 | 0 | 83 | 2 | 0 | 0 | 1 |
| Cyphocharax gilbert | 0 | 477 | 5 | 6 | 48 | 344 | 21 | 776 | 409 |
| Delturus parahybae | 0 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 1 |
| Deuterodon giton | 0 | 158 | 157 | 312 | 0 | 2221 | 243 | 752 | 1954 |
| Deuterodon hastatus | 193 | 269 | 0 | 32 | 2752 | 0 | 0 | 0 | 2 |
| Deuterodon heterostomus | 0 | 4 | 42 | 0 | 4 | 50 | 0 | 2 | 2 |
| Deuterodon intermedius | 154 | 2580 | 485 | 187 | 18 | 0 | 154 | 0 | 52 |
| Deuterodon janeiroensis | 0 | 5745 | 0 | 7 | 1190 | 512 | 17 | 0 | 0 |
| Deuterodon luetkenii | 0 | 0 | 0 | 0 | 35 | 98 | 6 | 6361 | 1451 |
| Deuterodon taeniatus | 0 | 27 | 9 | 64 | 8 | 984 | 349 | 3103 | 880 |
| Dormitator maculatus | 11 | 13 | 0 | 0 | 8 | 1 | 0 | 3 | 122 |
| Eigenmannia virescens | 0 | 20 | 13 | 17 | 15 | 43 | 127 | 14 | 32 |
| Eleotris pisonis | 32 | 10 | 0 | 0 | 29 | 27 | 5 | 28 | 36 |
| Geophagus brasiliensis | 4095 | 989 | 255 | 379 | 1836 | 223 | 364 | 1080 | 1289 |
| Glanidium melanopterum | 0 | 14 | 53 | 9 | 0 | 2 | 25 | 4 | 11 |
| Gymnotus carapo | 0 | 92 | 49 | 52 | 59 | 45 | 11 | 14 | 81 |
| Gymnotus pantherinus | 38 | 10 | 0 | 0 | 61 | 47 | 5 | 42 | 5 |
| Harttia carvalhoi | 0 | 88 | 11 | 24 | 0 | 0 | 0 | 0 | 23 |
| Harttia loricariformis | 0 | 32 | 18 | 54 | 0 | 0 | 55 | 0 | 9 |
| Hemipsilichthys gobio | 0 | 9 | 0 | 10 | 1 | 0 | 8 | 0 | 0 |
| Hemipsilichthys nimius | 145 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hemipsilichthys papillatus | 0 | 7 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hisonotus notatus | 0 | 205 | 6 | 3 | 776 | 31 | 31 | 2 | 113 |
| Hisonotus thayeri | 0 | 2 | 0 | 0 | 0 | 557 | 92 | 92 | 107 |
| Hollandichthys multifasciatus | 344 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Homodiaetus banguela | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 0 | 0 |
| Homodiaetus passarellii | 0 | 2 | 0 | 0 | 48 | 0 | 0 | 0 | 0 |
| Hoplerythrinus unitaeniatus | 0 | 2 | 1 | 0 | 17 | 31 | 0 | 13 | 157 |
| Hoplias lacerdae | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| Hoplias malabaricus | 14 | 46 | 28 | 20 | 120 | 100 | 24 | 163 | 284 |
| Hoplisoma nattereri | 0 | 68 | 34 | 99 | 321 | 87 | 2 | 7 | 163 |
| Hoplosternum littorale | 0 | 96 | 23 | 8 | 42 | 1 | 4 | 3 | 314 |
| Hyphessobrycon bifasciatus | 0 | 155 | 8 | 58 | 1077 | 192 | 11 | 5689 | 2997 |
| Hyphessobrycon eques | 0 | 70 | 40 | 2 | 18 | 0 | 17 | 0 | 194 |
| Hyphessobrycon flammeus | 0 | 48 | 0 | 1 | 40 | 12 | 0 | 0 | 4 |
| Hyphessobrycon reticulatus | 25 | 33 | 0 | 0 | 249 | 15 | 0 | 427 | 650 |
| Hypomasticus copelandii | 0 | 12 | 7 | 13 | 30 | 13 | 19 | 22 | 145 |
| Hypomasticus mormyrops | 0 | 10 | 18 | 12 | 0 | 0 | 17 | 1 | 4 |
| Hypomasticus thayeri | 0 | 0 | 6 | 3 | 0 | 0 | 0 | 0 | 3 |
| Hypostomus affinis | 0 | 21 | 20 | 109 | 5 | 98 | 117 | 69 | 61 |
| Hypostomus auroguttatus | 0 | 0 | 2 | 2 | 0 | 0 | 2 | 0 | 8 |
| Hypostomus luetkeni | 0 | 28 | 91 | 68 | 0 | 0 | 62 | 0 | 67 |
| Hypostomus punctatus | 3 | 95 | 4 | 0 | 263 | 5 | 17 | 0 | 4 |
| Imparfinis minutus | 0 | 61 | 1 | 4 | 0 | 0 | 6 | 0 | 7 |
| Imparfinis piperatus | 0 | 4 | 62 | 0 | 4 | 0 | 0 | 0 | 0 |
| Ituglanis parahybae | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 3 |
| Jenynsia darwini | 0 | 0 | 0 | 0 | 0 | 32 | 0 | 165 | 1549 |
| Jenynsia lineata | 0 | 0 | 0 | 0 | 350 | 72 | 0 | 0 | 0 |
| Knodus moenkhausii | 0 | 0 | 0 | 267 | 0 | 0 | 10 | 0 | 29 |
| Kronichthys heylandi | 667 | 167 | 0 | 1 | 151 | 5 | 0 | 0 | 0 |
| Kryptolebias brasiliensis | 0 | 59 | 0 | 0 | 304 | 22 | 0 | 0 | 1 |
| Kryptolebias caudomarginatus | 0 | 206 | 0 | 0 | 56 | 0 | 0 | 0 | 0 |
| Kryptolebias gracilis | 0 | 0 | 0 | 0 | 0 | 35 | 0 | 14 | 0 |
| Kryptolebias ocellatus | 0 | 86 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leptolebias marmoratus | 0 | 0 | 0 | 0 | 60 | 0 | 0 | 0 | 0 |
| Leptopanchax citrinipinnis | 0 | 0 | 0 | 0 | 225 | 0 | 0 | 0 | 0 |
| Leptopanchax opalescens | 0 | 7 | 0 | 0 | 83 | 0 | 0 | 0 | 0 |
| Leptopanchax sanguineus | 0 | 0 | 0 | 0 | 55 | 0 | 0 | 0 | 0 |
| Leptopanchax splendens | 0 | 0 | 0 | 0 | 69 | 0 | 0 | 0 | 0 |
| Listrura costai | 25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Listrura macacuensis | 0 | 0 | 0 | 0 | 48 | 0 | 0 | 0 | 0 |
| Listrura macaensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 1 |
| Listrura menezesi | 0 | 0 | 0 | 0 | 0 | 68 | 0 | 0 | 0 |
| Listrura nematopteryx | 0 | 0 | 0 | 0 | 120 | 0 | 0 | 0 | 0 |
| Listrura tetraradiata | 0 | 0 | 0 | 0 | 0 | 89 | 0 | 0 | 0 |
| Loricariichthys castaneus | 0 | 96 | 15 | 1 | 24 | 317 | 19 | 1 | 104 |
| Loricariichthys melanurus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 85 |
| Megaleporinus conirostris | 0 | 12 | 68 | 4 | 0 | 0 | 23 | 0 | 46 |
| Metynnis lippincottianus | 0 | 0 | 1 | 0 | 0 | 4 | 0 | 1 | 10 |
| Metynnis maculatus | 0 | 6 | 4 | 0 | 0 | 13 | 1 | 4 | 0 |
| Microcambeva barbata | 0 | 0 | 0 | 0 | 0 | 71 | 0 | 4 | 0 |
| Microcambeva bendego | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 0 | 0 |
| Microglanis nigripinnis | 0 | 0 | 0 | 0 | 17 | 0 | 0 | 0 | 0 |
| Microglanis parahybae | 0 | 32 | 0 | 0 | 34 | 0 | 46 | 0 | 72 |
| Microglanis pleriqueater | 0 | 0 | 0 | 0 | 0 | 57 | 0 | 13 | 0 |
| Mimagoniates microlepis | 810 | 260 | 0 | 646 | 1377 | 501 | 0 | 30 | 33 |
| Nannostomus beckfordi | 0 | 0 | 0 | 0 | 71 | 86 | 0 | 0 | 0 |
| Nematolebias catimbau | 0 | 0 | 0 | 0 | 0 | 102 | 0 | 0 | 0 |
| Nematolebias papilliferus | 0 | 0 | 0 | 0 | 205 | 0 | 0 | 0 | 0 |
| Nematolebias whitei | 0 | 0 | 0 | 0 | 0 | 510 | 0 | 39 | 0 |
| Neoplecostomus microps | 0 | 825 | 225 | 137 | 82 | 0 | 118 | 223 | 1 |
| Neoplecostomus paraty | 163 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neoplecostomus variipictus | 0 | 0 | 0 | 55 | 0 | 0 | 80 | 0 | 0 |
| Notholebias cruzi | 0 | 0 | 0 | 0 | 29 | 56 | 0 | 0 | 0 |
| Notholebias fractifasciatus | 0 | 0 | 0 | 0 | 141 | 2 | 0 | 1 | 0 |
| Notholebias minimus | 0 | 370 | 0 | 0 | 15 | 0 | 0 | 0 | 0 |
| Notholebias vermiculatus | 0 | 0 | 0 | 0 | 0 | 133 | 0 | 0 | 0 |
| Oligosarcus acutirostris | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Oligosarcus hepsetus | 10 | 347 | 114 | 82 | 78 | 25 | 92 | 167 | 262 |
| Oncorhynchus mykiss | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 0 |
| Ophthalmolebias constanciae | 0 | 0 | 0 | 0 | 0 | 71 | 0 | 65 | 4 |
| Oreochromis niloticus | 1 | 6 | 1 | 18 | 120 | 31 | 0 | 9 | 11 |
| Otocinclus affinis | 0 | 6 | 1 | 14 | 36 | 3 | 1 | 1 | 22 |
| Otothyris lophophanes | 2 | 24 | 4 | 3 | 53 | 135 | 3 | 130 | 52 |
| Pachyurus adspersus | 0 | 5 | 9 | 1 | 0 | 0 | 7 | 0 | 29 |
| Parachromis managuensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Paragenidens grandoculis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Pareiorhaphis garbei | 0 | 0 | 0 | 0 | 232 | 13 | 22 | 54 | 0 |
| Pareiorhina brachyrhyncha | 0 | 0 | 171 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pareiorhina rudolphi | 52 | 553 | 661 | 0 | 0 | 0 | 0 | 0 | 0 |
| Parotocinclus bidentatus | 0 | 0 | 22 | 1 | 0 | 0 | 0 | 0 | 0 |
| Parotocinclus fluminense | 0 | 0 | 0 | 0 | 0 | 520 | 0 | 0 | 0 |
| Parotocinclus maculicauda | 25 | 392 | 0 | 0 | 941 | 36 | 4 | 183 | 0 |
| Parotocinclus muriaensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 |
| Phalloceros anisophallos | 3423 | 1169 | 0 | 0 | 101 | 0 | 0 | 0 | 0 |
| Phalloceros enneaktinos | 701 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Phalloceros harpagos | 468 | 3262 | 276 | 1295 | 773 | 905 | 254 | 4394 | 1624 |
| Phalloceros leptokeras | 946 | 257 | 121 | 2216 | 1432 | 56 | 0 | 0 | 0 |
| Phalloptychus januarius | 0 | 33 | 0 | 0 | 1080 | 158 | 0 | 0 | 11157 |
| Piabina argentea | 0 | 0 | 2 | 0 | 0 | 0 | 10 | 0 | 0 |
| Pimelodella lateristriga | 103 | 63 | 4 | 34 | 226 | 204 | 82 | 181 | 246 |
| Pimelodus maculatus | 0 | 11 | 59 | 11 | 0 | 0 | 8 | 0 | 15 |
| Poecilia reticulata | 465 | 2025 | 271 | 1247 | 5239 | 30 | 95 | 5 | 159 |
| Poecilia vivipara | 120 | 419 | 3 | 106 | 2484 | 446 | 135 | 2072 | 10355 |
| Pogonopoma parahybae | 0 | 1 | 9 | 1 | 0 | 0 | 18 | 0 | 0 |
| Prochilodus lineatus | 0 | 2 | 4 | 0 | 0 | 0 | 13 | 6 | 233 |
| Prochilodus vimboides | 0 | 7 | 101 | 1 | 0 | 0 | 1 | 12 | 175 |
| Psalidodon parahybae | 0 | 144 | 213 | 105 | 104 | 196 | 410 | 10 | 1715 |
| Pseudobunocephalus iheringii | 0 | 0 | 0 | 0 | 6 | 8 | 0 | 0 | 0 |
| Pseudocorynopoma doriae | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 |
| Pseudotothyris janeirensis | 0 | 147 | 0 | 0 | 30 | 9 | 0 | 29 | 18 |
| Pyrrhulina australis | 0 | 0 | 0 | 0 | 13 | 0 | 0 | 2 | 0 |
| Pyrrhulina filamentosa | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 |
| Rhamdia quelen | 43 | 141 | 32 | 32 | 190 | 27 | 26 | 62 | 73 |
| Rhamdioglanis frenatus | 55 | 4 | 0 | 0 | 3 | 0 | 3 | 0 | 0 |
| Rhamdioglanis transfasciatus | 0 | 69 | 0 | 0 | 108 | 108 | 1 | 132 | 0 |
| Rineloricaria nigricauda | 0 | 282 | 189 | 366 | 0 | 0 | 4 | 0 | 4 |
| Rineloricaria nudipectoris | 0 | 25 | 0 | 265 | 1331 | 62 | 0 | 152 | 0 |
| Rineloricaria paraibensis | 0 | 273 | 129 | 15 | 0 | 0 | 0 | 0 | 0 |
| Rineloricaria steindachneri | 0 | 0 | 0 | 0 | 0 | 0 | 107 | 1 | 24 |
| Rineloricaria zawadzkii | 43 | 236 | 15 | 0 | 19 | 0 | 0 | 0 | 0 |
| Salminus brasiliensis | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 5 |
| Schizolecis guentheri | 2647 | 622 | 0 | 20 | 1394 | 123 | 42 | 563 | 0 |
| Scleromystax barbatus | 442 | 147 | 0 | 134 | 742 | 144 | 46 | 54 | 7 |
| Scleromystax prionotos | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 7 |
| Spintherobolus broccae | 0 | 10 | 0 | 0 | 159 | 39 | 0 | 17 | 0 |
| Steindachneridion parahybae | 0 | 0 | 15 | 4 | 0 | 0 | 1 | 0 | 5 |
| Synbranchus sp. | 3 | 7 | 11 | 2 | 59 | 14 | 4 | 12 | 8 |
| Taunayia bifasciata | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trachelyopterus striatulus | 0 | 31 | 1 | 4 | 5 | 25 | 9 | 81 | 140 |
| Trichogenes longipinnis | 627 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichomycterus albinotatus | 0 | 0 | 96 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichomycterus auroguttatus | 0 | 0 | 142 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichomycterus caipora | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 110 | 98 |
| Trichomycterus claudiae | 0 | 84 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichomycterus florensis | 0 | 0 | 16 | 16 | 0 | 0 | 0 | 0 | 0 |
| Trichomycterus fuliginosus | 0 | 0 | 0 | 0 | 0 | 0 | 64 | 0 | 0 |
| Trichomycterus giganteus | 0 | 242 | 0 | 0 | 153 | 0 | 0 | 0 | 0 |
| Trichomycterus goeldii | 0 | 0 | 0 | 179 | 0 | 0 | 0 | 0 | 0 |
| Trichomycterus itatiayae | 0 | 0 | 217 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichomycterus jacupiranga | 452 | 30 | 0 | 0 | 57 | 0 | 0 | 0 | 0 |
| Trichomycterus largoperculatus | 0 | 0 | 0 | 32 | 0 | 0 | 28 | 0 | 0 |
| Trichomycterus macrophthalmus | 0 | 142 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichomycterus mariamole | 0 | 1 | 83 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichomycterus mirissumba | 0 | 0 | 90 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichomycterus nigricans | 0 | 0 | 0 | 0 | 1099 | 16 | 0 | 0 | 0 |
| Trichomycterus nigroauratus | 0 | 238 | 16 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichomycterus paquequerense | 0 | 0 | 0 | 1013 | 0 | 0 | 0 | 0 | 0 |
| Trichomycterus potschi | 0 | 93 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichomycterus puriventris | 0 | 0 | 0 | 0 | 0 | 0 | 222 | 0 | 3 |
| Trichomycterus quintus | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichomycterus santaeritae | 0 | 0 | 9 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichomycterus saquarema | 0 | 0 | 0 | 0 | 0 | 14 | 0 | 0 | 0 |
| Trichomycterus travassosi | 0 | 0 | 114 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichomycterus vitalbrazili | 0 | 0 | 0 | 0 | 0 | 0 | 87 | 0 | 0 |
| Trichopodus trichopterus | 0 | 0 | 0 | 0 | 41 | 0 | 0 | 0 | 0 |
| Trichopsis vittata | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 |
| Xiphophorus helleri | 0 | 44 | 0 | 0 | 56 | 0 | 3 | 0 | 0 |
References
- Bizerril, C.R.S.F.; Primo, P.B.S. Peixes de Águas Interiores do Estado do Rio de Janeiro; FEMAR—SEMADS: Rio de Janeiro, Brazil, 2001; 417p. [Google Scholar]
- Bizerril, C.R.S.F.; Auraújo, R.M.C. Description d’une nouvelle espèce du genre Bryconamericus (Characidae, Tetragonopterinae) du Brésil oriental. Rev. Fr. Aquariol. 1992, 19, 65–68. [Google Scholar]
- Costa, W.J.E.M.; Campos-da-Paz, R. Description d’une nouvelle espèce de poisson électrique du genre néotropical Hypopomus (Siluriformes: Gymnotoidei: Hypopomidae) du sud-est du Brésil. Rev. Fr. Aquariol. 1992, 18, 117–120. [Google Scholar]
- Pereira, E.H.L.; Reis, R.E.; Souza, P.F.M.; Lazzarotto, H. A new species of the loricariid catfish genus Hemipsilichthys from southern Rio de Janeiro coastal rivers, southeastern Brazil (Teleostei: Siluriformes). Zootaxa 2003, 285, 1–10. [Google Scholar] [CrossRef]
- Gauger, M.F.W.; Buckup, P.A. Two new species of Hypoptopomatinae from the rio Paraíba do Sul basin, with comments on the monophyly of Parotocinclus and the Otothyrini (Siluriformes: Loricariidae). Neotrop. Ichthyol. 2005, 3, 509–518. [Google Scholar] [CrossRef][Green Version]
- Lucinda, P.H.F. Systematics and biogeography of the genus Phalloceros Eigenmann, 1907 (Cyprinodontiformes: Poeciliidae: Poeciliinae), with the description of twenty-one new species. Neotrop. Ichthyol. 2008, 6, 113–158. [Google Scholar] [CrossRef][Green Version]
- Barbosa, M.A.; Costa, W.J.E.M. Seven new species of the catfish genus Trichomycterus (Teleostei: Siluriformes: Trichomycteridae) from southeastern Brazil and redescription of T. brasiliensis. Ichthyol. Explor. Freshw. 2010, 21, 97–122. [Google Scholar]
- Costa, W.J.E.M. Four new species of the genus Atlantirivulus (Cyprinodontiformes: Rivulidae) from the Brazilian Atlantic Forest. Vertebr. Zool. 2014, 64, 9–21. [Google Scholar] [CrossRef]
- Leitão, R.P.; Buckup, P.A. A new species of Characidium (Characiformes: Crenuchidae) from coastal basins of Serra do Mar, southeastern Brazil. Copeia 2014, 2014, 14–22. [Google Scholar] [CrossRef]
- Silva, P.C.; Malabarba, M.C.; Malabarba, L.R. Integrative taxonomy: Morphology and ancient DNA barcoding reveals the true identity of Astyanax taeniatus, a tetra collected by Charles Darwin during the Beagle’s Voyage. Zool. Anz. 2019, 278, 110–120. [Google Scholar] [CrossRef]
- Medeiros, L.S.; Moreira, C.R.; Pinna, M.C.C.; Lima, S.M.Q. A new catfish species of Microcambeva Costa & Bockmann 1994 (Siluriformes: Trichomycteridae) from a coastal basin in Rio de Janeiro State, southeastern Brazil. Zootaxa 2020, 4895, 111–123. [Google Scholar] [CrossRef]
- Costa-Silva, G.J.; Silva, G.S.C.; Oliveira, C. A new species of spiny Rineloricaria (Siluriformes: Loricariidae) from the Rio Paraíba do Sul basin and coastal rivers from Rio de Janeiro State. Zootaxa 2022, 5175, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Mejia, E.; Ferraro, G.A.; Buckup, P.A. A new species of Rineloricaria (Siluriformes: Loricariidae) from coastal drainages of Rio de Janeiro, southeastern Brazil. Neotrop. Ichthyol. 2023, 21, e220083. [Google Scholar] [CrossRef]
- Gomes, J.R. Levantamento da ictiofauna do Maciço da Pedra Branca e arredores, Rio de Janeiro, estado do Rio de Janeiro. Arq. Mus. Nac. Rio J. 2006, 64, 309–320. [Google Scholar]
- Souza-Lima, R.; Miranda, J.C.; Portugal, A.S. Ictiofauna do Rio Aldeia, São Gonçalo. In Estudos Ambientais em Regiões Metropolitanas: São Gonçalo; Santos, M.G., Ed.; UERJ: Rio de Janeiro, Brazil, 2012; pp. 115–134. [Google Scholar]
- Di Dario, F.; Petry, A.C.; Pereira, M.M.S.; Mincarone, M.M.; Agostinho, L.S.; Camara, E.M.; Caramaschi, E.P.; Britto, M.R. An update on the fish composition (Teleostei) of the coastal lagoons of the Restinga de Jurubatiba National Park and the Imboassica Lagoon, northern Rio de Janeiro State. Acta Limnol. Bras. 2013, 25, 257–278. [Google Scholar] [CrossRef][Green Version]
- Buckup, P.A.; Britto, M.R.; Souza-Lima, R.; Pascoli, J.C.; Villa-Verde, L.; Ferraro, G.A.; Salgado, F.L.K.; Gomes, J.R. Guia de Identificação das Espécies de Peixes da Bacia do Rio das Pedras; The Nature Conservancy: Rio de Janeiro, Brazil, 2014; 79p. [Google Scholar]
- Camilo, G.S.; Terra, B.; Araújo, F.G. Ichthyofauna from the Parque Nacional da Serra dos Órgãos and its surrounding areas, Rio de Janeiro state, Brazil. Check List 2015, 11, 1696. [Google Scholar] [CrossRef]
- De Brito, V.; Buckup, P.A. The fish fauna of the upper Piraí drainage, a transposed mountain river system in southeastern Brazil. Check List 2019, 15, 235–247. [Google Scholar] [CrossRef]
- Guimarães, F.V.; Souza, T.M.; Rodrigues, R.R.; Souza-Lima, R. Composition and distribution of fishes from the Perequê-Açu river basin, Paraty, Rio de Janeiro, Southeastern Brazil. Biota Neotrop. 2021, 21, e20201096. [Google Scholar] [CrossRef]
- Dopazo, M.; Souto-Santos, I.C.A.; Britto, M.R.; Moreira, C.R.; Buckup, P.A. The freshwater fishes from the Costa Verde Fluminense region of southeastern Brazil. Biota Neotrop. 2023, 23, e20221422. [Google Scholar] [CrossRef]
- Araújo, F.G. Adaptação do índice de integridade biótica usando a comunidade de peixes para o rio Paraíba do Sul. Rev. Bras. Biol. 1998, 58, 547–558. [Google Scholar] [CrossRef]
- Mazzoni, R.; Iglesias-Rios, R. Distribution pattern of two fish species in a coastal stream in southeast Brazil. Braz. J. Biol. 2002, 62, 171–178. [Google Scholar] [CrossRef][Green Version]
- Mazzoni, R.; Iglesias-Rios, R. Environmentally related life history variations in Geophagus brasiliensis. J. Fish. Biol. 2002, 61, 1606–1618. [Google Scholar] [CrossRef]
- Mazzoni, R.; Verani-Fenerich, N.; Caramaschi, E.P.; Iglesias-Rios, R. Stream-dwelling fish communities from an Atlantic Rain Forest drainage. Braz. Arch. Biol. Technol. 2006, 49, 249–256. [Google Scholar] [CrossRef][Green Version]
- Schubart, S.A.; Mazzoni, R. Produtividade de peixes em um riacho costeiro da bacia do Leste, Rio de Janeiro, Brasil. Iheringia Ser. Zool. 2006, 96, 399–405. [Google Scholar] [CrossRef][Green Version]
- Araújo, F.G.; Pinto, B.C.T.; Teixeira, T.P. Longitudinal patterns of fish assemblages in a large tropical river in southeastern Brazil: Evaluating environmental influences and some concepts in river ecology. Hydrobiologia 2009, 618, 89–107. [Google Scholar] [CrossRef]
- de Macedo-Soares, P.H.M.; Petry, A.C.; Farjalla, V.F.; Caramaschi, E.P. Hydrological connectivity in coastal inland systems: Lessons from a Neotropical fish metacommunity. Ecol. Freshw. Fish. 2010, 19, 7–18. [Google Scholar] [CrossRef]
- Terra, B.F.; Hughes, R.M.; Francelino, M.R.; Araújo, F.G. Assessment of biotic condition of Atlantic Rain Forest streams: A fish-based multimetric approach. Ecol. Indic. 2013, 34, 136–148. [Google Scholar] [CrossRef]
- Terra, B.F.; Hughes, R.M.; Araújo, F.G. Fish assemblages in Atlantic Forest streams: The relative influence of local and catchment environments on taxonomic and functional species. Ecol. Freshw. Fish. 2016, 25, 527–544. [Google Scholar] [CrossRef]
- Reis, R.B.; Frota, A.; Deprá, G.C.; Ota, R.R.; Da Graça, W.J. Freshwater fishes from Paraná state, Brazil: An annotated list, with comments on biogeographic patterns, threats, and future perspectives. Zootaxa 2020, 4868, 451–494. [Google Scholar] [CrossRef]
- IBGE. Cidades e Estados. Rio de Janeiro. Available online: https://www.ibge.gov.br/cidades-e-estados/rj.html (accessed on 15 May 2024).
- Bizerril, C.R.S.F. A Ictiofauna da Bacia do Rio Paraíba do Sul. Biodiversidade e Padrões Biogeográficos. Braz. Arch. Biol. Technol. 1999, 42, 1–17. [Google Scholar] [CrossRef]
- Sabaj-Pérez, M.H. Codes for natural history collections in ichthyology and herpetology. Ichthyol. Herpetol. 2020, 108, 1–76. [Google Scholar] [CrossRef]
- Fricke, R.; Eschmeyer, W.N.; Van Der Laan, R. Eschmeyer’s Catalog of Fishes: Genera, Species, References. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 15 May 2024).
- Angrizani, R.C.; Malabarba, L.R. Genetic diversity and species delimitation in Rhamdia (Siluriformes: Heptapteridae) in South America, with a redescription of R. quelen (Quoy & Gaimard, 1824). Zootaxa 2020, 4801, 85–104. [Google Scholar] [CrossRef]
- Craig, J.M.; Crampton, W.G.R.; Albert, J.S. Revision of the polytypic electric fish Gymnotus carapo (Gymnotiformes, Teleostei), with descriptions of seven subspecies. Zootaxa 2017, 4318, 401–438. [Google Scholar] [CrossRef]
- Dergam, J.A.; Suzuki, H.I.; Shibatta, O.A.; Duboc, L.F.; Júlio, H.F., Jr.; Giuliano-Caetano, L.; Black, W.C., IV. Molecular biogeography of the neotropical fish Hoplias malabaricus (Erythrinidae: Characiformes) in the Iguaçu, Tibagi and Paraná Rivers. Genet. Mol. Biol. 1998, 21, 493–496. [Google Scholar] [CrossRef]
- Bertaco, V.A.; Ferrer, J.; Carvalho, F.R.; Malabarba, L.R. Inventory of the freshwater fishes from a densely collected area in South America—A case study of the current knowledge of Neotropical fish diversity. Zootaxa 2016, 4138, 401–440. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, K.L.A.; Rosso, J.J.; González-Castro, M.; Souza, M.F.B.; Díaz de Astarloa, J.M.; Rodrigues, L.R.R. A new species of Hoplias malabaricus species complex (Characiformes: Erythrinidae) from the Crepori River, Amazon basin, Brazil. J. Fish. Biol. 2022, 100, 425–443. [Google Scholar] [CrossRef]
- Torres, R.A.; Roper, J.J.; Foresti, F.; Oliveira, C. Surprising genomic diversity in the Neotropical fish Synbranchus marmoratus (Teleostei: Synbranchidae): How many species? Neotrop. Ichthyol. 2005, 3, 277–284. [Google Scholar] [CrossRef]
- Ferreira Júnior, O. GPS Software for Mapping and Innovative Solutions for Asset Tracking: GPS Trackmaker Pro v. 5.1; Geo Studio Tecnologia Ltd.: Belo Horizonte, Brazil, 2012. [Google Scholar]
- Instituto Brasileiro de Geografia e Estatística (IBGE). Base Cartográfica Vetorial Contínua do Estado do Rio de Janeiro na Escala 1:25.000—BC25_RJ. Available online: https://www.ibge.gov.br/geociencias/downloads-geociencias.html?caminho=cartas_e_mapas/bases_cartograficas_continuas/bc250/versao2023/ (accessed on 1 June 2024).
- Instituto Brasileiro de Geografia e Estatística (IBGE). Área Territorial—Brasil, Grandes Regiões, Unidades da Federação e Municípios. Available online: https://geoftp.ibge.gov.br/organizacao_do_territorio/estrutura_territorial/areas_territoriais/2022/AR_BR_RG_UF_RGINT_MES_MIC_MUN_2022.xls (accessed on 1 June 2024).
- Silva, T.N.; Sarmento-Soares, L.M.; Martins-Pinheiro, R.F.; Santos, A.C.A. Composition and distribution of the fish fauna in the Rio Jacuípe, northernmost tributary of the Rio Paraguaçu basin, Bahia, Brazil. Stud. Neotrop. Fauna Environ. 2021, 58, 300–316. [Google Scholar] [CrossRef]
- Dajoz, R. Ecologia Geral, 4th ed.; Vozes: Petrópolis, Brazil, 1983; 472p. [Google Scholar]
- Harper, D.A.T. Numerical Palaeobiology: Computer-Based Modelling and Analysis of Fossils and Their Distributions; John Wiley & Sons: Chichester, NH, USA, 1999; 468p. [Google Scholar]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Eletron 2001, 4, 1–9. [Google Scholar]
- Walther, B.A.; Moore, J.L. The concepts of bias, precision and accuracy, and their use in testing the performance of species richness estimators, with a literature review of estimator performance. Ecography 2005, 28, 815–829. [Google Scholar] [CrossRef]
- Chao, A. Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Chao, A. Estimating the population size for capture-recapture data with unequal catchability. Biometrics 1987, 43, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.H.; Wang, Y.T.; Walther, B.A.; Chao, A. An improved nonparametric lower bound of species richness via a modified Good-Turing frequency formula. Biometrics 2014, 70, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Lee, S.-M. Estimating the number of classes via sample coverage. J. Am. Stat. Assoc. 1992, 87, 210–217. [Google Scholar] [CrossRef]
- Chao, A.; Hwang, W.-H.; Chen, Y.-C.; Kuo, C.-Y. Estimating the number of shared species in two communities. Stat. Sin. 2000, 10, 227–246. [Google Scholar]
- Alroy, J. Limits to species richness in terrestrial communities. Ecol. Lett. 2018, 21, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Zizka, A.; Silvestro, D.; Andermann, T.; Azevedo, J.; Duarte Ritter, C.; Edler, D.; Farooq, H.; Herdean, A.; Ariza, M.; Scharn, R.; et al. CoordinateCleaner: Standardized cleaning of occurrence records from biological collection databases. Methods Ecol. Evol. 2019, 10, 744–751. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing v. 4.3; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org (accessed on 14 August 2024).
- Edler, D.; Guedes, T.; Zizka, A.; Rosvall, M.; Antonelli, A. Infomap bioregions: Interactive mapping of biogeographical regions from species distributions. Syst. Biol. 2017, 66, 197–204. [Google Scholar] [CrossRef]
- Oliveira, U.; Soares-Filho, B.; Leitão, R.F.M.; Rodrigues, H.O. BioDinamica: A toolkit for analyses of biodiversity and biogeography on the Dinamica-EGO modelling platform. PeerJ 2019, 7, e7213. [Google Scholar] [CrossRef]
- Ferreira, B.M.; Soares-Filho, B.S.; Pereira, F.M.Q. The Dinamica EGO virtual machine. Sci. Comput. Program. 2019, 173, 3–20. [Google Scholar] [CrossRef]
- Williams, P.H.; Humphries, C.J. Biodiversity, Taxonomic Relatedness, and Endemism in Conservation; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Oliveira, U.; Vasconcelos, M.F.; Santos, A.J. Biogeography of Amazon birds: Rivers limit species composition, but not areas of endemism. Sci. Rep. 2017, 7, 2992. [Google Scholar] [CrossRef]
- Melo, F.A.G. Revisão taxonômica das espécies do gênero Astyanax Baird & Girard, 1854 (Teleostei: Characiformes: Characidae) da região da serra dos Órgãos. Arq. Mus. Nac. Rio J. 2001, 59, 1–46. [Google Scholar]
- Bertaco, V.A.; Lucena, C.A.S. Two new species of Astyanax (Ostariophysi: Characiformes: Characidae) from eastern Brazil, with a synopsis of the Astyanax scabripinnis species complex. Neotrop. Ichthyol. 2006, 4, 53–60. [Google Scholar] [CrossRef]
- Oyakawa, O.T.; Fischberg, I.; Langeani, F. Harttia absaberi, a new species of loricariid catfish (Siluriformes: Loricariidae: Loricariinae) from the upper Rio Paraná basin, Brazil. Neotrop. Ichthyol. 2013, 11, 779–786. [Google Scholar] [CrossRef]
- Botelho, G. Síntese da História da Aquariofilia; Interciência: Rio de Janeiro, Brazil, 1990. [Google Scholar]
- Souto-Santos, I.C.A.; Beltrão, G.B.M. Resgate histórico de lotes provenientes da primeira firma de aquarismo do Brasil na Coleção Ictiológica do Museu Nacional. Bol. Soc. Bras. Ictiol. 2018, 125, 2–6. [Google Scholar]
- Menezes, N.A.; Weitzman, S.H.; Oyakawa, O.T.; Lima, F.C.; Castro, R.M.C.; Weitzman, M.J. Peixes de água doce da Mata Atlântica; Neotrópica: São Paulo, Brazil, 2007; 407p. [Google Scholar]
- Lowe-McConnell, R.H. Estudos Ecológicos de Comunidades de Peixes Tropicais; EDUSP: São Paulo, Brazil, 1999; 536p. [Google Scholar]
- Bizerril, C.R.S.F. Análise taxonômica e biogeográfica da ictiofauna de água doce do leste brasileiro. Acta Biol. Leopoldensia 1994, 16, 51–80. [Google Scholar]
- Abell, R.; Thieme, M.L.; Revenga, C.; Bryer, M.; Kottelat, M.; Bogutskaya, N.; Coad, B.; Mandrak, N.; Balderas, S.C.; Bussing, W.; et al. Freshwater ecoregions of the world: A new map of biogeographic units for freshwater biodiversity conservation. BioScience 2008, 58, 403–414. [Google Scholar] [CrossRef]
- Dagosta, F.C.P.; de Pinna, M. Biogeography of Amazonian fishes: Deconstructing river basins as biogeographic units. Neotrop. Ichthyol. 2017, 15, e170034. [Google Scholar] [CrossRef]
- Thomaz, A.T.; Knowles, L.L. Flowing into the unknown: Inferred paleodrainages for studying the ichthyofauna of Brazilian coastal rivers. Neotrop. Ichthyol. 2018, 16, e180019. [Google Scholar] [CrossRef]
- Abreu, J.M.S.; Craig, J.M.; Albert, J.S.; Piorski, N.M. Historical biogeography of fishes from coastal basins of Maranhão State, northeastern Brazil. Neotrop. Ichthyol. 2019, 17, e180156. [Google Scholar] [CrossRef]
- Dagosta, F.C.P.; de Pinna, M.; Peres, C.A.; Tagliacollo, V.A. Existing protected areas provide a poor safety-net for threatened Amazonian fish species. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 1167–1189. [Google Scholar] [CrossRef]
- Buckup, P.A. The Eastern Brazilian Shield. In Historical Biogeography of Neotropical Freshwater Fishes; Albert, J.S., Reis, R.E., Eds.; University of California Press: Los Angeles, CA, USA, 2011; pp. 203–210. [Google Scholar] [CrossRef]
- Cassemiro, F.A.S.; Albert, J.S.; Antonelli, A.; Menegotto, A.; Wüest, R.O.; Cerezer, F.; Coelho, M.T.P.; Reis, R.E.; Tan, M.; Tagliacollo, V.; et al. Landscape dynamics and diversification of the megadiverse South American freshwater fish fauna. Proc. Natl. Acad. Sci. USA 2023, 120, e2211974120. [Google Scholar] [CrossRef] [PubMed]
- Igor, C.A.S.; Jennings, W.B.; Buckup, P.A. Testing palaeodrainage hypotheses in south-eastern Brazil: Phylogeography of the sinistral livebearer fish of the genus Phalloceros (Cyprinodontiformes: Poeciliidae). Zool. J. Linn. Soc. 2023, 197, 514–531. [Google Scholar] [CrossRef]
- Salgado, F.L.K. Astyanax viridis (Characiformes: Characidae), new species from southeastern Brazil. Int. J. Zool. Anim. Biol. 2021, 4, 327. [Google Scholar] [CrossRef]
- Castro, R.M.C.; Polaz, C.N.M. Small-sized fish: The largest and most threatened portion of the megadiverse neotropical freshwater fish fauna. Biota Neotrop. 2020, 20, e20180683. [Google Scholar] [CrossRef]
- Pio, N.L.; Carvalho, T.P. Evidence on the paleodrainage connectivity during Pleistocene: Phylogeography of a hypoptopomatine endemic to southeastern Brazilian coastal drainages. Neotrop. Ichthyol. 2021, 19, e200128. [Google Scholar] [CrossRef]
- Lima, S.M.Q.; Vasconcellos, A.V.; Berbel-Filho, W.M.; Lazoski, C.; Russo, C.A.; Sazima, I.; Solé-Cava, A.M. Effects of Pleistocene climatic and geomorphological changes on the population structure of the restricted-range catfish Trichogenes longipinnis (Siluriformes: Trichomycteridae). Syst. Biodivers. 2017, 14, 155–170. [Google Scholar] [CrossRef]
- Zalán, P.V.; Oliveira, J.A.B. Origem e evolução estrutural do Sistema de Riftes Cenozóicos do Sudeste do Brasil. Bol. Geoci. Petrobras 2005, 13, 269–300. [Google Scholar]
- Polaz, C.N.M.; Bataus, Y.S.L.; Desbiez, A.; Reis, M.L. Plano de Ação Nacional para a Conservação das Espécies Aquáticas Ameaçadas de Extinção da Bacia do Rio Paraíba do Sul; Instituto Chico Mendes de Conservação da Biodiversidade, ICMBio: Brasília, Brazil, 2011; 140p.
- Lamego, A.R. O homem e a Guanabara, 2nd ed.; IBGE—Conselho Nacional de Geografia: Rio de Janeiro, Brazil, 1964; 408p.
- Santos, A.B.I.; Terra, B.F.; Araújo, F.G. Influence of the river flow on the structure of fish assemblage along the longitudinal gradient from river to reservoir. Zoologia 2010, 27, 732–740. [Google Scholar] [CrossRef][Green Version]
- Oliveira, C.A.M. Revisão Taxonômica do Complexo de Espécies Astyanax Scabripinnis sensu Bertaco & Lucena (2006) (Ostariophysi: Characiformes: Characidae). Ph.D. Thesis, Programa de Pós-Graduação em Ecologia de Ambientes Aquáticos Continentais. Universidade Estadual de Maringá, Maringá, Brazil, 2017. [Google Scholar]
- Carvalho, F.R.; Jesus, G.C.; Langeani, F. Redescription of Hyphessobrycon flammeus Myers, 1924 (Ostariophysi: Characidae), a threatened species from Brazil. Neotrop. Ichthyol. 2014, 12, 247–256. [Google Scholar] [CrossRef]
- Vari, R.P.; Harold, A.S. Phylogenetic Study of the Neotropical Fish Genera Creagrutus Gunther and Piabina Reinhardt (Teleostei: Ostariophysi: Characiformes), with a Revision of the Cis-Andean Species. Smithson. Contrib. Zool. 2001, 613, 1–239. [Google Scholar] [CrossRef]
- São-Thiago, H. Composição e Distribuição Longitudinal da Ictiofauna do Rio Parati-Mirim (RJ) e Aspectos Sobre a Reprodução das Principais Espécies. Master’s Thesis, Programa de Pós-graduação em Zoologia. Museu Nacional da Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, 1990. [Google Scholar]
- Sazima, I. Natural history of Trichogenes longipinnis, a threatened trichomycterid catfish endemic to Atlantic forest streams in southeast Brazil. Ichthyol. Explor. Freshw. 2004, 15, 49–60. [Google Scholar]
- de Pinna, M.C.C.; Wosiacki, W. Family Trichomycteridae (Pencil or parasitic catfishes). In Check List of the Freshwater Fishes of South and Central America; Reis, R.E., Kullander, S.O., Ferraris, C.J., Jr., Eds.; EDIPUCRS: Porto Alegre, Brazil, 2003; pp. 270–290. [Google Scholar]
- Lima, S.M.Q.; Lazzarotto, H.; Costa, W.J.E.M. A new species of Trichomycterus (Siluriformes: Trichomycteridae) from Lagoa Feia drainage, southeastern Brazil. Neotrop. Ichthyol. 2008, 6, 315–322. [Google Scholar] [CrossRef]
- Costa, W.J.E.M.; Katz, A.M.; Mattos, J.L.O.; Amorim, P.F.; Mesquita, B.O.; Vilardo, P.J.; Barbosa, M.A. Historical review and redescription of three poorly known species of the catfish genus Trichomycterus from south-eastern Brazil (Siluriformes: Trichomycteridae). J. Nat. Hist. 2019, 53, 2905–2928. [Google Scholar] [CrossRef]
- Lima, S.M.Q.; Costa, W.J.E.M. Trichomycterus giganteus (Siluriformes: Loricarioidea: Trichomycteridae): A new catfish from the Rio Guandu basin, southeastern Brazil. Zootaxa 2004, 761, 1–6. [Google Scholar] [CrossRef]
- Barbosa, M.A.; Costa, W.J.E.M. Description of a new species of catfish from the upper Rio Paraíba do Sul basin, south-eastern Brazil (Teleostei: Siluriformes: Trichomycteridae) and re-description of Trichomycterus itatiayae. Int. J. Ichthyol. 2008, 14, 175–186. [Google Scholar]
- Costa, W.J.E.M.; Katz, A.M. A new catfish of the genus Trichomycterus from the Rio Paraíba do Sul Basin, south-eastern Brazil, a supposedly migrating species (Siluriformes: Trichomycteridae). Zoosyst. Evol. 2022, 98, 13–21. [Google Scholar] [CrossRef]
- Barbosa, M.A.; Costa, W.J.E.M. Trichomycterus macrophthalmus (Teleostei: Siluriformes: Trichomycteridae), a new species of catfish from the Paraíba do Sul river basin, southeastern Brazil. Vertebr. Zool. 2012, 62, 79–82. [Google Scholar] [CrossRef]
- Barbosa, M.A.; Costa, W.J.E.M. Trichomycterus potschi (Siluriformes: Loricarioidei): A new trichomycterid catfish from coastal streams of southeastern Brazil. Ichthyol. Explor. Freshw. 2003, 14, 281–287. [Google Scholar]
- Barbosa, M.A.; Costa, W.J.E.M. Trichomycterus puriventris (Teleostei: Siluriformes: Trichomycteridae), a new species of catfish from the Rio Paraíba do Sul basin, southeastern Brazil. Vertebr. Zool. 2012, 62, 155–160. [Google Scholar] [CrossRef]
- Costa, W.J.E.M.; Mattos, J.L.O.; Amorim, P.F.; Vilardo, P.J.; Katz, A.M. Relationships of a new species support multiple origin of melanism in Trichomycterus from the Atlantic Forest of south-eastern Brazil (Siluriformes: Trichomycteridae). Zool. Anz. 2020, 288, 74–83. [Google Scholar] [CrossRef]
- Costa, W.J.E.M.; Mattos, J.L.O.; Vilardo, P.J.; Amorim, P.F.; Katz, A.M. Perils of underestimating species diversity: Revisiting systematics of Psammocambeva catfishes (Siluriformes: Trichomycteridae) from the Rio Paraíba do Sul basin, south-eastern Brazil. Taxonomy 2022, 2, 491–523. [Google Scholar] [CrossRef]
- Vilardo, P.J.; Katz, A.M.; Costa, W.J.E.M. Relationships and description of a new species of Trichomycterus (Siluriformes: Trichomycteridae) from the Rio Paraíba do Sul basin, south-eastern Brazil. Zool. Stud. 2020, 59, e53. [Google Scholar] [CrossRef]
- Costa, W.J.E.M.; Katz, A.M. Comparative morphology, phylogeny, classification and evolution of interstitial habits in microcambevine catfishes (Siluriformes: Trichomycteridae). Taxonomy 2021, 1, 313–344. [Google Scholar] [CrossRef]
- Medeiros, L.S.; Donin, L.M.; Ferrer, J.; Lima, S.M.Q.; de Pinna, M. A new and threatened species of Listrura (Siluriformes: Trichomycteridae), a rare catfish from an Atlantic Forest continental island. Neotrop. Ichthyol. 2024, 22, e230136. [Google Scholar] [CrossRef]
- Villa-Verde, L.; de Pinna, M.C.C.; Reis, V.J.C.; Oyakawa, O.T. Secretive fish diversity: A new species of Listrura (Siluriformes: Trichomycteridae) from a supposedly well-known river in south-eastern Brazil. J. Fish. Biol. 2022, 100, 1299–1310. [Google Scholar] [CrossRef]
- Sarmento-Soares, L.M.; Pessali, T.C.; Reis, V.J.C.; de Medeiros, L.S.; Lima, S.M.Q.; Silva, J.P.; Martins-Pinheiro, R.F.; de Pinna, M.C.C. Distribution, morphological notes and conservation status of the psammophilus Microcambeva catfishes (Siluriformes: Trichomycteridae). Zootaxa 2019, 4712, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Koch, W.R. Revisão taxonômica do gênero Homodiaetus (Teleostei: Siluriformes: Trichomycteridae). Iheringia Série Zool. 2002, 92, 33–46. [Google Scholar] [CrossRef]
- Britto, M.R. Phylogeny of the subfamily Corydoradinae (Siluriformes: Callichthyidae), with a definition of its genera. Proc. Acad. Nat. Sci. Phila. 2003, 153, 119–154. [Google Scholar] [CrossRef]
- Bizerril, C.R.S.F. Composição Taxonômica e Análise Ecológica da Ictiofauna da Bacia Hidrográfica do Rio São João, RJ, Brasil. Master’s Thesis, Pós-graduação em Zoologia. Museu Nacional, UFRJ, Rio de Janeiro, Brazil, 1995. [Google Scholar]
- Reis, R.E.; Vieira, F.; Pereira, E.H.L. A new species of the loricariid catfish genus Loricariichthys (Teleostei: Siluriformes) from eastern Brazil. Ichthyol. Herpetol. 2021, 109, 557–566. [Google Scholar] [CrossRef]
- Mejia, E.; Buckup, P.A. Species boundaries of the whiptail catfish Rineloricaria (Siluriformes: Loricariidae) from the Paraíba do Sul River drainage, southeastern Brazil, with species redescriptions and description of a new species. J. Fish. Biol. 2024, 105, 288–313. [Google Scholar] [CrossRef]
- Martins, F.d.O.; Langeani, F. Redescription of Hisonotus notatus Eigenmann & Eigenmann, 1889 (Loricariidae: Hypoptopomatinae), the type species of the genus, and description of a new species from coastal drainages of southeastern Brazil. Neotrop. Ichthyol. 2016, 14, e150100. [Google Scholar] [CrossRef]
- Bizerril, C.R.S.F. Relação entre geomorfologia fluvial e biodiversidade e sua aplicação no processo de avaliação ambiental. Braz. Arch. Biol. Technol. 1998, 41, 67–81. [Google Scholar] [CrossRef][Green Version]
- Cherobim, A.M.; Lazzarotto, H.; Langeani, F. A new species of the catfish Neoplecostomus (Loricariidae: Neoplecostominae) from a coastal drainage in southeastern Brazil. Neotrop. Ichthyol. 2017, 14, e160015. [Google Scholar] [CrossRef]
- Pereira, E.H.L. Resurrection of Pareiorhaphis Miranda Ribeiro, 1918 (Teleostei: Siluriformes: Loricariidae), and description of a new species from the Rio Iguaçu basin, Brazil. Neotrop. Ichthyol. 2005, 3, 271–276. [Google Scholar] [CrossRef][Green Version]
- Chamon, C.C.; Aranda, A.T.; Buckup, P.A. Pareiorhina brachyrhyncha (Loricariidae: Siluriformes): A new species of fish from the Paraíba do Sul slope of Serra da Mantiqueira, southeastern Brazil. Copeia 2005, 3, 550–558. [Google Scholar] [CrossRef]
- Roxo, F.F.; Melo, B.F.; Silva, G.S.C.; Oliveira, C. New species of Parotocinclus (Siluriformes: Loricariidae) from coastal drainages of Rio de Janeiro, southeastern Brazil. Zootaxa 2017, 4232, 260–270. [Google Scholar] [CrossRef]
- Medeiros, L.S.; Ferreira-Araújo, T.; Lazzarotto, H.; Lima, S.M.Q. Type locality, distribution and conservation of the threatened catfish Taunayia bifasciata (Eigenmann & Norris 1900) (Siluriformes: Heptapteridae) in the Atlantic Forest streams of southeastern Brazil. Zootaxa 2022, 5154, 595–600. [Google Scholar] [CrossRef]
- Mattos, J.L.O.; Ottoni, F.P.; Barbosa, M.A. Microglanis pleriqueater, a new species of catfish from the São João river basin, eastern Brazil (Teleostei: Pseudopimelodidae). Ichthyol. Explor. Freshw. 2013, 24, 147–154. [Google Scholar]
- Marceniuk, A.P.; Ingenito, L.F.S.; Lima, F.C.T.; Gasparini, J.L.; Oliveira, C. Systematics, biogeography and conservation of Paragenidens grandoculis n. gen. and n. comb. (Siluriformes; Ariidae), a critically endangered species from southeastern Brazil. Zootaxa 2019, 4586, 425–444. [Google Scholar] [CrossRef]
- Lucena, C.A.S.de; Kullander, S.O.; Norén, M.; Calegari, B. Conjectures and refutations: Species diversity and phylogeny of Australoheros from coastal rivers of southern South America (Teleostei: Cichlidae). PLoS ONE 2022, 17, e0261027. [Google Scholar] [CrossRef]
- Kullander, S.O.; Ferreira, E.J.G. A review of the South American cichlid genus Cichla, with descriptions of nine new species (Teleostei: Cichlidae). Ichthyol. Explor. Freshw. 2006, 17, 289–398. [Google Scholar]
- Casatti, L. Taxonomia do gênero Sul-Americano Pachyurus Agassiz, 1831 (Teleostei: Perciformes: Sciaenidae) e descrição de duas novas espécies. Comun. Mus. Ciênc. PUCRS 2001, 14, 133–178. [Google Scholar]
- Costa, W.J.E.M. Redescription of Kryptolebias ocellatus (Hensel) and K. caudomarginatus (Seegers) (Teleostei: Cyprinodontiformes: Rivulidae), two killifishes from mangroves of south-eastern Brazil. aqua, J. Ichthyol. 2006, 11, 5–12. [Google Scholar]
- Costa, W.J.E.M. Kryptolebias gracilis n. sp. (Teleostei: Cyprinodontiformes: Rivulidae): A new killifish from the Saquarema Lagoon basin, southeastern Brazil. Aqua. Int. J. Ichthyol. 2007, 13, 7–12. [Google Scholar]
- Costa, W.J.E.M. Description of a new species of cynopoeciline killifish (Cyprinodontiformes, Aplocheilidae), possibly extinct, from the Atlantic Forest of south-eastern Brazil. ZooKeys 2019, 867, 73–85. [Google Scholar] [CrossRef]
- Costa, W.J.E.M. Comparative morphology and classification of South American cynopoeciline killifishes (Cyprinodontiformes: Aplocheilidae), with notes on family-group names used for aplocheiloids. Vertebr. Zool. 2016, 66, 125–140. [Google Scholar] [CrossRef]
- Costa, W.J.E.M.; Amorim, P.F.; Aranha, G.N. Species limits and DNA barcodes in Nematolebias, a genus of seasonal killifishes threatened with extinction from the Atlantic Forest of south-eastern Brazil, with description of a new species (Teleostei: Rivulidae). Ichthyol. Explor. Freshw. 2014, 24, 225–236. [Google Scholar]
- Costa, W.J.E.M. Taxonomic revision of the seasonal South American killifish genus Simpsonichthys (Teleostei: Cyprinodontiformes: Aplocheiloidei: Rivulidae). Zootaxa 2007, 1669, 1–134. [Google Scholar] [CrossRef]
- Petry, A.C.; Guimarães, T.F.R.; Vasconcellos, F.M.; Hartz, S.M.; Becker, F.G.; Rosa, R.S.; Goyenola, G.; Caramaschi, E.P.; Díaz de Astarloa, J.M.; Sarmento-Soares, L.M.; et al. Fish composition and species richness in eastern South American coastal lagoons: Additional support for the freshwater ecoregions of the world. J. Fish. Biol. 2016, 89, 280–314. [Google Scholar] [CrossRef]
- Amorim, P.F. Jenynsia lineata species complex, revision and new species description (Cyprinodontiformes: Anablepidae). J. Fish. Biol. 2018, 92, 1312–1332. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).