Abstract
Background: Peritoneal metastasis (PM) from colorectal cancer (CRC) carries a poor prognosis. The Peritoneal Cancer Index (PCI) is among the principal prognostic stratification tools, yet the prognostic value of the anatomical distribution of disease beyond total PCI is underexplored. This pilot study evaluated whether quadrant-specific involvement adds prognostic information in patients undergoing cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC), with a focused analysis of oligometastatic disease (PCI ≤ 6). Methods: A single-institution cohort of 48 CRC-PM patients treated with CRS + HIPEC was analyzed. Primary endpoints were OS, DFS, and PRFS, with a focused evaluation of the oligometastatic subset (PCI ≤ 6). Comparative statistics used Student’s two-sample t test for continuous variables and chi-square or two-sided Fisher’s exact tests for categorical variables. Survival was estimated by Kaplan–Meier with log-rank tests, and prognostic factors were evaluated using Cox regression. Results: Median follow-up was 177 months (IQR 87–224). Outcomes favored PCI ≤ 6: 5-year OS and DFS were 54% and 37.5% versus 6.6% and 0% for PCI > 6, and median OS 64 vs. 29 months (log-rank p = 0.007), median DFS 30 vs. 7 months (p = 0.0002), and median PRFS 26 vs. 8 months (p = 0.0002). In the PCI ≤ 6 subset (n = 27), quadrant 3 (left upper quadrant) was associated with higher recurrence risk and shorter DFS, remaining independently prognostic for DFS (p = 0.005) and PRFS (p = 0.005). For PRFS, quadrants 7 and 8 also showed associations on univariable analysis; Q7 remained independent (p = 0.047), whereas Q8 was borderline (p = 0.077). A histology-related signal at Q8 (p = 0.011) was exploratory due to very small mucinous and signet-ring strata. Sidedness and synchronicity yielded no significant differences in quadrant involvement within PCI ≤ 6. No quadrant effects were observed in PCI > 6. Conclusions: PCI remains the dominant prognostic determinant after CRS + HIPEC, yet in oligometastatic disease, the anatomical distribution adds complementary prognostic information, particularly involvement of Q3 and Q7. These findings are hypothesis-generating and warrant validation in larger, preferably multicenter cohorts with standardized quadrant mapping. If confirmed, quadrant-directed operative planning, including consideration of prophylactic resection in selected high-risk regions, could be prospectively evaluated.
1. Introduction
Peritoneal metastasis (PM) from colorectal cancer (CRC) is a well-established negative prognostic factor that significantly affects both short- and long-term outcomes. Approximately 15% of patients present with synchronous PM, while 4–19% develop metachronous disease []. In up to 24% of cases, PM is the sole site of recurrence []. Compared to other metastatic patterns, PM carries a higher overall mortality risk in CRC patients []. The majority of patients with PM are still managed with palliative intent. The introduction of modern chemotherapeutic agents (oxaliplatin, irinotecan) and targeted therapies, including anti-EGFR agents (cetuximab, panitumumab) and anti-VEGF therapy (bevacizumab), has improved median overall survival (OS) to 16–24 months [,].
Over the past two decades, cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) has emerged as a potentially effective treatment for selected patients with peritoneal metastases, achieving median OS rates of up to 40–43 months [] and lower recurrence rates compared to systemic therapy or palliative care.
Despite its potential benefits, CRS + HIPEC is a complex procedure associated with significant morbidity and quality-of-life implications. Optimizing patient selection is essential to maximize outcomes while minimizing unnecessary harm. Nevertheless, outcomes remain heterogeneous, with nearly two-thirds of patients experiencing recurrence within the first year after surgery []. In particular, peritoneal recurrence strongly influences post-relapse quality of life and life expectancy.
A wide range of prognostic factors has been explored to improve patient stratification. Among the most relevant are a high Peritoneal Cancer Index (PCI), incomplete cytoreduction, lymph node involvement [,] and molecular markers, as specific mutational patterns may pave the way for more targeted therapeutic strategies [].
The PCI quantifies tumor burden by assessing lesion size in nine abdominopelvic regions and four small bowel segments, commonly referred to as “quadrants”. It is an established tool to stratify patients and identify those with limited disease who are most likely to benefit from complete cytoreduction. While various PCI thresholds have been proposed to define eligibility for CRS + HIPEC, consensus has yet to be reached. A PCI > 15 is often considered a relative contraindication, and involvement of the lower ileum has been identified as an additional negative prognostic factor []. A recent multicenter study by the Italian Peritoneal Surface Malignancies Oncoteam (SICO) [] reported encouraging results: patients with a completeness of cytoreduction score (CCS) of 0 and PCI ≤ 6 experienced significantly better long-term survival (median OS 40.7 and 44.3 months, respectively) compared to those with incomplete cytoreduction (median OS 10.7 months, p = 0.003) or PCI > 6 (median OS 13.4 months, p = 0.005). Given that peritoneal recurrence is a key determinant of prognosis after relapse, further investigation is warranted to assess the prognostic relevance of the specific anatomical distribution of carcinomatosis.
Peritoneal fluid circulation plays a crucial role in shaping the distribution of peritoneal disease. The peritoneum, the largest serosal membrane in the body, lines the abdominal cavity and viscera, creating compartments through the omentum, mesentery, and ligaments. It secretes a sterile fluid that facilitates frictionless organ movement and contributes to immune defense [].
The direction of fluid flow is determined by diaphragmatic motion, bowel peristalsis, hydrostatic pressure gradients, and gravity, a phenomenon described as cephalic circulation []. Dynamic peritoneographic studies have demonstrated predictable routes of peritoneal fluid movement [,].
Fluid ascends from the pelvis into the right paracolic gutter, reaching Morrison’s pouch and the right subphrenic space, while the phrenicocolic ligament restricts communication between the left paracolic gutter and the perisplenic space. In the left subcolic space, flow is directed towards the pelvis, with stagnation along the superior border of the sigmoid mesocolon before continuing to the rectum. In the right submesocolic space, fluid courses through mesenteric recesses, accumulating at the ileocecal junction before descending into the pelvis. Flow across mesenteric attachments may also facilitate disease spread. From the pelvis, fluid collects in the pouch of Douglas and paravesical fossae.
Overall, four sites have been identified as preferential areas for fluid stasis and potential metastatic implantation: the pouch of Douglas at the rectosigmoid level (Q6 according to PCI score); the right lower quadrant at the termination of the small bowel mesentery (Q7 according to PCI score); the left lower quadrant along the upper border of the sigmoid mesocolon (Q5 according to PCI score); and the right paracolic gutter adjacent to the cecum and ascending colon (Q8 according to PCI score) [].
The aim of this study is to evaluate the impact of quadrant-specific peritoneal involvement on OS, disease-free survival (DFS), and peritoneal recurrence-free survival (PRFS) in patients undergoing CRS and HIPEC for colorectal cancer, with a particular focus on whether patients with a PCI ≤ 6 or >6 derive greater benefit from the procedure or are more adversely affected by the involvement of specific peritoneal regions. Given the paucity of data addressing the prognostic value of the anatomical distribution of colorectal peritoneal metastases, and the limited number of eligible cases treated with CRS + HIPEC even in high-volume centers, the present work was conceived as a pilot, hypothesis-generating study. Its primary intent is to explore potential quadrant-specific prognostic signals rather than to provide confirmatory evidence. As the analysis was restricted to patients who achieved complete cytoreduction (CC-0), the conclusions drawn from this study apply specifically to this population.
2. Patients and Methods
2.1. Study Design and Selection Criteria
This study included patients with synchronous or metachronous CRC-PM who underwent curative-intent CRS and HIPEC in the Surgical Department of Morgagni-Pierantoni Hospital, Forlì, Italy, from March 2004 to January 2024.
Patients with appendiceal cancer were excluded due to their distinct molecular and biological behavior compared to colorectal malignancies. We also excluded patients who received prophylactic HIPEC (with a PCI of 0) and patients undergoing repeat HIPEC procedures for recurrent disease. Patients with insufficient follow-up were excluded when postoperative or oncologic surveillance was conducted at external institutions, precluding reliable survival assessment. These exclusions reflected missing follow-up data rather than outcome-related selection bias.
Patients with a CCS greater than 0 (i.e., CC-1, indicating residual macroscopic nodules ≤ 2.5 mm) were excluded from quadrant-wise analyses because residual macroscopic disease is a major confounder for PRFS and could obscure the independent prognostic contribution of preoperative disease distribution among patients rendered macroscopically disease-free (CC-0).
All cases were evaluated preoperatively by a dedicated multidisciplinary team (MDT) including at least one oncologist, one radiologist, two surgical oncologists, one endoscopist, and, as needed, specialists in thoracic, hepatobiliary, or urological surgery. Patients with unresectable extra-peritoneal metastases or nodal disease outside the primary field were considered ineligible. Since 2019, patients with limited, stable pulmonary metastases have been considered for CRS/HIPEC on a case-by-case basis.
The following clinical data were collected: age, sex, vital status and cause of death (cancer-related vs. other), peritoneal cancer index (PCI), intraoperative quadrant involvement, cytoreduction status, surgical details, molecular profile when available, and receipt of pre- or post-HIPEC.
PCI was dichotomized as ≤6 or >6 based on literature supporting improved prognosis in patients with lower disease burden (oligometastatic). Abdominal quadrant involvement was recorded for each of the 13 anatomical regions (Q0–Q12) in accordance with the Sugarbaker PCI map. For the purpose of the analysis, we considered only the presence or absence of quadrant involvement rather than the PCI subscore assigned to each region, as further stratification would have excessively fragmented the cohort and precluded meaningful statistical comparisons.
2.1.1. Surgical Details
HIPEC was performed using a semiclosed coliseum technique. The preferred protocol included intravenous 5-fluorouracil (400 mg/m2) and folinic acid (20 mg/m2), followed by intraperitoneal oxaliplatin (460 mg/m2) administered over 30 min at 41.5 °C, based on the Elias protocol. Mitomycin C (3.4 mg/m2) was used as an alternative regimen in selected cases. The chemotherapeutic protocol was chosen by MDT oncologists based on patients’ prior treatments and clinical characteristics. During hyperthermia, urinary output was maintained above 10 cc/min with intravenous hydration, and amifostine was administered as nephroprotection until 2020. Fresh frozen plasma was routinely transfused at the end of HIPEC. All patients were transferred to the intensive care unit (ICU) for 24–48 h postoperatively.
2.1.2. Post-Resection Follow-Up
Histopathologic examination of all peritonectomy and nodule specimens confirmed metastatic colorectal adenocarcinoma, consistent with the intraoperative PCI findings. Patients were followed every 6 months for 2 years, then annually. Follow-up included thoracoabdominal-pelvic CT, hepatic ultrasonography, and serum tumor markers (CEA- Ca 19-9).
2.2. Statistical Analysis
Descriptive statistics were performed using means and standard deviations for continuous variables, and proportions for categorical variables. Comparisons between PCI groups (≤6 vs. >6) were performed using Student’s two-sample t test (two-sided) for continuous variables and the χ2 or Fisher’s exact tests (two-sided) for categorical variables, applying Fisher when any expected cell count was <5. The normality of continuous variables was assessed using the Shapiro–Wilk test. A two-sided α = 0.05 was adopted.
The primary outcomes were overall survival (OS), disease-free survival (DFS), and peritoneal recurrence-free survival (PRFS). OS was defined as the time from CRS/HIPEC to death or last follow-up. DFS was defined as the time from surgery to any recurrence (peritoneal or distant), and PRFS as the time from surgery to documented peritoneal recurrence only. Patients who died from non-oncological causes were censored for OS, DFS and PRFS analyses.
Survival analyses were performed using the Kaplan–Meier method and log-rank tests. Time-to-event analyses were stratified by PCI (≤6 vs. >6), and univariate Cox proportional hazards models were fitted to assess the prognostic impact of quadrant involvement and other covariates on OS, DFS, and PRFS. Multivariate models included only variables with p < 0.05 in univariate analysis and were stratified by PCI category. To minimize overfitting, multivariable models were limited to a maximum of three covariates. To account for multiple quadrant-wise comparisons, p-values were adjusted using the Benjamini–Hochberg false discovery rate (BH-FDR) method, and adjusted p-values (q-values) were reported for both univariable and multivariable analyses.
Hazard ratios (HRs) and 95% confidence intervals (CIs) were reported. Statistical analyses were performed using Stata/SE version 18.0 (StataCorp, College Station, TX, USA).
3. Results
A total of 65 patients with CRC-PM underwent CRS and HIPEC at our institution (Morgagni-Pierantoni Hospital, Forlì, Italy). Among these, complete data on all prognostic variables of interest were available for 48 patients, who constituted the study population. Data were obtained from prospectively maintained institutional databases and retrospectively reviewed.
In all 48 patients, complete cytoreduction was achieved (CC-0). Of the 48 patients included, 33 received neoadjuvant chemotherapy before HIPEC, and 32 received chemotherapy post-HIPEC. Twenty patients received both pre- and post-HIPEC (baseline characteristics are available in Table 1).

Table 1.
Comparative analysis of PCI-stratified baseline characteristics; patients (n = 48).
3.1. Baseline Characteristics and Comparative Analysis by PCI Stratum
The mean age of the cohort was 62.6 ± 9.3 years (range, 31–74). The study included 27 patients with PCI ≤ 6 and 21 with PCI > 6. At last follow-up, 13 patients were alive, 84.6% of whom were in the PCI ≤ 6 group; among the 35 deaths, 54.3% occurred in patients with PCI > 6. Synchronicity was comparable across PCI strata, with synchronous peritoneal metastases present in 62.5% of patients overall. Molecular profiling (KRAS, BRAF, MSI) was available only for a subset, as routine testing was introduced after 2011; the high proportion of missing data, combined with the small sample size, likely reduced power to detect molecular associations. Baseline characteristics are summarized in Table 1.
Regarding systemic therapy, a greater proportion of patients in the PCI > 6 group received chemotherapy before HIPEC (85.7% vs. 55.6%; p = 0.025), and receipt of chemotherapy both before and after CRS + HIPEC also differed (38.1% vs. 26.0%; p = 0.012), whereas post-HIPEC alone did not (71.4% vs. 63.0%; p = 0.537).
Recurrence patterns were more favorable in the low-PCI group: peritoneal recurrence occurred in 40.7% vs. 81.0% (p = 0.005) and extraperitoneal recurrence in 64.0% vs. 90.5% (p = 0.036).
With respect to disease distribution, involvement of individual peritoneal regions differed significantly between PCI groups, with a higher burden of quadrant involvement in patients with PCI > 6 (χ2 test, p < 0.05 for multiple regions; Table 2).

Table 2.
Comparative analysis of quadrant involvement stratified by PCI; patients (n = 48).
3.2. Quadrant-Specific Distribution in Oligometastatic Subgroup, with Comparative Findings by Histology, Sidedness and Prior Colorectal Surgery
After excluding patients with PCI > 6, 27 patients remained and were stratified by histotype (Adenocarcinoma n = 21; Mucinous n = 2; Signet-ring n = 4). Given the very small cell counts and high variability in the mucinous/signet groups, between-histology comparisons of quadrant involvement were performed using two-sided Fisher’s exact tests. As shown in Table 3, only Q8 reached statistical significance (p = 0.011), suggesting a possible predilection of this region in non-adenocarcinoma subtypes. Given the extremely small numbers in the mucinous and signet-ring strata, these findings should be considered exploratory and hypothesis-generating, pending confirmation in larger cohorts.

Table 3.
Oligometastatic patients (PCI ≤ 6) stratified by primary tumor histology; patients (n = 27).
Ten patients had right-sided primaries (cecum to the proximal two-thirds of the transverse colon) and 17 had left-sided disease, including the rectum (n = 5). For topographic consistency and given the limited sample, rectal cancers were grouped with the left-sided category. Comparisons of quadrant involvement by sidedness showed no statistically significant differences on two-sided Fisher’s exact tests. The largest absolute contrast was for Q0 (right 50.0% [,,,,,] vs. left 11.8% [,,,,,,,,,,,,,,,]; p = 0.065). To explore whether prior surgery might alter dissemination patterns, patients were further stratified by synchronicity within each side. On the right (n = 10), 6 were synchronous and 4 metachronous; no quadrant reached significance. On the left (n = 17), 7 were synchronous and 10 metachronous; all tests were non-significant. These analyses are exploratory and limited by sparse cells and small group sizes; confirmation in larger cohorts is warranted.
3.3. Overall, Disease-Free and Peritoneal Recurrence-Free Survival
The median follow-up was 177 months (IQR: 87–224).
OS rates at 1, 3, 5, and 10 years were 91.6%, 74.3%, 54%, and 43% for patients with PCI ≤ 6, versus 90%, 37%, 6.6%, and 0% for those with PCI > 6. Median OS was 64 vs. 29 months, respectively (log-rank p = 0.007), as shown in Figure 1.
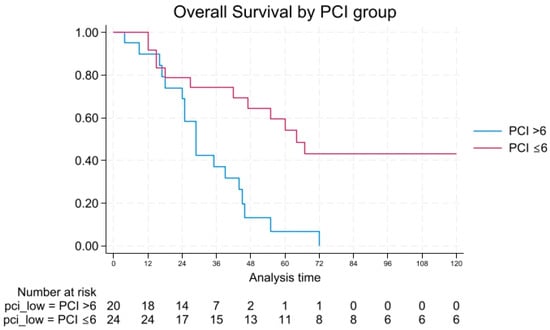
Figure 1.
Overall survival by PCI.
DFS followed a similar trend, with 1- and 3-year rates of 66.1% and 45.8% vs. 15.0% and 5.0%. No patients with PCI > 6 remained disease-free at 5 or 10 years, while DFS in the PCI ≤ 6 group was 37.5% and 32.8%, respectively. Median DFS was 30 vs. 7 months (log-rank p = 0.0002), as shown in Figure 2.
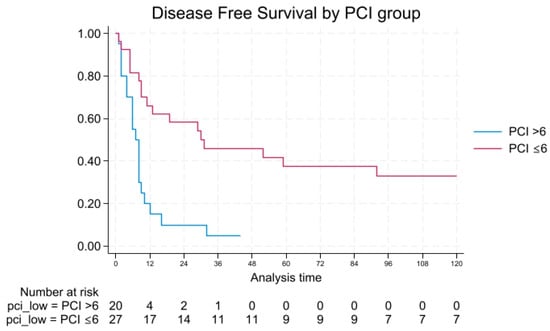
Figure 2.
Disease-free survival by PCI.
PRFS was also significantly longer in patients with low PCI, with a median of 26 months vs. 8 months (log-rank p = 0.0002), as shown in Figure 3.
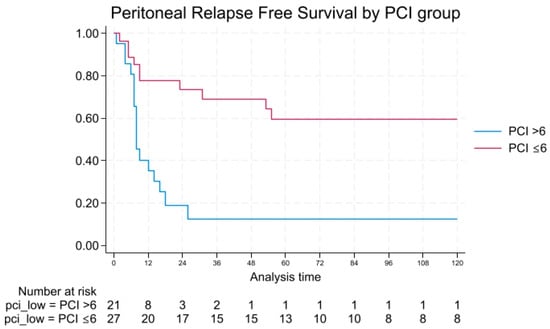
Figure 3.
Peritoneal relapse-free survival by PCI.
3.4. Univariable and Multivariable Analysis of Overall Survival
OS was significantly associated with PCI. In univariate analysis, a PCI ≤ 6 was predictive of prolonged OS (p = 0.005; 95% CI: 0.18–0.74). In the PCI > 6 subgroup, involvement of Q0 emerged as a protective factor (p = 0.02; 95% CI: 0.07–0.84), although this association may reflect residual confounding.
KRAS mutation showed a borderline association with improved OS in the overall model (p = 0.064; 95% CI: 0.06–1.08). However, nearly half of the cohort (47.9%) lacked molecular data and were excluded from the model, potentially introducing selection bias.
At multivariate analysis, low PCI remained independently associated with longer OS (p = 0.016; 95% CI: 0.01–0.62). When stratified by PCI, no variables were independently associated with OS among patients with PCI ≤ 6 or PCI > 6.
3.5. Univariable and Multivariable Analysis of Disease-Free Survival
DFS was significantly associated with PCI. In univariate analysis, patients with PCI ≤ 6 had improved DFS (p = 0.001; 95% CI: 0.14–0.58), while no significant associations were found for KRAS status or specific quadrants when considering the entire cohort.
When stratifying by PCI, Q3 involvement emerged as a significant risk factor for recurrence in the PCI ≤ 6 subgroup (p = 0.05; 95% CI: 1.12–11.67). This association was confirmed in multivariate analysis (p = 0.005; 95% CI: 1.76–26.76), suggesting a possible locoregional prognostic relevance in patients with limited peritoneal disease (Table 4). After BH-FDR correction across the 13 quadrant-wise tests within the PCI ≤ 6 subgroup, no associations reached statistical significance. For DFS, the lowest adjusted p-value was observed for Q3 (q = 0.41). No significant predictors were identified in the PCI > 6 subgroup.

Table 4.
Univariate and multivariate analysis of DFS.
3.6. Univariable and Multivariable Analysis of Peritoneal Relapse-Free Survival (PRFS)
Unsurprisingly, PRFS was significantly associated with PCI (p = 0.0004, 95% CI: 0.1–0.54). PRFS analysis revealed no significant predictors in the PCI > 6 group. In contrast, univariate analysis within the PCI ≤ 6 group identified involvement of quadrants Q3 (p = 0.060; 95% CI: 0.95–14.4), Q7 (p = 0.05; 95% CI: 1.01–11.18), and Q8 (p = 0.05; 95% CI: 1.04–16.02) as potential risk factors for peritoneal relapse. After BH-FDR correction across the 13 quadrant-wise tests within the PCI ≤ 6 subgroup for PRFS, the lowest adjusted p-values were seen for Q3, Q7, and Q8 (q = 0.26 for each), while all other regions showed q ≥ 0.67.
In univariable Cox analysis, the model-level likelihood ratio test suggested association for KRAS (p = 0.020), but the hazard ratio was not reliably estimable (zero events in one stratum; degenerate CI). Given the small number of events and high missingness, KRAS was not included in multivariable models.
Multivariate analysis confirmed Q3 (p = 0.005; 95% CI: 2.31–120.06) and Q7 (p = 0.047; 95% CI: 1.02–31.78) as independent prognostic factors for peritoneal recurrence in patients with limited PCI, while Q8 retained borderline significance (p = 0.077; 95% CI: 0.85–22.5) (Table 5). In multivariable analysis within the PCI ≤ 6 subgroup, Q3 involvement remained significantly associated with shorter peritoneal relapse-free survival after BH-FDR correction (q = 0.016), whereas Q7 (q = 0.071) and Q8 (q = 0.077) showed borderline, non-significant trends.

Table 5.
Univariate and multivariate analysis of peritoneal relapse-free survival (PRFS); p-values adjusted for multiple comparisons using the Benjamini–Hochberg FDR (BH-FDR) across the three tested quadrants (Q3, Q7, Q8).
Due to the small sample size and the resulting sparse distribution across several quadrants, often with 2–4 patients per stratum, the statistical power to detect true associations is limited, and the confidence intervals around hazard ratios are necessarily wide. These analyses should therefore be interpreted as exploratory and descriptive in nature, intended to identify potential prognostic patterns that warrant validation in larger cohorts.
4. Discussion
The peritoneum is the third most common site of CRC metastasis after the liver and lungs []. The peritoneal microenvironment, shaped by immune cells, fibroblasts, and extracellular matrix components, plays an active role in supporting tumor progression and may contribute to both biological aggressiveness and limited drug penetration in the peritoneal cavity [].
Unlike hematogenous or lymphatic spread, peritoneal metastases represent a distinct pattern of dissemination, typically arising from direct serosal seeding and modulated by peritoneal fluid dynamics.
To quantify disease burden, PCI remains the most widely adopted prognostic tool, combining lesion size and anatomical distribution across 13 regions. While the prognostic value of total PCI is well established, the independent relevance of tumor location within specific peritoneal regions remains underexplored.
In the oligometastatic subset, the hypothesis that histology shapes peritoneal dissemination patterns with preferential quadrant involvement was evaluated. Based on the peritoneal fluid-flow dynamics theory, tumor histology, sidedness and prior colorectal surgery in metachronous disease were considered potential modifiers of peritoneal spread. Mesenteric reorientation may disrupt physiological peritoneal flow and thereby alter the topography of peritoneal deposits.
In patients with higher PCI, the disease burden inherently entails involvement of more than three quadrants, which prevents a meaningful assessment of single-quadrant effects; by contrast, in the oligometastatic subset, limited topographic spread makes quadrant-level analyses statistically feasible.
After excluding PCI > 6, only one quadrant-histology association met conventional significance: Q8 differed across subtypes (p = 0.011), with higher involvement in mucinous and signet-ring cancers than in adenocarcinoma. Q8 corresponds to the right paracolic gutter, a recognized area of cephalad flow and fluid pooling, which offers a plausible anatomical rationale. However, the mucinous (n = 2) and signet-ring (n = 4) strata were extremely small, generating wide uncertainty and a high risk of chance findings in the context of multiple comparisons. This signal should therefore be viewed as exploratory and hypothesis-generating.
By contrast, sidedness did not show statistically significant differences in quadrant involvement within PCI ≤ 6. The largest absolute contrast was observed for Q0 (right 50.0% vs. left 11.8%; p = 0.065), but this did not reach significance. Further stratification by synchronicity within each side also yielded no significant differences. These negative results are compatible with limited statistical power and sparse cells, rather than evidence of equivalence.
Consistent with previous literature, in our study, PCI was strongly associated with OS, DFS, and PRFS. Patients with PCI ≤ 6 demonstrated markedly improved outcomes compared to those with higher disease burden. Specifically, 5-year OS and DFS rates were 54% and 37.5%, respectively, and 10-year OS remained as high as 43%, indicating durable long-term survival in a substantial proportion of patients. Conversely, the PCI > 6 group showed 5-year OS and DFS rates of 6.6% and 0%, respectively, with no survivors at 10 years. Median OS was 64 months for PCI ≤ 6 versus 29 months for PCI > 6 (log-rank p = 0.007), and median DFS was 30 versus 7 months (log-rank p = 0.0002).
PRFS followed a similar pattern, with patients in the PCI ≤ 6 group showing a median PRFS of 26 months compared to 8 months in the PCI > 6 group (log-rank p = 0.0002). These differences underscore the central prognostic role of PCI in determining both oncologic efficacy and durability of disease control following CRS and HIPEC. Notably, no patients with PCI > 6 remained disease-free at 5 years, further emphasizing the challenge of achieving long-term control in extensive peritoneal disease despite complete cytoreduction.
Beyond the global PCI score, we identified that, in oligometastatic, the involvement of specific regions carried additional prognostic relevance. Notably, Q3, corresponding to the left upper quadrant, was independently associated with both decreased DFS and increased risk of peritoneal recurrence. In the PCI ≤ 6 subgroup, Q3 involvement was significantly associated with recurrence in univariate analysis (p = 0.031; 95% CI: 1.12–11.67) and retained strong significance in multivariate models for both DFS (p = 0.005; 95% CI: 1.76–26.76) and PRFS (p = 0.005; 95% CI: 2.31–120.06). The association between Q3 involvement and poorer peritoneal recurrence-free survival, although counterintuitive compared to the commonly reported predominance of right diaphragmatic disease, may be related to the greater technical complexity of exploring the left upper quadrant during CRS. The splenic recess and left subphrenic space are anatomically constrained areas where microscopic nodules may be more easily missed to avoid splenic injury. Quadrants 7 and 8, which include the right lower quadrant and the right colic gutter respectively, also emerged as relevant prognostic indicators in the low-PCI group. In univariate analysis for PRFS, Q7 and Q8 involvement were both significantly associated with increased risk of peritoneal relapse (Q7: p = 0.048; 95% CI: 1.01–11.18; Q8: p = 0.043; 95% CI: 1.04–16.02). Multivariate analysis confirmed Q7 as an independent predictor (p = 0.047; 95% CI: 1.02–31.78), while Q8 retained borderline significance (p = 0.077; 95% CI: 0.85–22.51), suggesting a potential prognostic trend that may warrant further exploration.
The observed predilection of Q8 involvement may also be partly explained by the higher frequency of mucinous and signet-ring cell histologies originating from right-sided colorectal primaries, which tend to disseminate along the right paracolic gutter toward this region.
After applying the BH-FDR correction across all 13 quadrant-wise tests within the PCI ≤ 6 subgroup, no associations reached formal statistical significance in the univariable analyses. For DFS, the lowest adjusted p-value was observed for Q3 (q = 0.41), whereas for PRFS, the lowest q-values were found for Q3, Q7, and Q8 (q = 0.26 each). In the multivariable PRFS model including these three quadrants, Q3 involvement remained significant after FDR adjustment (q = 0.016), while Q7 (q = 0.071) and Q8 (q = 0.077) displayed borderline trends. These results support the presence of a reproducible signal for Q3 involvement in oligometastatic patients, suggesting that even within this favorable subgroup, the anatomical distribution of peritoneal disease may influence recurrence dynamics and contribute to further risk stratification. Nevertheless, all findings should be interpreted cautiously within the exploratory, hypothesis-generating framework of this pilot study. Involvement of these specific quadrants, particularly Q3 and Q7, may reflect areas more prone to microscopic residual disease or less amenable to complete cytoreduction, despite achieving CC-0 status. This observation aligns with prior hypotheses [] suggesting that not all peritoneal regions carry the same prognostic weight. Localization, especially in areas involving intestinal or pelvic structures, may affect both the technical complexity of resection and the likelihood of recurrence. However, most existing studies have focused primarily on total PCI rather than the prognostic implications of its anatomical subcomponents. Our findings that certain quadrants may be associated with poorer outcomes in patients with low disease burden suggest a potential refinement in the prognostic relevance of peritoneal tumor distribution, warranting further investigation.
In contrast, among patients with PCI > 6, no quadrant-specific associations emerged for survival or recurrence, indicating that in widespread disease, the influence of anatomical site is likely eclipsed by total tumor burden. Systemic disease control may be the primary driver of outcomes in advanced-stage presentations, whereas the impact of local tumor distribution becomes more relevant in patients with limited disease.
KRAS mutation showed a borderline association with improved OS in univariate analysis but was not retained in multivariable models. The high proportion of missing molecular data limits our ability to draw definitive conclusions.
These observations are hypothesis-generating and require confirmation in larger, prospective cohorts with standardized quadrant mapping and pathology.
Should future studies validate these findings, the concept of quadrant-specific prognostic assessment could contribute to refining patient selection or guiding operative planning. However, no changes in current surgical practice can be inferred from this exploratory analysis.
The study benefits from a homogeneous, single-institution cohort treated under standardized protocols with long-term follow-up, but several limitations temper inference. The limited sample size, particularly within the PCI ≤ 6 subgroup, and the large number of quadrant-wise comparisons inherently increase the risk of both type I and II errors across multiple quadrant-wise tests. The retrospective design may introduce selection bias. Grouping rectal primaries with left-sided colon tumors, although anatomically pragmatic, may blur biological differences specific to rectal disease. Residual confounding by treatment timing and prior surgery is also possible. Molecular profiling was incomplete for a substantial proportion of patients, reducing the power to detect associations between genotype and outcome. Although the inclusion period spans two decades, the CRS and HIPEC protocols remained largely unchanged over time; however, era-related improvements in systemic therapy or perioperative management cannot be entirely excluded and are acknowledged as a potential limitation.
The findings should therefore be regarded as preliminary signals consistent with a pilot, hypothesis-generating analysis rather than definitive associations. These results specifically pertain to colorectal peritoneal metastases, as other primary tumors (e.g., gastric or ovarian) exhibit distinct biological behavior, dissemination patterns, and prognostic trajectories, and extrapolation beyond this histologic context should therefore be avoided.
These limitations underscore the need for validation in larger, preferably multicenter cohorts with adequate representation of non-adenocarcinoma histologies and pre-specified, flow-based hypotheses to enable a more definitive assessment of pattern-phenotype relationships and to clarify the role of prophylactic resection.
5. Conclusions
In colorectal peritoneal metastasis treated with CRS + HIPEC, PCI remains the dominant prognostic determinant, with PCI ≤ 6 associated with durable disease control and PCI > 6 with poor long-term outcomes. Within the oligometastatic subset, however, the anatomical distribution of disease added prognostic information: involvement of specific regions, particularly Q3 and Q7, correlated with inferior DFS and higher risk of peritoneal relapse, while Q8 showed a borderline signal and a small, exploratory histology-related difference. No quadrant effects were observed in PCI > 6, consistent with outcomes being driven primarily by overall tumor burden in extensive disease. These findings support a biologically grounded view of peritoneal spread shaped by fluid flow and regional stasis, and they raise the hypothesis that quadrant-directed operative planning might refine selection and local control in oligometastatic candidates. In selected high-risk regions, evaluation of prophylactic peritonectomy despite the absence of macroscopic deposits could be considered in prospective studies, provided oncologic benefit and acceptable morbidity are demonstrated. Given the retrospective design, small group sizes with sparse cells, incomplete molecular profiling, and the pragmatic grouping of rectal with left-sided primaries, validation in larger, preferably multicenter cohorts with standardized quadrant mapping, adequate representation of non-adenocarcinoma histologies, and pre-specified flow-based hypotheses is warranted.
Author Contributions
Conceptualization, V.Z. and F.D.; methodology, V.Z.; formal analysis, V.Z.; data curation, G.E.C., G.M., E.P., R.T. and M.B.; writing—original draft preparation, V.Z.; writing—review and editing, F.D., V.Z. and M.F.; supervision, G.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The diagnostic–therapeutic protocol and data collection were approved by CEROM, Hospital Ethics Committee Protocol code 0/23453/F2RP (date of approval 4 June 2004), and written informed consent was signed by all patients.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
In accordance with current privacy legislation, the database generated 448 and analyzed during this study cannot be publicly shared.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lurvink, R.J.; Bakkers, C.; Rijken, A.; van Erning, F.N.; Nienhuijs, S.W.; Burger, J.W.; Creemers, G.J.; Verhoef, C.; Lemmens, V.E.; De Hingh, I.H. Increase in the incidence of synchronous and metachronous peritoneal metastases in patients with colorectal cancer: A nationwide study. Eur. J. Surg. Oncol. 2021, 47, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Breuer, E.; Hebeisen, M.; Schneider, M.A.; Roth, L.; Pauli, C.; Frischer-Ordu, K.; Eden, J.; Pache, B.; Steffen, T.; Huebner, M.; et al. Site of Recurrence and Survival After Surgery for Colorectal Peritoneal Metastasis. JNCI-J. Natl. Cancer Inst. 2021, 113, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Franko, J.; Shi, Q.; Goldman, C.D.; Pockaj, B.A.; Nelson, G.D.; Goldberg, R.M.; Pitot, H.C.; Grothey, A.; Alberts, S.R.; Sargent, D.J. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: A pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J. Clin. Oncol. 2012, 30, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Franko, J.; Shi, Q.; Meyers, J.P.; Maughan, T.S.; Adams, R.A.; Seymour, M.T.; Saltz, L.; Punt, C.J.A.; Koopman, M.; Tournigand, C.; et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: An analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016, 17, 1709–1719. [Google Scholar] [CrossRef] [PubMed]
- van Oudheusden, T.R.; Razenberg, L.G.; van Gestel, Y.R.; Creemers, G.J.; Lemmens, V.E.; de Hingh, I.H. Systemic treatment of patients with metachronous peritoneal carcinomatosis of colorectal origin. Sci. Rep. 2015, 5, 18632. [Google Scholar] [CrossRef] [PubMed]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Malcomson, L.; Soh, Y.J.; Wilson, M.S.; Clouston, H.; O’Dwyer, S.T.; Kochhar, R.; Aziz, O. Patterns and Timing of Recurrence following CRS and HIPEC in Colorectal Cancer Peritoneal Metastasis. Eur. J. Surg. Oncol. 2023, 49, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, V.; Tan, S.; Kong, J.; Pham, T.; Michael, M.; Ramsay, R.; Warrier, S.; Heriot, A. Prognostic factors influencing survival in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for isolated colorectal peritoneal metastases: A systematic review and meta-analysis. Color. Dis. 2020, 22, 1482–1495. [Google Scholar] [CrossRef] [PubMed]
- Hallam, S.; Tyler, R.; Price, M.; Beggs, A.; Youssef, H. Meta-analysis of prognostic factors for patients with colorectal peritoneal metastasis undergoing cytoreductive surgery and heated intraperitoneal chemotherapy. BJS Open 2019, 3, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Zucchini, V.; D’Acapito, F.; Rapposelli, I.G.; Framarini, M.; Di Pietrantonio, D.; Turrini, R.; Pozzi, E.; Ercolani, G. Impact of RAS, BRAF mutations and microsatellite status in peritoneal metastases from colorectal cancer treated with cytoreduction + HIPEC: Scoping review. Int. J. Hyperth. 2025, 42, 2479527. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Mariani, A.; Cloutier, A.-S.; Blot, F.; Goéré, D.; Dumont, F.; Honoré, C.; Billard, V.; Dartigues, P.; Ducreux, M. Modified selection criteria for complete cytoreductive surgery plus HIPEC based on peritoneal cancer index and small bowel involvement for peritoneal carcinomatosis of colorectal origin. Eur. J. Surg. Oncol. 2014, 40, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Marano, L.; Marrelli, D.; Sammartino, P.; Biacchi, D.; Graziosi, L.; Marino, E.; Coccolini, F.; Fugazzola, P.; Valle, M.; Federici, O.; et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer with Synchronous Peritoneal Metastases: Multicenter Study of ‘Italian Peritoneal Surface Malignancies Oncoteam—S.I.C.O.’. Ann. Surg. Oncol. 2021, 28, 9060–9070. [Google Scholar] [CrossRef] [PubMed]
- Montanarella, M.; Boldig, K.; Virarkar, M.; Kumar, S.; Elsherif, S.; Lall, C.; Gopireddy, D.R. Intraperitoneal anatomy with the aid of pathologic fluid and gas: An imaging pictorial review. J. Clin. Imaging Sci. 2023, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Wasnik, A.P.; Maturen, K.E.; Kaza, R.K.; Al-Hawary, M.M.; Francis, I.R. Primary and secondary disease of the peritoneum and mesentery: Review of anatomy and imaging features. Abdom. Imaging 2015, 40, 626–642. [Google Scholar] [CrossRef] [PubMed]
- Meyers, M.A. Distribution of intra-abdominal malignant seeding: Dependency on dynamics of flow of ascitic fluid. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1973, 119, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.D.; Shaw, J.C.; Sobin, L.H. Secondary Tumors and Tumorlike Lesions of the Peritoneal Cavity: Imaging Features with Pathologic Correlation. RadioGraphics 2009, 29, 347–373. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Liu, Q.; Yu, W.; Ma, Y.; Zhu, J.; Lian, P.; Cai, S.; Li, Q.; Li, X. Prognostic value of distant metastasis sites and surgery in stage IV colorectal cancer: A population-based study. Int. J. Color. Dis. 2018, 33, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Klaver, Y.L.B.; Simkens, L.H.J.; Lemmens, V.E.P.P.; Koopman, M.; Teerenstra, S.; Bleichrodt, R.P.; de Hingh, I.H.J.T.; Punt, C.J.A. Outcomes of colorectal cancer patients with peritoneal carcinomatosis treated with chemotherapy with and without targeted therapy. Eur. J. Surg. Oncol. 2012, 38, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl, M.; Harter, P.; Bjørn, S.F.; Høgdall, C. Specific Regions, Rather than the Entire Peritoneal Carcinosis Index, are Predictive of Complete Resection and Survival in Advanced Epithelial Ovarian Cancer. Int. J. Gynecol. Cancer 2018, 28, 316–322. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).