Abstract
Herniation of the transplanted ureter into the inguinal canal is an exceptionally rare complication following renal transplantation. Most cases present as delayed-onset obstructions, typically occurring more than one year post-transplant and often involving the ipsilateral inguinal canal. We presented the case of a 49-year-old male kidney transplant recipient who developed obstructive uropathy due to herniation of the graft ureter into the ipsilateral inguinal canal. Diagnosis was confirmed by computed tomography (CT), which proved superior to ultrasonography in delineating the ureteral course. A JJ ureteral stent was successfully placed, followed by inguinal hernia repair using the Lichtenstein technique. The postoperative course was uneventful, with complete resolution of symptoms and preservation of graft function. Transplanted ureteral herniation is a rare but important cause of late post-transplant obstruction. Cross-sectional imaging, particularly CT, offers greater diagnostic accuracy than ultrasound alone in identifying ureteral displacement. When feasible, primary ureteral stenting may obviate the need for nephrostomy, thereby reducing patient morbidity.
1. Introduction
Inguinal hernias represent one of the most prevalent types of hernias encountered in adult patients [1,2,3,4], with surgical repair of inguinal hernias being among the most frequently performed procedures worldwide [5]. This condition constitutes a substantial portion of the surgical workload within general and visceral surgery departments [6,7]. While the vast majority of inguinal hernias are composed of abdominal contents such as intestine or omentum, rarer occurrences involve other structures, including components of the urinary tract [8,9]. Among these, herniation of the ureter through the inguinal canal is an exceptionally uncommon event, yet one that carries significant clinical implications [10,11].
Ureteral herniation into the inguinal canal may give rise to hydronephrosis either as a primary event (primary type), directly caused by the herniated segment, or as a secondary condition (secondary type), occurring as a consequence of distal urinary outflow obstruction [12].
Renal transplantation, a common and life-saving procedure for patients with end-stage renal disease, carries its own set of complications [13]. Among these, urologic issues—specifically ureteral obstructions—are observed in approximately 10% of transplant recipients, with half of these cases being obstructive in nature [14]. Transplanted-Ureter Inguinal Herniation (TUIH), an exceptionally rare manifestation [14,15,16], typically occurs in the late postoperative period, often more than a year after the transplant [17,18]. This complication is most frequently seen on the ipsilateral side of the transplanted kidney [17,18].
Given its rarity and often indolent clinical course, TUIH is frequently underrecognized or misdiagnosed [19]. Clinical signs may be subtle, such as vague groin discomfort, swelling, or unexplained renal graft dysfunction, making the diagnosis particularly challenging [19]. Without timely recognition and intervention, the patient may suffer from progressive hydronephrosis, irreversible damage to the transplanted kidney, and even graft loss [19,20,21].
Therefore, a high index of suspicion is essential, particularly in patients with a history of renal transplantation presenting with inguinal hernia or obstructive uropathy [20]. Early diagnosis, often aided by advanced imaging modalities such as CT, plays a crucial role in confirming the presence of ureteral herniation, delineating its anatomical course, and guiding appropriate surgical intervention [21,22].
Prompt and accurate diagnosis is essential to prevent long-term complications, preserve graft function, and improve patient outcomes [21].
This paper seeks to advance understanding of transplanted-ureter inguinal herniation (TUIH) by integrating an illustrative case report with a focused systematic review of the literature, with the aim of enhancing clinical recognition of this uncommon yet consequential complication after renal transplantation.
2. Materials and Methods
2.1. Search Strategy
A comprehensive and systematic literature search was conducted to identify studies pertaining to the herniation of the transplanted ureter into the inguinal canal.
This review has been registered in the International Prospective Register for Systematic Reviews—PROSPERO, with registration number CRD420251146767.
The search was performed using the PubMed database (US National Library of Medicine, http://www.ncbi.nlm.nih.gov/PubMed, accessed on 9 January 2024), a recognized repository for medical literature. We utilized a combination of relevant medical subject headings (MeSH) and keywords to optimize the search, specifically: inguinal hernia AND transplanted ureter OR ureteral complications OR ureter complications AND kidney transplantation. This strategy was designed to capture all articles that addressed the primary focus of this review, which is the rare occurrence of ureteral herniation following renal transplantation.
2.2. Inclusion Criteria
To ensure the relevance and quality of the included studies, the review was restricted to original research articles published in the English language or in the mother tongue of authors (Italian). Only studies that specifically addressed the herniation of the transplanted ureter into the inguinal canal were included in the analysis. Articles were initially selected based on their titles and abstracts. Following this, a full-text review was conducted to confirm their eligibility. This multi-step selection process was implemented to ensure that only studies directly relevant to the topic of interest were incorporated. The studies were required to provide sufficient clinical data on the herniation of transplanted ureters, including patient demographics, surgical outcomes, and other key clinical variables.
2.3. Exclusion Criteria
Studies published in languages other than English or Italian were excluded from the analysis to maintain consistency in data interpretation and to avoid potential biases related to translation. Furthermore, while the number of patients reported in each study was not an exclusion criterium, we excluded articles that did not substantially contribute to the overall understanding of the subject matter. For example, studies focused on herniations occurring in anatomical locations other than the inguinal canal, such as the femoral foramen, obturator foramen, or incisional hernias, were also excluded.
2.4. Data Extraction
Following the initial screening of titles and abstracts, the full-text versions of eligible articles were retrieved for in-depth analysis. Data were systematically extracted from each study in a standardized format to ensure consistency and comprehensiveness. Extracted data included the year of publication, patient demographics (age and gender), and the time interval between kidney transplantation and the occurrence of ureteral herniation. Additionally, the side of the inguinal herniation (left or right) was recorded, along with the presence of any nephrostomy or ureteral stent in place at the time of herniation. These variables were considered critical to understanding the context and clinical outcomes of transplanted ureter herniation.
For each study, we applied the CARE checklist [23] to derive a structured quality grade (A = Outstanding, B = Strong, C = Moderate), which informed the evidence synthesis and contextualized outcomes across reports (Table 1).
Moreover, detailed information regarding the surgical management of the hernia was extracted, including whether mesh reinforcement was used in the hernia repair, and if so, the type of mesh employed. The choice of surgical technique, particularly the use of mesh, may influence long-term outcomes and recurrence rates, making this a crucial data point.
The final inclusion and assessment of studies were completed in December 2024, yielding a total of 35 studies. Discrepancies in the study selection were resolved through discussion and consensus among all authors, ensuring the final list of included articles met the criteria for high-quality evidence. A PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [24] flow chart was created to document the search and selection process, visually summarizing the progression from initial identification to final inclusion (Figure 1).
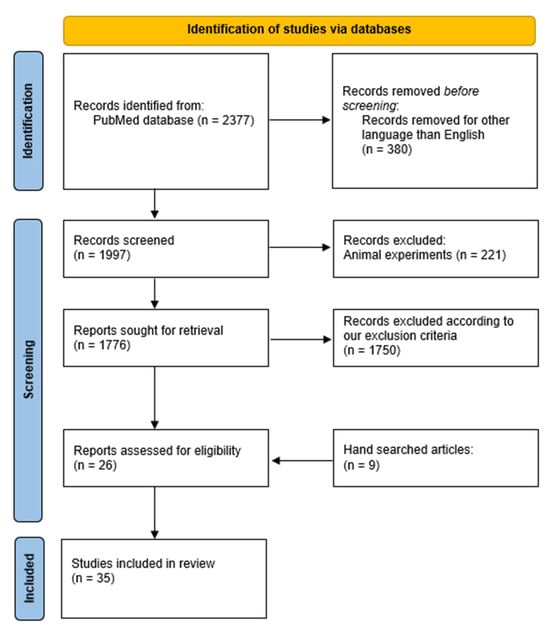
Figure 1.
PRISMA Flow chart documented the literature review process.
2.5. Statistical Analysis and Synthesis
The studies were then analyzed to identify common trends, clinical patterns, and outcomes. The findings were synthesized to provide an evidence-based summary of the incidence, diagnostic challenges, and treatment options for transplanted ureter inguinal herniation, with particular attention to surgical techniques and long-term patient outcomes.
2.6. Case-Report Methodology
In the preparation of this case report, we have adhered to the CARE (CAse REport) [23] guidelines, which provide a standardized framework for reporting clinical cases with clarity and rigor. By following these guidelines, we aim to enhance the reproducibility, clinical relevance, and scientific quality of the report, ensuring its utility for both clinicians and researchers in the field.
2.7. PICO Framework
To ensure clarity and methodological rigor, the research question was structured according to the PICO framework [25]:
- -
- P (Population/Patient/Problem): Kidney transplant recipients who developed in guinal herniation of the transplanted ureter.
- -
- I (Intervention/Exposure): Diagnostic and therapeutic interventions, including cross-sectional imaging (CT, ultrasound), ureteral stenting, nephrostomy placement, and surgical hernia repair (with or without mesh).
- -
- C (Comparison): Alternative or conservative management strategies, or different surgical techniques (e.g., tissue repair versus mesh reinforcement).
- -
- (Outcomes): Clinical outcomes including resolution of obstructive uropathy, preservation of graft function, recurrence of hernia, perioperative complications.
3. Results
3.1. Case Report
A 49-year-old male patient presented to the Emergency Department with complaints of rectal bleeding and anemia, which were further complicated by acute kidney injury (AKI). On admission, his hemoglobin level was critically low at 6.4 g/dL, and serum creatinine was markedly elevated at 6 mg/dL, reflecting significant renal impairment. His past medical history was notable for an appendectomy and a kidney transplant performed 15 years earlier, with the graft originating from a living donor. The patient had previously undergone kidney transplantation due to end-stage chronic renal disease for type II Diabetes. His medical history was also notable for arterial hypertension as a comorbidity. The immunosuppressive regimen consisted of variable dosage of tacrolimus based on tacrolemia values regularly followed by the nephrology transplant center. In the days preceding admission, he had reported persistent diarrhea accompanied by episodes of bright red blood loss per rectum, raising immediate concern for lower gastrointestinal pathology in addition to his renal dysfunction. Given the severity of the presentation, urgent blood transfusions and intravenous fluid resuscitation were promptly initiated to stabilize his hemodynamic and metabolic condition.
On detailed physical examination, a primary non-reducible left inguinal hernia was palpated, prompting further imaging. Ultrasonography demonstrated grade III hydronephrosis, according to the Society of Fetal Urology grading system of hydronephrosis [26], of the transplanted kidney, findings that necessitated additional evaluation by CT. The CT scan confirmed the diagnosis of a left inguinal hernia containing an incarcerated intestinal loop and also revealed kinking and entrapment of the transplanted ureter, which explained the hydronephrosis of the grafted kidney (Figure 2).
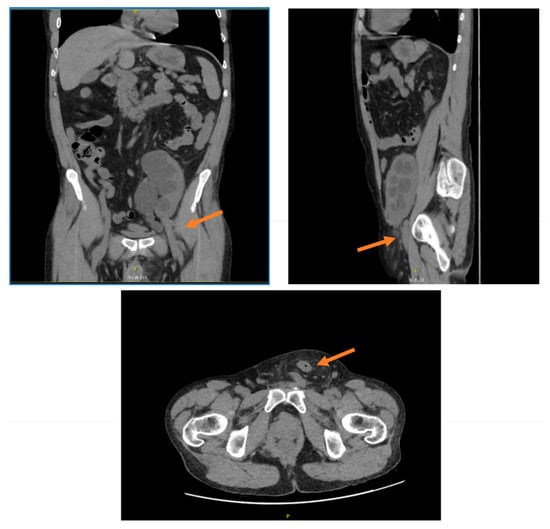
Figure 2.
CT scan images showing left inguinal hernia of transplanted ureter. The arrow indicates the herniation of the ureter.
In light of these complex findings, the patient was immediately transferred to the operating room for combined urological and surgical intervention. A cystoscopy was performed, during which the neo-ostium located on the left anterolateral bladder wall was identified and selectively cannulated. Under pyelographic guidance, a double-J (JJ) ureteral stent was successfully placed by the urology team (Figure 3), thereby ensuring decompression of the obstructed renal unit. Following this, open anterior hernioplasty, for a primary indirect inguinal hernia, was undertaken using the standard Lichtenstein technique, with the placement of a polypropylene mesh to reinforce the repair. Importantly, the transplanted ureter was preserved intact, without the need for resection or reimplantation.
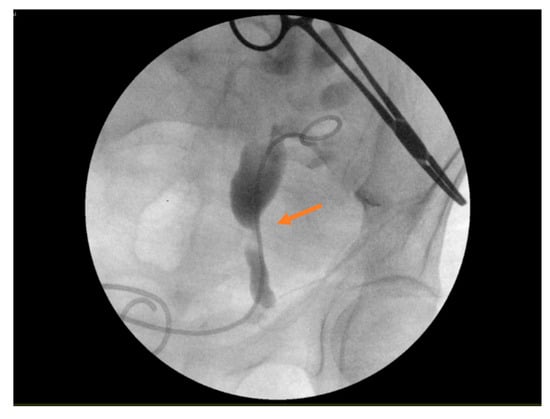
Figure 3.
The positioning of a JJ ureteral stent during an intraoperative cystoscopy. The arrows indicates the positioning of the stent in the obstructed ureter.
Postoperatively, the patient was admitted to the nephrology ward for close surveillance of renal function and careful monitoring of urine output. His clinical recovery was uneventful, and progressive improvement in renal parameters was observed. On the 11th postoperative day, both the bladder catheter and the ureteral stent were removed without complications. At the time of discharge, his serum creatinine had decreased to 3.8 mg/dL, approximating his baseline pre-admission levels, and his overall condition was stable. The successful resolution of this complex clinical scenario highlighted the importance of prompt multidisciplinary management and timely surgical intervention in transplant recipients presenting with unusual hernia-related complications.
3.2. Literature Review
A comprehensive literature search was conducted using the PubMed database, which initially yielded a total of 2377 records. After the removal of duplicate entries and the application of predefined inclusion and exclusion criteria during the screening of titles and abstracts, 26 articles were deemed eligible for inclusion in the final analysis. In addition, a manual search of reference lists and literature identified a further 9 case reports that satisfied the eligibility criteria. Taken together, a total of 35 articles were ultimately reviewed, encompassing a cumulative total of 40 documented cases of inguinal herniation involving the transplanted ureter (Table 1) [12,14,17,18,19,20,21,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54]. The methodology of the search process, including the identification, screening, and selection phases, is illustrated in the PRISMA flow chart presented in Figure 1.
The demographic profile of the reported patients was notably skewed toward males, with only two female cases identified across all studies. The median age of affected patients was 61.8 years, while the median interval between kidney transplantation and the diagnosis of ureteral herniation was 12.9 years. With respect to management strategies, of the 40 cases identified, 24 involved the placement of a nephrostomy, and 22 required ureteral stenting (Table 1). In 16 instances, both nephrostomy and stenting were performed, underscoring the frequent need for combined interventions. Hernia repair techniques varied considerably: 38 cases were managed with simple hernia repair, whereas mesh reinforcement was applied in 21 cases (Table 1). Among these, polypropylene mesh represented the most frequently reported material, utilized in nine studies [17,28,29,33,37,45,47,48,54]. Eight cases, however, did not specify the type of mesh employed [35,44,49,52,53]. In isolated reports, alternative materials or not clearly specified were described, including polyglactin 910 mesh [20], a non-absorbable mesh [40], a non-absorbable preperitoneal mesh [43], and a biological mesh [49]. Notably, two rare cases of contralateral ureteral herniation were identified, in which the herniated ureter was located on the side opposite to the transplanted kidney [18,44].
In 27 cases, as shown in Table 2, no post-operative complications were described by the authors. Only 14 cases reported post-operative length of stay with a median of 4.1 days (Table 2). No Hernia recurrence during the follow up after the discharge from hospital was reported in 11 studies [12,18,27,37,43,44,47,49,50,53,54]. In 35 cases (Table 2), no graft failure was described.
This heterogeneity in presentation and management reflects both the rarity of the condition and the absence of standardized therapeutic protocols, further highlighting the need for heightened clinical awareness and multidisciplinary expertise in addressing such complex scenarios.
4. Discussion
TUIH is an uncommon but clinically important complication that carries substantial implications for both urological and transplant outcomes and, although rare, the condition warrants careful attention due to its potential to precipitate a spectrum of adverse events, including obstructive uropathy, progressive graft dysfunction, and, in extreme cases, graft loss [19,20,33,39]. The rarity of TUIH often contributes to delays in recognition, as it may initially present with vague or nonspecific symptoms such as groin swelling, local discomfort, or unexplained renal function decline [19,20]. Timely diagnosis and management are therefore crucial to avoid irreversible consequences [19,20,21].
The condition is particularly relevant in transplant recipients, who represent a unique surgical population with multiple vulnerabilities [55]. These patients are often immunosuppressed, frequently have coexisting comorbidities, and typically require complex perioperative management [56]. Within this setting, even complications considered rare in the general surgical population can assume heightened importance [57]. TUIH exemplifies such a scenario, underscoring the need for heightened clinical awareness and multidisciplinary collaboration in its detection and treatment.
Several risk factors have been consistently associated with the development of TUIH. Among the most frequently cited are male sex [28,29,30,31,45] and older age, particularly in individuals aged 50 years or above [14,17,31,32,52]. Additionally, patients who have lived with a kidney transplant for five years or more appear to have an elevated risk [21,36,40,44].
Other anatomical and technical factors also play a role. Excessive ureteral length has been identified as a significant risk factor, as redundant segments are more likely to herniate through the inguinal canal [18,21,29].
Similarly, the placement of the donor ureter anterior to the spermatic cord during transplantation has been implicated as a predisposing factor, given its proximity to the inguinal canal and the potential for abnormal trajectories [18,20,21,29].
Additional contributors include infection with Polyomavirus BK [58], which has been associated with graft dysfunction and may weaken the integrity of ureteral tissue, thereby predisposing to herniation. Obesity also constitutes an important risk factor, not only because of increased intra-abdominal pressure but also due to the technical challenges it poses during both transplant and hernia repair surgeries [17,18,21,30].
The aggregation of these risk factors substantially raises the likelihood of ureteral herniation into the inguinal canal, a condition that frequently progresses to ureteral obstruction if not promptly identified [41,45]. Particularly in older patients, who often present with multiple comorbidities and diminished physiological reserves, the consequences can be profound that is why meticulous preoperative evaluation and comprehensive postoperative care are indispensable for minimizing complications and shortening hospitalization [59,60,61,62,63].
In this regard, the feasibility of performing tension-free hernia repair under ultrasound-guided local nerve block in elderly patients, as demonstrated by Wang et al. [64], provides meaningful insights into adapting surgical strategies for frail and high-risk populations.
The primary mechanism of clinical deterioration in TUIH is ureteral obstruction [45,65]. If left unrecognized, such obstruction can compromise graft function, sometimes irreversibly [45,65]. Early detection and treatment are therefore fundamental in preventing further decline or complete loss of the transplant kidney [41,45].
The timing of obstruction provides important diagnostic insights. According to literature experience most cases of transplanted ureter obstruction occur early—within the first three months after transplantation [19,28]. These are usually unrelated to TUIH and instead result from ischemic ureteral strictures, external compression caused by hematomas or lymphoceles, or technical complications such as an excessively narrow anti-reflux tunnel, suboptimal uretero-vesical anastomosis, or ureteral kinking [19,28].
One of the greatest difficulties in managing TUIH lies in its diagnosis [46]. In the initial evaluation of acute kidney allograft dysfunction, abdominal ultrasound is often the first-line imaging modality [45]. Ultrasound is useful for detecting hydronephrosis; however, its ability to detect ureteral herniation into the inguinal canal is limited [45]. Indeed, hernias may not always be clinically evident or easily visualized with sonography, leading to delays in the identification of TUIH as the underlying cause of hydronephrosis [18,45].
While ultrasound can reliably confirm hydroureteronephrosis, the inguinal component of transplanted ureter herniation is frequently overlooked [45]. In contrast, CT offers a more robust diagnostic modality [19,28]. Abdominal CT, which can be performed without intravenous contrast in patients with renal impairment, provides detailed anatomical information on both the transplanted kidney and the ureter [19,28]. This level of precision facilitates accurate mapping of the ureter’s course and its relationship to the inguinal canal, thereby enabling definitive diagnosis [19,28].
Once obstructive uropathy has been confirmed, urgent decompression of the urinary tract is indicated [66,67]. Depending on the clinical context, decompression may be achieved through percutaneous nephrostomy, ureteral stent placement, or a combination of the two [66,67]. The placement of a nephrostomy not only relieves obstruction but can also facilitate stent placement, while serving as a useful intraoperative landmark during subsequent hernia repair procedures [43].
Individual patient features and surgical history play a decisive role in guiding the choice of operative technique, with previous pelvic or infra-abdominal procedures being of particular relevance [68].
Historically, the use of mesh in hernia repair for transplant recipients has been approached with caution, largely due to concerns regarding mesh-related infection and impaired wound healing in immunosuppressed patients [11,69,70]. Nevertheless, primary hernia repair without mesh carries a significant risk of recurrence, estimated at 10–30% [19,71]. More recent evidence supports the safety and efficacy of polypropylene mesh in transplant patients [72,73,74,75], despite concern that immunosuppression drugs may affect the wound-related complications [75].
Alternative biologic meshes have also been explored. Catena et al. [76] evaluated the use of porcine small intestinal submucosa (Surgisis) in ten immunocompromised patients, including four transplant recipients (three kidney, one liver), undergoing elective Lichtenstein hernioplasty. The study reported no postoperative complications, wound infections, or recurrences, suggesting that Surgisis is a safe alternative to polypropylene mesh, particularly in high-risk immunosuppressed populations [76].
Beyond material safety, attention has increasingly shifted toward the broader evaluation of surgical quality. In this context, Willms et al. [77] showed that systematic analysis of surgical quality indicators after structured improvements in practice was associated with significant gains in hernia repair outcomes.
Laparoscopic approaches have similarly gained traction. Yannam et al. [75] assessed laparoscopic incisional hernia repair (LIHR) with mesh in 36 kidney and/or pancreas transplant recipients. With a mean follow-up of 2.2 years, outcomes—including complication and recurrence rates—were comparable to those observed in nontransplant patients (n = 62) [75]. Importantly, all transplant patients remained on immunosuppressive therapy, yet mesh-related infections and graft dysfunction were rare [75]. These findings support the feasibility of mesh-based laparoscopic hernia repair as a viable surgical strategy in transplant recipients [75].
According to Yin et al. [78], although inguinal herniorrhaphy is one of the most common operations worldwide, expected complication might be encountered in kidney transplantation recipients as the grafted kidney and ureter are positioned in the pre-peritoneal space.
Although ureteral obstruction remains the most significant concern in TUIH, other postoperative complications following inguinal hernia repair have been documented [79,80]. These include rare but potentially serious events such as acute kidney injury secondary to iatrogenic ureteral injury [79,80].
Acute renal failure due to ureter compression after hernia repair is uncommon in kidney transplant recipients [81].
Selman et al. [82] reported a case of a transplant patient who developed anuria following inguinal hernia repair due to inadvertent ureteral ligation. The complication was successfully managed with nephrostomy placement followed by ureteral reimplantation, ultimately restoring renal function [82].
Similarly, Veroux et al. [81] described acute renal failure caused by ureteral compression from a mesh plug placed in the preperitoneal space. Prompt surgical reintervention to remove the plug led to rapid recovery of graft function, with full normalization occurring within four days [81].
These literature experiences underscore the importance of recognizing the distinct anatomical and physiological context of transplant recipients.
Repair with polypropylene mesh has proven to be both safe and effective, even in immunocompromised recipients, significantly reducing the risk of recurrence when com-pared with primary tissue-based hernia repairs. While mesh inguinal hernia repair is generally safe in the broader population, it carries a heightened risk of urological complications in patients with renal transplants. Surgeons must therefore exercise particular caution, considering the unique anatomical course of the transplanted ureter.
Adjunctive measures, including nephrostomy and ureteral stenting, play pivotal roles in relieving obstruction, protecting graft function, and ensuring stability during the perioperative period. Furthermore, meticulous postoperative surveillance is fundamental, as early recognition of complications allows for prompt corrective intervention and minimizes the likelihood of irreversible renal impairment.
Prompt recognition of complications through graft-targeted ultrasonography and abdominal CT, followed by timely surgical intervention, may obviate the need for nephrostomy placement and contribute to the preservation and recovery of graft function. In this regard, the development of artificial intelligence tools has opened new perspectives for the early detection of complex clinical conditions, supporting clinicians in anticipating complications before overt clinical deterioration occurs [83].
In summary, the clinical management of TUIH requires an integrated, multidisciplinary approach that combines high clinical suspicion, advanced imaging, timely urinary decompression, meticulous surgical planning, and vigilant postoperative monitoring. Adherence to these principles can substantially reduce the risk of graft dysfunction and enhance patient prognosis.

Table 1.
Reported cases of inguinal herniation of the transplanted ureter. Abbreviation: M, male; F, female; PT, post-transplantation; R, right; L, left; NA, Not available.
Table 1.
Reported cases of inguinal herniation of the transplanted ureter. Abbreviation: M, male; F, female; PT, post-transplantation; R, right; L, left; NA, Not available.
| Case | Year | Grading Score | Gender | Age | Years PT | Side | Nephrostomy | Stent | Hernia Repair | Mesh | Type |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ciancio et al. [27] | 1995 | B (Strong) | M | 59 | 1.5 | R | Yes | Yes | Yes | No | \ |
| Osman et al. [28] | 2004 | B (Strong) | F | 44 | 8 | R | Yes | No | Yes | Yes | Polypropylene |
| Salinas Sanchez et al. [29] | 2005 | B (Strong) | M | 70 | 5 | R | Yes | Yes | Yes | Yes | Polypropylene |
| Furtado et al. [30] | 2006 | B (Strong) | M | 44 | 12 | L | Yes | No | Yes | No | \ |
| Verbeeck et al. [31] | 2007 | B (Strong) | M | 75 | 11 | R | No | No | Yes | No | \ |
| Ingber et al. [17] | 2007 | B (Strong) | M | 72 | 12 | L | Yes | Yes | Yes | Yes | Polypropylene |
| Otani et al. [32] | 2008 | A (Outstanding) | M | 53 | 9 | R | Yes | No | Yes | No | \ |
| Di Cocco et al. [12] | 2009 | A (Outstanding) | M | 41 | 19 | R | Yes | Yes | Yes | No | \ |
| Azhar et al. [14] | 2009 | B (Strong) | M | 76 | 20 | R | Yes | Yes | Yes | No | \ |
| Odisho et al. [33] | 2010 | A (Outstanding) | M | 58 | 15 | R | Yes | Yes | Yes | Yes | Polypropylene |
| Tran et al. [34] | 2011 | B (Strong) | M | 52 | 8 | L | Yes | Yes | Yes | No | \ |
| Eng et al. [35] | 2011 | B (Strong) | M | 50 | 7 | R | Yes | Yes | Yes | Yes | NA |
| Pourafkari et al. [21] | 2013 | B (Strong) | M | 50 | 12 | R | Yes | Yes | No | No | \ |
| Vyas et al. [36] | 2014 | B (Strong) | M | 32 | 7 | R | No | No | Yes | No | \ |
| Kondo et al. [37] | 2015 | A (Outstanding) | M | 52 | 7 | R | No | Yes | Yes | Yes | Polypropylene |
| Hakeem et al. [20] | 2016 | B (Strong) | M | 72 | 9 | R | Yes | Yes | Yes | Yes | Polyglactin 910 |
| Cheung et al. [38] | 2016 | A (Outstanding) | M | 44 | 18 | L | No | No | Yes | No | \ |
| Cheung et al. [38] | 2016 | B (Strong) | M | 88 | 15 | R | Yes | No | Yes | No | \ |
| Soleymanian et al. [39] | 2016 | B (Strong) | M | 48 | <1 [4 months] | L | No | No | No | No | \ |
| Coelho et al. [40] | 2016 | B [Strong] | M | 77 | 25 | L | Yes | No | Yes | Yes | Non absorbable |
| Vigo et al. [41] | 2016 | A [Outstanding] | M | 51 | 12 | R | No | No | Yes | No | \ |
| Ross et al. [42] | 2017 | B (Strong) | M | 63 | 30 | R | Yes | Yes | Yes | No | \ |
| Ghielmini et al. [43] | 2017 | A (Outstanding) | M | 82 | 14 | L | Yes | Yes | Yes | Yes | Preperitoneal Non absorbable |
| Du Toit et al. [44] | 2017 | A (Outstanding) | M | 39 | 11 | R | Yes | No | Yes | Yes | NA |
| Lobo et al. [18] | 2017 | B (Strong) | M | 73 | 29 | L | Yes | Yes | Yes | No | \ |
| Bugeja et al. [45] | 2018 | A (Outstanding) | M | 75 | 6 | R | No | Yes | Yes | Yes | Polypropylene |
| Areda et al. [46] | 2019 | C (Moderate) | M | 81 | NA | R | Yes | No | Yes | No | \ |
| Bosmans et al. [19] | 2019 | A (Outstanding) | M | 61 | 9 | R | Yes | No | Yes | No | \ |
| Kobayashi et al. [47] | 2020 | B (Strong) | M | 68 | 8 | R | No | Yes | Yes | Yes | Polypropylene |
| Giacomoni et al. [48] | 2021 | B (Strong) | M | 56 | 37 | L | No | No | Yes | Yes | Polypropylene |
| Merani et al. [49] | 2021 | A (Outstanding) | M | 62 | 11 | L | Yes | Yes | Yes | Yes | NA |
| Merani et al. [49] | 2021 | B (Strong) | F | 72 | 2 | R | Yes | Yes | Yes | Yes | Biological |
| Chang et al. [50] | 2021 | B (Strong) | M | 76 | 3 | R | Yes | Yes | Yes | No | \ |
| Mongera et al. [51] | 2023 | A (Outstanding) | M | 78 | 17 | R | No | Yes | Yes | NA | \ |
| Lima et al. [52] | 2023 | A (Outstanding) | M | 63 | 9 | L | No | Yes | Yes | Yes | NA |
| Ishikawa et al. [53] | 2024 | B (Strong) | M | 68 | 10.6 | R | No | No | Yes | Yes | NA |
| Ishikawa et al. [53] | 2024 | B (Strong) | M | 68 | 8.3 | R | No | No | Yes | Yes | NA |
| Ishikawa et al. [53] | 2024 | B (Strong) | M | 58 | 2.5 | R | No | No | Yes | Yes | NA |
| Ishikawa et al. [53] | 2024 | B (Strong) | M | 60 | 32.7 | R | No | No | Yes | Yes | NA |
| Shaheen et al. [54] | 2024 | B (Strong) | M | 53 | 25 | R | No | No | Yes | Yes | Polypropylene |
| Present case | 2024 | M | 49 | 15 | L | No | Yes | Yes | Yes | Polypropylene |

Table 2.
Post operative characteristics of reported cases of inguinal herniation of the transplanted ureter. Abbreviation: NA, Not available.
Table 2.
Post operative characteristics of reported cases of inguinal herniation of the transplanted ureter. Abbreviation: NA, Not available.
| Case | Post Operative Complications | Post Operative Stay (Days) | Hernia Recurrence During the Follow Up | Graft Failure |
|---|---|---|---|---|
| Ciancio et al. [27] | NA | NA | No | No |
| Osman et al. [28] | No | NA | NA | No |
| Salinas Sanchez et al. [29] | NA | NA | NA | No |
| Furtado et al. [30] | NA | NA | NA | NA |
| Verbeeck et al. [31] | No | NA | NA | No |
| Ingber et al. [17] | NA | NA | NA | No |
| Otani et al. [32] | No | 3 | NA | No |
| Di Cocco et al. [12] | No | 4 | No | No |
| Azhar et al. [14] | NA | NA | NA | No |
| Odisho et al. [33] | No | 1 | NA | NA |
| Tran et al. [34] | NA | NA | NA | No |
| Eng et al. [35] | No | NA | NA | No |
| Pourafkari et al. [21] | / | / | / | / |
| Vyas et al. [36] | No | NA | NA | No |
| Kondo et al. [37] | No | 4 | No | No |
| Hakeem et al. [20] | No | NA | NA | No |
| Cheung et al. [38] | No | 2 | NA | No |
| Cheung et al. [38] | No | 7 | NA | No |
| Soleymanian et al. [39] | NA | NA | NA | NA |
| Coelho et al. [40] | No | NA | NA | No |
| Vigo et al. [41] | No | NA | NA | No |
| Ross et al. [42] | NA | NA | NA | NA |
| Ghielmini et al. [43] | No | NA | No | No |
| Du Toit et al. [44] | No | 6 | No | No |
| Lobo et al. [18] | No | NA | No | No |
| Bugeja et al. [45] | NA | NA | NA | No |
| Areda et al. [46] | NA | NA | NA | No |
| Bosmans et al. [19] | No | 5 | NA | No |
| Kobayashi et al. [47] | No | 7 | No | No |
| Giacomoni et al. [48] | No | NA | NA | No |
| Merani et al. [49] | NA | NA | NA | No |
| Merani et al. [49] | NA | NA | No | No |
| Chang et al. [50] | No | NA | No | No |
| Mongera et al. [51] | No | NA | NA | No |
| Lima et al. [52] | No | 0 (discharged the same day of the surgery) | NA | No |
| Ishikawa et al. [53] | No | 4 | No | No |
| Ishikawa et al. [53] | No | 7 | No | No |
| Ishikawa et al. [53] | No | 3 | No | No |
| Ishikawa et al. [53] | No | 5 | No | No |
| Shaheen et al. [54] | No | NA | No | No |
| Present case | No | 11 | No | No |
5. Limitations
The present review is subject to several limitations that reflect the current state of the literature. First, transplanted-ureter inguinal herniation is an exceptionally rare condition, and the available evidence is limited to isolated case reports and small case series, which restricts the generalizability of findings. Second, the cases reported are highly heterogeneous with regard to clinical presentation, diagnostic modalities, and surgical management, making direct comparisons challenging and precluding standardized recommendations. Third, most studies are retrospective and descriptive in nature, often lacking systematic data collection or long-term follow-up, which limits conclusions regarding recurrence rates, graft survival, and overall patient prognosis. Finally, since this review did not aim to perform a cost analysis of the meshes used, we did not investigate the economic aspect of mesh selection.
6. Conclusions
Transplanted-ureter inguinal herniation is a rare but clinically important entity that endangers renal allograft function and can culminate in severe complications, including graft loss, if not identified and treated in a timely manner. Early recognition through advanced imaging modalities, particularly CT, is essential, as it not only establishes an accurate diagnosis but also provides detailed anatomical information that is indispensable for preoperative assessment and surgical planning.
Repair with polypropylene mesh has proven to be both safe and effective, even in immunocompromised recipients, significantly reducing the risk of recurrence when compared with primary tissue-based hernia repairs. Adjunctive measures, including nephrostomy and ureteral stenting, play pivotal roles in relieving obstruction, protecting graft function, and ensuring stability during the perioperative period. Furthermore, meticulous postoperative surveillance is fundamental, as early recognition of complications allows for prompt corrective intervention and minimizes the likelihood of irreversible renal impairment. Ultimately, optimal outcomes require a comprehensive, multidisciplinary approach that integrates precise diagnosis, timely surgical management, perioperative urinary tract protection, and long-term follow-up, thereby safeguarding graft survival and improving quality of life in this highly vulnerable patient population.
Author Contributions
Conceptualization, P.E., R.S., S.S. and P.A.; methodology, S.S. and P.A.; validation, G.G. and G.C.; formal analysis, P.E., S.S. and P.A.; investigation, P.E., S.S., P.A. and L.R.; resources, R.S., S.S., L.R. and M.D.R.; data curation, S.S. and P.A.; writing—original draft preparation, P.E., S.S. and P.A.; writing—review and editing, S.S. and P.A.; supervision: G.G., G.C. and M.D.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was carried out according to the Declaration of Helsinki guidelines and was approved by the Institutional Review Board of the University of Molise (protocol number 10/21, approved date: 12 May 2021).
Informed Consent Statement
A written informed consent for the treatment of personal and sensible data was obtained from all patients prior to the data collection and evaluation. Patients signed informed consent regarding publishing their data and photographs.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
All authors declare no conflicts of interest.
References
- Shi, H.; Li, S.; Lin, Y.; Yang, D.; Dong, W.; Song, Z.; Song, H.; Gu, Y. Suture repair versus mesh repair in elderly populations with incarcerated or strangulated groin hernia. Updates Surg. 2024, 76, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, J.T.; O’dwyer, P.J. Inguinal hernias. BMJ 2008, 336, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Zeng, H. Two-port (single incision plus one port) versus single-port laparoscopic totally extraperitoneal repair for inguinal hernia: A retrospective comparative study. Updates Surg. 2024, 76, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Burcharth, J. The epidemiology and risk factors for recurrence after inguinal hernia surgery. Dan. Med. J. 2014, 61, B4846. [Google Scholar]
- Usmani, S.U.R.; Bin Sultan, S.M.M.; Islam, M.B.; Abbas, S.; Choudhry, M.S. TAPP versus lichtenstein techniques for bilateral inguinal hernia repair: A systematic review and meta-analysis. Updates Surg. 2024, 76, 2583–2591. [Google Scholar] [CrossRef]
- Fan, X.; Ding, Y.; Sun, N.; Chen, Y. Ultra-micro instrument in laparoscopic transabdominal preperitoneal (TAPP) hernioplasty. Updates Surg. 2024, 76, 601–605. [Google Scholar] [CrossRef]
- Aydoğdu, Y.F.; Kubat, Ö.; Büyükkasap, Ç.; Göbüt, H.; Dikmen, K. A new approach to mesh fixation in laparoscopic transabdominal technique, “suture passer”, superior or not? Updates Surg. 2024, 76, 2617–2625. [Google Scholar] [CrossRef]
- Liechty, S.; Eiref, A.D.; Vengatesan, K.; Barasch, S.P.; Dong, X.D.; Zimmerman, P.W.; Nicoara, M.; Patel, K.; Walden, H.; Eiref, S.D. An inguinal hernia ‘hard to stomach’. J. Surg. Case Rep. 2023, 2023, rjad416. [Google Scholar] [CrossRef]
- Khan, K.; Chaudhry, A.; Feinman, M.B. Inguinoscrotal hernia containing the urinary bladder. BMJ Case Rep. 2016, 2016, bcr2016217408. [Google Scholar] [CrossRef]
- Wendler, J.J.; Baumunk, D.; Liehr, U.B.; Schostak, M. Kidney dislocation in a monstrous inguinal intestinal hernia with ureteropelvic junction obstruction and acute on chronic renal failure. Urol. Int. 2013, 91, 370–372. [Google Scholar] [CrossRef]
- Saidi, R.F.; Elias, N.; Hertl, M.; Kawai, T.; Cosimi, A.B.; Ko, D.S. Urinary reconstruction after kidney transplantation: Pyeloureterostomy or ureteroneocystostomy. J. Surg. Res. 2013, 181, 156–159. [Google Scholar] [CrossRef]
- Di Cocco, P.; Orlando, G.; Bonanni, L.; D’ANgelo, M.; Mazzotta, C.; Rizza, V.; Clementi, K.; Greco, S.; Famulari, A.; Pisani, F. Scrotal herniation of the ureter: A rare late complication after renal transplantation. Transplant. Proc. 2009, 41, 1393–1397. [Google Scholar] [CrossRef]
- Alotaibi, N.E. Incidence and risk factors of infections following kidney transplantation. J. Infect. Public Health 2024, 17, 102491. [Google Scholar] [CrossRef] [PubMed]
- Azhar, R.; Boutros, M.; Hassanain, M.; Polyhronopoulos, G.; Chaudhury, P.; Tchervenkov, J.; Cabrera, T. A rare case of obstructive uropathy in renal transplantation: Ipsilateral indirect inguinal herniation of a transplant ureter. Transplantation 2009, 88, 1038–1039. [Google Scholar] [CrossRef] [PubMed]
- Shoskes, D.A.; Hanbury, D.; Cranston, D.; Morris, P.J. Urological complications in 1,000 consecutive renal transplant recipients. J. Urol. 1995, 153, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Ballard, J.L.; Dobbs, R.M.; Malone, J.M. Ureteroinguinal hernia: A rare companion of sliding inguinal hernias. Am. Surg. 1991, 57, 720–722. [Google Scholar]
- Ingber, M.S.; Girdler, B.J.; Moy, J.F.; Frikker, M.J.; Hollander, J.B. Inguinal herniation of a transplant ureter: Rare cause of obstructive uropathy. Urology 2007, 70, 1224.e1–1224.e3. [Google Scholar] [CrossRef]
- Lobo, N.; McCaig, F.; Olsburgh, J. Contralateral inguinal herniation of a transplant ureter causing obstructive uropathy in a renal transplant recipient. BMJ Case Rep. 2017, 2017, bcr2016218071. [Google Scholar] [CrossRef]
- Bosmans, I.; De Boe, V.; Wissing, K.M.; Vanhoeij, M.; Jacobs-Tulleneers-Thevissen, D. A preventable cause of transplant hydroureteronephrosis: Inguinal herniation of the transplant ureter: Case report and review of the literature. Acta Chir. Belg. 2021, 121, 340–345. [Google Scholar] [CrossRef]
- Hakeem, A.R.; Gopalakrishnan, P.; Dooldeniya, M.D.; Irving, H.C.; Ahmad, N. Inguinal Herniation of a Transplant Ureter: Lessons Learned from a Case of “Water Over the Bridge”. Exp. Clin. Transplant. 2016, 14, 103–105. [Google Scholar] [CrossRef][Green Version]
- Pourafkari, M.; Ghofrani, M.; Riahi, M. Inguinal herniation of a transplant kidney ureter: A case report. Iran. J. Radiol. 2012, 10, 48–50. [Google Scholar] [CrossRef]
- Gupta, P.; Sharma, P.; Maurya, V.; Bhatia, M. Herniation of ureter: A rare cause of hydroureteronephrosis. Egypt. J. Radiol. Nucl. Med. 2021, 52, 178. [Google Scholar] [CrossRef]
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D. The CARE guidelines: Consensus-based clinical case report guideline development. J. Clin. Epidemiol. 2014, 67, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Eldawlatly, A.; Alshehri, H.; Alqahtani, A.; Ahmad, A.; Al-Dammas, F.; Marzouk, A. Appearance of Population, Intervention, Comparison, and Outcome as research question in the title of articles of three different anesthesia journals: A pilot study. Saudi J. Anaesth. 2018, 12, 283–286. [Google Scholar] [CrossRef]
- Fernbach, S.K.; Maizels, M.; Conway, J.J. Ultrasound grading of hydronephrosis: Introduction to the system used by the Society for Fetal Urology. Pediatr. Radiol. 1993, 23, 478–480. [Google Scholar] [CrossRef]
- Ciancio, G.; Burke, G.W.; Nery, J.; Huson, H.; Coker, D.; Miller, J. Positional obstructive uropathy secondary to ureteroneocystostomy herniation in a renal transplant recipient. J. Urol. 1995, 154, 1471–1472. [Google Scholar] [CrossRef]
- Osman, Y.; Ali-El-Dein, B.; El-Leithy, R.; Shokeir, A. Sliding hernia containing the ureter—A rare cause of graft hydroureteronephrosis: A case report. Transplant. Proc. 2004, 36, 1402–1404. [Google Scholar] [CrossRef]
- Sánchez, A.S.S.; Tebar, J.C.; Martín, M.S.; Bachs, J.M.G.; Moreno, M.J.D.; Navarro, H.P.; Rodríguez, J.A.V. Obstructive uropathy secondary to ureteral herniation in a pediatric en bloc renal graft. Am. J. Transplant. 2005, 5, 2074–2077. [Google Scholar] [CrossRef]
- Furtado, C.D.; Sirlin, C.; Precht, A.; Casola, G. Unusual cause of ureteral obstruction in transplant kidney. Abdom. Imaging 2006, 31, 379–382. [Google Scholar] [CrossRef]
- Verbeeck, N.; Niedercorn, J.B.; Mc Intyre, D.; Pouthier, D.; Lamy, S. Assessment of renal graft obstruction due to ureteral inguinal hernia: US detection and 3D MR confirmation. JBR-BTR 2007, 90, 132–134. [Google Scholar]
- Otani, L.H.; Jayanthi, S.K.; Chiarantano, R.S.; Amaral, A.M.; Menezes, M.R.; Cerri, G.G. Sonographic diagnosis of a ureteral inguinal hernia in a renal transplant. J. Ultrasound Med. 2008, 27, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Odisho, A.Y.; Freise, C.E.; Tomlanovich, S.J.; Vagefi, P.A. Inguinal herniation of a transplant ureter. Kidney Int. 2010, 78, 115. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.; Gaboriault, J.; Collette, S.; Senécal, L.; Morin, M.; Boucher, A.; Dandavino, R. Obstructive uropathy caused by an inguinal hernia in a kidney transplant recipient: Report of hernia cure by the shouldice technique. Dial. Transplant. 2011, 40, 413–414. [Google Scholar] [CrossRef]
- Eng, M.; Schiffman, S.; Ravindra, K. Graft Hydroureteronephrosis Secondary to Inguinal Hernia: A Report of Two Unusual Cases. Nephro-Urol. Mon. 2011, 3, 301–303. [Google Scholar]
- Vyas, S.; Chabra, N.; Singh, S.K.; Khandelwal, N. Inguinal herniation of the bladder and ureter: An unusual cause of obstructive uropathy in a transplant kidney. Saudi J. Kidney Dis. Transplant. 2014, 25, 153–155. [Google Scholar] [CrossRef]
- Kondo, A.; Nishizawa, Y.; Akamoto, S.; Fujiwara, M.; Okano, K.; Suzuki, Y. Internal inguinal hernia on the transplant side after kidney transplantation: A case report. Surg. Case Rep. 2015, 1, 108. [Google Scholar] [CrossRef][Green Version]
- Cheung, F.; Debartolo, M.M.; Copertino, L.M.; Szafran, A.A.; Estrada, C.C.; Lynch, P.G.; Darras, F.S. Different Management Options for Transplant Ureteral Obstructions within an Inguinal Hernia. Case Rep. Transplant. 2016, 2016, 4730494. [Google Scholar] [CrossRef]
- Soleymanian, T.; Kalantarian, T.; Radmard, A. Report of a kidney recipient with inguinal herniation of the transplant ureter. Saudi J. Kidney Dis. Transplant. 2016, 27, 614–616. [Google Scholar] [CrossRef]
- Coelho, H.; Nunes, P.; Canhoto, C.; Temido, P. Inguinal hernia containing bladder and ureteroneocystostomy: A rare cause for acute renal graft dysfunction. BMJ Case Rep. 2016, 2016, bcr2016214466. [Google Scholar] [CrossRef]
- Vigo, V.; Rossi, L.; Lisi, P.; Antonelli, M.; Lomonte, C.; Basile, C. An unusual cause of ureteral obstruction in kidney transplant. G. Ital. Nefrol. 2016, 33, 1–5. [Google Scholar]
- Ross, J.T.; Orandi, B.J.; Lin, M.Y.; Roll, G.R. Ureteral Obstruction and New Groin Pain in a Kidney Transplant Recipient. Am. J. Transplant. 2017, 17, 1139–1141. [Google Scholar] [CrossRef][Green Version]
- Ghielmini, E.; Julita, L.; Cerantola, Y.; Matter, M.; Zingg, T. Inguinal Bladder Hernia With Acute Ureteral Obstruction 14 Years After Kidney Transplantation: A Case Report. Transplant. Proc. 2017, 49, 1593–1595. [Google Scholar] [CrossRef] [PubMed]
- du Toit, T.; Kaestner, L.; Muller, E.; Kahn, D. Inguinal Herniation Containing Bladder, Causing Contralateral Allograft Hydroureteronephrosis—A Case Report and Literature Review. Am. J. Transplant. 2017, 17, 565–568. [Google Scholar] [CrossRef]
- Bugeja, A.; Clark, E.G.; Sood, M.M.; Ali, S.N. As in Real Estate, Location Is What Matters: A Case Report of Transplant Ureteral Obstruction Due to an Inguinal Hernia. Can. J. Kidney Health Dis. 2018, 5, 205435811775362. [Google Scholar] [CrossRef]
- Areda, M.A.; Bailey, C.R.; O’Mara, D.; Weiss, C.R. Transplant uretero-inguinal hernia resulting in urosepsis. Radiol. Case Rep. 2019, 14, 14–17. [Google Scholar] [CrossRef]
- Kobayashi, T.; Miura, K.; Saito, K.; Tasaki, M.; Saito, K.; Sakata, J.; Takizawa, K.; Katada, T.; Hirose, Y.; Yuza, K.; et al. Inguinal Herniation After Living Donor Kidney Transplantation: A Case Report. Transplant. Proc. 2020, 52, 1940–1943. [Google Scholar] [CrossRef]
- Giacomoni, A.; Hassan, R.; Vanzulli, A.; De Carlis, L. Obstructive Uropathy Due to an Unusual Inguinal Hernia 35 Years After Kidney Transplant. Exp. Clin. Transplant. 2021, 19, 80–82. [Google Scholar] [CrossRef]
- Merani, S.; Aufhauser, D.D.; Maskin, A.T.; Mezrich, J.; Al-Adra, D. Not as Rare as Initially Described: Transplant Ureter Incarceration Within Inguinal Hernia. Two Cases, Literature Review, and Management Algorithm. Transplant. Proc. 2021, 53, 2285–2290. [Google Scholar] [CrossRef]
- Chang, T.-Y.; Chang, C.-H.; Lai, P.-C.; Lin, W.-C. Graft kidney hydronephrosis caused by transplant ureter inguinal hernia: A case report. Medicine 2021, 100, e25965. [Google Scholar] [CrossRef]
- Mongera, N.; Vezzali, N.; Passler, W. An unusual ureteral dynamic obstruction in a kidney transplant patient. J. Ultrasound 2023, 26, 615–618. [Google Scholar] [CrossRef]
- Lima, D.L.; Viscarret, V.; Nogueira, R.; Watts, K.; Malcher, F. Transplant Ureter Inguinal Herniation Treated by Robotic Inguinal Hernia Repair. CRSLS MIS Case Rep. SLS 2023, 10, e2023.00020. [Google Scholar] [CrossRef]
- Ishikawa, H.; Kobayashi, T.; Miura, K.; Tasaki, M.; Saito, K.; Takizawa, K.; Sakata, J.; Wakai, T. Surgical Outcomes of Ipsilateral Inguinal Hernia After Kidney Transplantation. Transplant. Proc. 2024, 56, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, M.F.; Almalki, F.J.; Altheaby, A.; Alsaikhan, B. An unusual hernia postkidney transplantation led to intermittent ureteric obstruction. J. Surg. Case Rep. 2024, 2024, rjae060. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.M.; Woldesenbet, S.; Munir, M.M.; Khalil, M.; Endo, Y.; Katayama, E.; Altaf, A.; Rashid, Z.; Schenk, A.; Pawlik, T.M. Association of transplant recipient status with clinical and financial outcomes among patients undergoing major surgery. Surgery 2025, 179, 108938. [Google Scholar] [CrossRef] [PubMed]
- Magro, P.S.D.; Meinerz, G.; Garcia, V.D.; Mendes, F.F.; Marques, M.E.C.; Keitel, E. Kidney transplantation and perioperative complications: A prospective cohort study. Braz. J. Anesthesiol. 2024, 74, 844556. [Google Scholar] [CrossRef]
- Palamuthusingam, D.; Kunarajah, K.; Pascoe, E.M.; Johnson, D.W.; Hawley, C.M.; Fahim, M. Postoperative outcomes of kidney transplant recipients undergoing non-transplant-related elective surgery: A systematic review and meta-analysis. BMC Nephrol. 2020, 21, 365. [Google Scholar] [CrossRef]
- Greco, F.; Fornara, P.; Mirone, V. Renal transplantation: Technical aspects, diagnosis and management of early and late urological complications. Panminerva Med. 2014, 56, 17–29. [Google Scholar]
- Rocca, A.; Brunese, M.C.; Cappuccio, M.; Scacchi, A.; Martucci, G.; Buondonno, A.; Perrotta, F.M.; Quarto, G.; Avella, P.; Amato, B. Impact of Physical Activity on Disability Risk in Elderly Patients Hospitalized for Mild Acute Diverticulitis and Diverticular Bleeding Undergone Conservative Management. Medicina 2021, 57, 360. [Google Scholar] [CrossRef]
- Luciani, C.; Scacchi, A.; Vaschetti, R.; Di Marzo, G.; Fatica, I.; Cappuccio, M.; Guerra, G.; Ceccarelli, G.; Avella, P.; Rocca, A. The uniportal VATS in the treatment of stage II pleural empyema: A safe and effective approach for adults and elderly patients—A single-center experience and literature review. World J. Emerg. Surg. 2022, 17, 46. [Google Scholar] [CrossRef]
- Rocca, A.; Porfidia, C.; Russo, R.; Tamburrino, A.; Avella, P.; Vaschetti, R.; Bianco, P.; Calise, F. Neuraxial anesthesia in hepato-pancreatic-bilio surgery: A first western pilot study of 46 patients. Updates Surg. 2023, 75, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Marcellinaro, R.; Rocca, A.; Avella, P.; Grieco, M.; Spoletini, D.; Carlini, M. How aging may impact the failure to rescue after colorectal laparoscopic surgery. Analysis of 1000 patients in a single high-volume center. Updates Surg. 2025, 77, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Marcellinaro, R.; Grieco, M.; Spoletini, D.; Troiano, R.; Avella, P.; Brachini, G.; Mingoli, A.; Carlini, M. How to reduce the colorectal anastomotic leakage? The MIRACLe protocol experience in a cohort in a single high-volume centre. Updates Surg. 2023, 75, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Wu, Z.; Sun, H.; Zhang, W.; Cai, A.; Cui, Z.; Sun, S. Feasibility of tension-free repair of inguinal hernia in senile patients under ultrasound-guided local nerve block. Updates Surg. 2024, 76, 1461–1465. [Google Scholar] [CrossRef]
- Kumar, S.; Ameli-Renani, S.; Hakim, A.; Jeon, J.H.; Shrivastava, S.; Patel, U. Ureteral obstruction following renal transplantation: Causes, diagnosis and management. Br. J. Radiol. 2014, 87, 20140169. [Google Scholar] [CrossRef]
- Cozma, C.; Georgescu, D.; Popescu, R.; Geavlete, B.; Geavlete, P. Double-J stent versus percutaneous nephrostomy for emergency upper urinary tract decompression. J. Med. Life 2023, 16, 663–667. [Google Scholar] [CrossRef]
- Zul Khairul Azwadi, I.; Norhayati, M.N.; Abdullah, M.S. Percutaneous nephrostomy versus retrograde ureteral stenting for acute upper obstructive uropathy: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 6613. [Google Scholar] [CrossRef]
- Hayward, R.; Smith, J.J.; Kontovounisios, C.; Qiu, S.; Warren, O.J. Laparoscopic totally extraperitoneal hernia repair in patients with a history of previous abdominopelvic surgery. Updates Surg. 2024, 76, 2387–2393. [Google Scholar] [CrossRef]
- Kanella, I.; Kengadaran, K.; Papalois, V. Management of incisional hernias in renal transplant patients. Transplant. Rep. 2023, 8, 100148. [Google Scholar] [CrossRef]
- Quiroga-Centeno, A.C.; Quiroga-Centeno, C.A.; Guerrero-Macías, S.; Navas-Quintero, O.; Gómez-Ochoa, S.A. Systematic review and meta-analysis of risk factors for Mesh infection following Abdominal Wall Hernia Repair Surgery. Am. J. Surg. 2022, 224, 239–246. [Google Scholar] [CrossRef]
- Gopal, S.V.; Warrier, A. Recurrence after groin hernia repair-revisited. Int. J. Surg. 2013, 11, 374–377. [Google Scholar] [CrossRef]
- Mazzucchi, E.; Nahas, W.C.; Antonopoulos, I.; Ianhez, L.E.; Arap, S. Incisional hernia and its repair with polypropylene mesh in renal transplant recipients. J. Urol. 2001, 166, 816–819. [Google Scholar] [CrossRef]
- Antonopoulos, I.M.; Nahas, W.C.; Mazzucchi, E.; Piovesan, A.C.; Birolini, C.; Lucon, A.M. Is polypropylene mesh safe and effective for repairing infected incisional hernia in renal transplant recipients? Urology 2005, 66, 874–877. [Google Scholar] [CrossRef] [PubMed]
- Vardanian, A.J.; Farmer, D.G.; Ghobrial, R.M.; Busuttil, R.W.; Hiatt, J.R. Incisional hernia after liver transplantation. J. Am. Coll. Surg. 2006, 203, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Yannam, G.R.; Gutti, T.L.; High, R.; Stevens, R.B.; Thompson, J.S.; Morris, M.C. Experience of laparoscopic incisional hernia repair in kidney and/or pancreas transplant recipients. Am. J. Transplant. 2011, 11, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Catena, F.; Ansaloni, L.; Leone, A.; De Cataldis, A.; Gagliardi, S.; Gazzotti, F.; Peruzzi, S.; Agrusti, S.; D’aLessandro, L.; Taffurelli, M. Lichtenstein repair of inguinal hernia with Surgisis inguinal hernia matrix soft-tissue graft in immunodepressed patients. Hernia 2005, 9, 29–31. [Google Scholar] [CrossRef]
- Willms, A.G.; Schaaf, S.; Schwab, R. Analysis of surgical quality indicators after certification as a Hernia Center. Updates Surg. 2024, 76, 255–264. [Google Scholar] [CrossRef]
- Yin, S.-M.; Yang, C.-M.; Wu, Y.-J.; Liu, Y.-W. Inguinal herniorrhaphy related ureteral obstruction in kidney transplant recipient: A rare but crucial complication. Asian J. Surg. 2020, 43, 716–717. [Google Scholar] [CrossRef]
- Unver, M.; Firat, O.; Sezer, T.O.; Hoscoskun, C. Iatrogenic Ureteral Injury During Incisional Hernia Repair in a Kidney Recipient. J. Curr. Surg. 2017, 7, 20–22. [Google Scholar] [CrossRef]
- Yahya, Z.; Al-Habbal, Y.; Hassen, S. Ureteral inguinal hernia: An uncommon trap for general surgeons. BMJ Case Rep. 2017, 2017, bcr2017219288. [Google Scholar] [CrossRef]
- Veroux, M.; Ardita, V.; Zerbo, D.; Caglià, P.; Palmucci, S.; Sinagra, N.; Giaquinta, A.; Veroux, P. First Case Report of Acute Renal Failure After Mesh-Plug Inguinal Hernia Repair in a Kidney Transplant Recipient. Medicine 2016, 95, e3199. [Google Scholar] [CrossRef]
- Selman, S.H.; Grecos, G.P.; Koo, B.C. Anuria in transplant patient following inguinal herniorrhaphy. J. Urol. 1985, 133, 669–670. [Google Scholar] [CrossRef]
- Avella, P.; Cappuccio, M.; Cappuccio, T.; Rotondo, M.; Fumarulo, D.; Guerra, G.; Sciaudone, G.; Santone, A.; Cammilleri, F.; Bianco, P.; et al. Artificial Intelligence to Early Predict Liver Metastases in Patients with Colorectal Cancer: Current Status and Future Prospectives. Life 2023, 13, 2027. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).