Abstract
There is evidence to suggest that restoration of major/rare biominerals by supplementation can produce osteogenic and anti-resorptive effects in humans. LithoLexal® is a natural extract harvested from a marine alga, Lithothamnion sp., with a porous microstructure and multimolecular composition rich in calcium (32% w/w) and magnesium (2.2% w/w) together with ~72 trace bioelements. In vitro, LithoLexal® demonstrated cellular-level osteogenic efficacy through enhancing the maturation and activity of pre-osteoblasts. This extract also expressed the ability to suppress osteoclastogenesis by downregulating the pro-resorptive cytokines TNF-α and IL-1β and the master regulator of inflammation NF-κB. Parathyroid hormone inhibition of parathyroid hormone secretion is another bioactivity of LithoLexal® Bone reported with both short- and long-term administration at a longer duration and higher magnitude than what calcium carbonate could induce. Due to these bioactivities that affect pathogenetic factors of osteoporosis, LithoLexal® Bone is referred to as a disease-modifying adjunctive therapy (DMAT). In postmenopausal animal models, LithoLexal® monotherapy preserved bone mineral density, microarchitecture, and biomechanical properties, while calcium carbonate failed to produce significant outcomes. The pro-resorptive effect of a high-fat diet was also efficiently counteracted in vivo by supplementary LithoLexal®. A large clinical trial on postmenopausal women verified the mitigating effects of LithoLexal® Bone on bone resorption and turnover rate. The characteristic composition of LithoLexal® together with its lattice microstructure are suggested to underlie its in vivo bioactivities. In conclusion, adjunctive therapy with LithoLexal® Bone is an attractive option for clinical prevention and treatment of osteopenia/osteoporosis.
1. Mineral Supplementation in Clinical Management of Osteoporosis
Osteoporosis is the single most common bone disease in the general population with an age- and sex-dependent prevalence. This ‘progressive’ but ‘reversible’ skeletal disease is operationally defined as a demonstration of a low energy fracture and/or bone density T-score of −2.5 or lower in the lumbar spine, femoral neck, or total hip measured by dual-energy X-ray absorptiometry (DXA). The key clinical feature of osteoporosis may be its clinically silent nature, which often presents itself with a sentinel low-energy fracture of hip, spine, or wrist. This type of pathological fracture is the most significant clinical complication of osteoporosis, which affects one in three women and one in five men after the age of 50 years. Hence, osteoporosis deserves special attention as an important public health issue with exceptionally high cumulative rates of psychological and physical complications. Since the elderly population is the fastest growing age group and the rate of obesity is rising worldwide, the global prevalence of osteoporosis and its complications are expected to upsurge in the upcoming years [1].
Similar to other multifactorial, noncommunicable diseases, optimal clinical management of osteoporosis entails lifestyle as well as pharmacological interventions. In light of this, major clinical guidelines recommend including a bioavailable source of elemental calcium in the dietary regimen of affected individuals as a priority measure [2]. In practice, clinicians cannot rely merely on dietary sources, particularly in individuals at high risk of osteoporosis and fragility fracture given that 51% of the world population is at risk of Ca deficiency according to a global-scale survey. The rates are especially high in Africa (80%) and Asia (57%) [3]. In addition, the bowel’s capacity to absorb calcium deteriorates after the sixth decade of life, leaving almost everybody above the age of 80 with some degree of calcium malabsorption [4]. Therefore, the daily calcium requirement of high-risk individuals is amplified, while the majority even fail to supply their basic needs from food sources. Members of this large group are hence considered appropriate candidates for supplementation therapy. The clinical value of calcium supplementation is supported (albeit not without controversy) by research on the effects of calcium adjunctive therapy on bone mineral density (BMD) and risk of fracture [5]. In a meta-analysis of 59 randomized controlled trials, calcium supplementation increased BMD at all studied skeletal sites by 0.7–1.4% after one year and by 0.8–1.5% after two years in persons over the age of 50. Notably, the effect size of supplementary calcium was equal to that of calcium from dietary sources [6]. This class of treatments is also viewed as an essential add-on to support the action of anti-resorptive treatments, with some clinical trial evidence for their efficacy [7].
Based on the above, calcium-based supplements with/without vitamin D have traditionally been a fixed part of the standard clinical management of osteopenia/osteoporosis in almost all patients with low BMD or increased risk of fragility fracture. Continuous efforts have also been made to optimize the efficacy or develop new forms of bone mineral supplements suitable for the treatment of bone loss disorders. Among the more successful candidates, a multimineral extract from a genus of red algae, known as Lithothamnion, deserves particular attention. The bone cell mineralization and osteogenic efficacy of this extract have been evidenced by several in vitro, in vivo, and clinical studies and reviewed previously by Carson and Clarke [8]. This paper aims to provide an updated review of the existing evidence and delve further into the underlying action mechanisms of this promising adjunctive therapy.
2. Mono- vs. Multimineral Adjunctive Therapy of Osteoporosis
It is widely known that calcium salts from different sources exhibit various levels of bioavailability and bioactivity, thus having disparate influences on bone metabolism [9,10,11,12,13]. Apart from calcium bioavailability, whether or not other active elements are present in a given supplement may considerably affect its overall bioactivity. Evidence suggests that co-administration of trace biominerals can enhance the bioactivity of calcium in living tissues [14]. A typical example of such a synergistic effect has been reported with a commercial product branded as LithoLexal® Bone (Nordic Medical LTD, London, UK, previously marketed as Aquamin®). LithoLexal® is a marine plant extract harvested from the calcareous cytoskeleton of Lithothamnion sp. of the Corallinacea family. This multimolecular complex is molded naturally by the deposition of marine minerals on organic cell walls and hence retains a unique porous microstructure. By utilizing a proprietary extraction technology, the microstructure of the LithoLexal® source is largely preserved during harvest and processing (Figure 1). The safety of this plant extract was evaluated by an acute/subacute toxicity study on male and female Sprague Dawley rats. No mortality or signs of toxicity were observed after administering a single dose of 10 g/kg BW of Lithothamnion sp. to the test animals. Escalating dosages of 0.625%, 1.25%, and 2.50% were used in the subacute arm, none of which caused any mortality or treatment-related changes in body weight, organ weight, feed consumption, feed utilization rate, urinalysis, hematological and biochemical blood analysis, gross necropsy, or histopathologic examinations.

Figure 1.
Scanning electron microscopic images of the mineralized cellular structure of Lithothamnion after being extracted and milled. As shown, the original microstructure of the harvested fragments used for making LithoLexal® is preserved. Images are courtesy of Marigot Ltd. (West Sussex, UK).
LithoLexal® Bone has been proven to be more bioactive than pure calcium supplements in various research settings in both splanchnic and peripheral tissues. For instance, LithoLexal® treatment reduced the number of pre-neoplastic/neoplastic liver lesions and exhibited tumor-suppressing effects in low- and high-fat diet mouse models [15]. Modulating uncontrolled inflammation is another bioactivity of LithoLexal®, observed in different disease models. The long-term administration of this complex delayed both the development and progression of chronic spontaneous colitis and diminished its associated tissue damage and mortality [16]. Peripheral anti-inflammatory properties of LithoLexal® have been reported using a model of ulcerative dermatitis, where oral treatment effectively reduced the incidence and severity of cutaneous symptoms [17]. In humans, a special formulation of this plant extract expressed considerable therapeutic effects in patients with osteoporosis, as reviewed by Eriksen et al. [18]. This distinctive bioactivity may also contribute to modifying the pathogenesis of osteoporosis as a disorder with pivotal inflammatory components [19].
Based on the above, LithoLexal® Bone is suggested as a preferred option for adjunctive therapy for osteopenic/osteoporotic patients, with additional clinical benefits compared to conventional monomineralic supplements. In this review, we elaborate on the available evidence for the efficacy of this natural extract and strive to touch upon some of its potential disease-modifying activities relevant to the pathogenesis of osteoporosis. Finally, we discuss the position and utilization of DMAT with LithoLexal® Bone in the standard clinical management of osteopenia and osteoporosis.
3. Pro-Mineralization and Osteogenic Effects of LithoLexal® Bone
As mentioned, having a multimolecular composition rich in calcium, magnesium, and several trace bioelements is a distinctive feature of LithoLexal® Bone [20]. Since several of these bioelements are essential for bone formation and mineralization, LithoLexal® Bone is a promising candidate for adjunctive therapy of osteoporosis [8]. The osteogenic efficiency of this treatment was verified at the cellular level by replicated experiments on bone cells. O’Gorman et al. reported that LithoLexal® improves the mineralization ability and osteogenic differentiation of pre-osteoblasts. Von Kossa and Alizarin Red S staining revealed widespread, cell-mediated deposition of calcium after treatment, which was significantly more prominent than that in the control group. At the endpoint, alkaline phosphatase (ALP) expression was higher in LithoLexal®-treated osteoblasts than controls at the same level of metabolic activity, indicating more advanced cellular maturation and osteogenesis [21]. In another experiment, adding LithoLexal® to pre-osteoblasts on fabricated scaffolds caused a significant dose- and time-dependent increase in the mineralization ability of cells and augmented the expression of the bone formation biomarkers osteopontin and osteocalcin [22]. Widaa et al. highlighted that simultaneous administration of vitamin D can enhance the osteogenic activity of LithoLexal® and result in more efficient mineralization and higher levels of ALP [23]. Overall, studies on bone cells are conclusive as to LithoLexal® having potent pro-mineralization and osteogenic efficiency. In Section 4, we describe the extent to which these anabolic properties translate into in vivo efficacy in postmenopausal and diet-induced osteoporotic subjects.
4. Suppression of Osteoclastogenesis
Inhibition of osteoclastogenesis is a key mechanism that can shift the balance of bone remodeling in favor of bone formation. The multistep process of osteoclastogenesis is initiated by the secretion of receptor activator of nuclear factor-κB ligand (RANKL) and macrophage colony-stimulating factor (M-CSF). The expression of these cytokines is necessary for the differentiation, maturation, function, and survival of osteoclasts. In addition to RANKL and M-CSF, other pro-inflammatory cytokines also contribute to the complex regulation of osteoclast formation, the more important ones being tumor necrosis factor alpha (TNF-α), interleukin 1 (IL-1), and IL-6 [24]. Notably, the induced secretion of some of these key cytokines, i.e., TNF-α and IL-1β, is suppressed by LithoLexal®, as reported by two independent research groups [16,25]. This prominent effect has subsequently been verified through a clinical trial in which the circulating level of TNF-α was reduced by a 6-week course of LithoLexal® therapy [26]. Furthermore, direct clues from the literature indicate that LithoLexal® downregulates nuclear factor κB (NF-κB), which prevents the overactivation of osteoclasts. This complex of transcription factors modulates the expression of osteoclastogenic cytokines; thus, its hyperactivity plays a central role in the pathogenesis of osteoporosis. There is evidence to show that inhibition of NF-κB is an effective approach to inhibit osteoclast formation and bone resorption [27]. Based on a mechanistic study on macrophages, LithoLexal® appears to possess NF-κB inhibitory properties. Physiological concentrations of LithoLexal® exerted significant inhibitory effects on induced NF-κB activation, which evidently originated from the increased activation of its inhibitory protein, I-kBα [28]. Hence, LithoLexal® Bone is suggested to block inflammation-induced osteoclastogenesis at different levels, which is particularly relevant in postmenopausal women with increased levels of resorptive cytokines [29]. The term disease-modifying adjunctive therapy (DMAT) refers mainly to this range of pharmacological activities of LithoLexal® Bone.
Suppression of parathyroid hormone (PTH) secretion is another potential anti-osteoclastogenic action mechanism of LithoLexal® Bone. PTH stimulates bone resorption through the action of RANKL. Hence, normalizing excess secretion of PTH (secondary hyperparathyroidism) can balance the bone turnover and moderate bone loss. According to a population-based study, serum PTH level is a function of age and estrogen availability and thus rises in older adults and postmenopausal women [30]. In controlled clinical trials, LithoLexal® induced efficient PTH suppression, which was of a higher magnitude and duration compared to conventional sources of calcium. Both acute and long-term effect sizes of LithoLexal® in suppressing PTH secretion were compared with commonly used calcium salts in head-to-head clinical studies. In an acute crossover trial, healthy subjects were given a single dose of either LithoLexal® or calcium carbonate, each containing 720 mg of elemental calcium. Serum PTH and urinary calcium excretion were monitored for 12 h post-intervention. Outcome analysis indicated that the PTH-suppressive effect of LithoLexal® was more potent and prolonged compared to that induced by calcium carbonate, and only LithoLexal® produced significantly greater urinary clearance of calcium than the placebo [13]. These findings suggest a superior and more sustained bioavailability for LithoLexal® in comparison with calcium carbonate. Our yet unpublished clinical study on postmenopausal women also supported the higher impact of LithoLexal® on PTH compared with a placebo and active controls. Randomly selected subjects (per-protocol sample size = 21) received either placebo, LithoLexal® Bone, calcium carbonate, or tricalcium phosphate (TCP) pills three times a day (all active treatments contained 600 mg/day of elemental calcium) for 3 months. In conformity with the findings of Zenk et al., the PTH suppression level by LithoLexal® Bone was 2–3 times greater than that by calcium carbonate and tricalcium phosphate and significantly higher than that induced by the placebo (p = 0.036) (Figure 2A). Changes in the urinary level of a biomarker of osteoclastic activity, deoxypyridinoline (DPD), were also of note. LithoLexal® Bone reduced the excretion of the bone resorption marker deoxypyridinoline (DPD) by more than 21% after three months, while monomineralic supplements had almost no effect (Figure 2B). This observation suggests that the multimineral complex LithoLexal® Bone may cause higher levels of osteoclastogenic suppression compared with traditional monomineralic supplements.
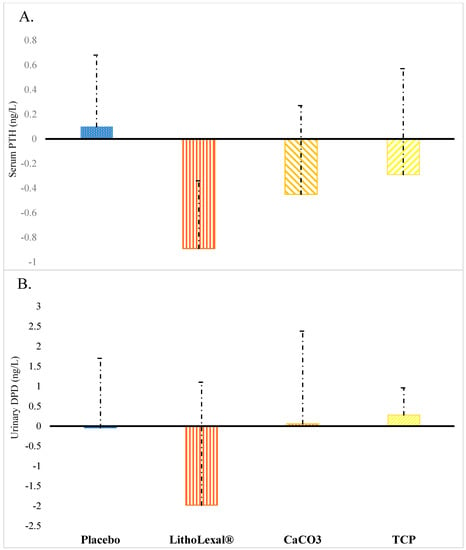
Figure 2.
Changes observed in postmenopausal women after three months of treatment with placebo or active intervention in (A) serum PTH and (B) urinary DPD (a biomarker of osteoclastic activity). Error bars display standard deviation (SD). Abbreviations: CaCO3 = calcium carbonate; TCP = tricalcium phosphate; DPD = deoxypyridinoline. This figure is based on data from a clinical study by O’Gorman and colleagues.
5. In Vivo and Clinical Anti-Resorptive Efficacy of LithoLexal® Bone
5.1. Improving Bone Density and Structure in Postmenopausal Osteoporosis
While osteoporosis is a multifactorial disorder, estrogen deficiency is accountable for a major part of its progression in both sexes. Estrogen is the most potent anti-resorptive steroid hormone; thus, its depletion due to ageing or menopause accelerates the rate of bone loss by increasing the activity of RANKL and the expression of pro-inflammatory cytokines and reducing the retention of calcium [31,32]. The pathogenetic significance of cytokines is to the extent that postmenopausal osteoporosis has been recognized as a ‘cytokine-driven disease’ [33]. Multiple lines of evidence suggest that LithoLexal® Bone can contribute to the prevention and treatment of postmenopausal osteopenia/osteoporosis through its osteogenic and anti-inflammatory properties (see below).
In a controlled in vivo study, LithoLexal® (50 mg/kg/day) was given to Sprague Dawley rats for 14 weeks, starting 4 weeks after ovariectomy. Endpoint bone densitometry examination confirmed that LithoLexal® treatment increased tibial bone mineral content (BMC) and BMD by 51% and 73%, respectively, compared with the ovariectomized control rats (p < 0.0001 for both comparisons). Additional significant improvements were observed in the microstructure of metaphyseal trabecular bones only in the treated groups. Measurement of serum ALP indicated a considerable decline in the rate of bone turnover in LithoLexal® groups. Interestingly, adding lactic acid bacteria improved the bone preservation efficacy of LithoLexal® [34]. A subsequent study replicated these observations and compared the efficacy of early and late (after 8 weeks) postmenopausal treatment with either LithoLexal® or calcium carbonate. The control group was not ovariectomized and received an unsupplemented regular diet. Early initiation of LithoLexal® prevented the loss of bone volume fraction and mineral/matrix ratio and preserved tibial modulus and hardness with significantly higher potency than that of calcium carbonate. Markedly, the bone hydroxyapatite content and trabecular number in the LithoLexal®-treated subjects were comparable to those of the non-ovariectomized subjects at the endpoint, which reflects its higher pro-mineralization efficacy than calcium carbonate. Most importantly, the maximum load and stiffness of the tibia in LithoLexal® groups were higher than those in both the control and calcium carbonate groups. A notable finding of this study was that even a delayed treatment with LithoLexal® post-ovariectomy was able to prevent the loss of bone minerals and stiffness [12]. Enhancing the expression of osteoprotegerin (OPG) and thus modulating the RANKL/OPG ratio is suggested by some authors to mediate the anti-resorptive impact of multimineral supplements under estrogen deprivation [35].
To validate the in vivo evidence in humans, monotherapy with LithoLexal® was studied in a large, 2-year clinical trial on postmenopausal women (average age = 61). In order to enhance bioavailability, LithoLexal® was mixed with short-chain fructo-oligosaccharides (scFOS) in one group. Twelve months into the study, LithoLexal® prevented the surge in C-telopeptide of type I collagen (CTX), a bone resorption biomarker, that occurred in controls. Lower bone resorption expectedly led to reduced bone turnover and osteocalcin release after 24 months in the LithoLexal® group compared to the controls (p = 0.02). At the end of the trial, the patients who received LithoLexal® + scFOS had the highest average lumbar, femoral, and total BMD. Importantly, the best treatment response was recorded in women with osteopenia, who started the treatment with higher baseline BMD [36]. Altogether, the published data suggest that LithoLexal® Bone therapy contributes to controlling the accelerated rate of bone turnover after menopause and helps to achieve a positive balance for major bone minerals in the body. Moreover, it appears that the therapeutic effects of LithoLexal® can be augmented by co-administration of scFOS prebiotics.
5.2. Preventing Bone Loss Secondary to Diet-Induced Obesity
Poor nutritional status is a major factor that leads to pathological changes in bone mass and density. Specifically, a high-fat diet (rich in saturated fat) can induce resorption mainly in the trabecular areas of bones. This effect is partly due to the formation of insoluble calcium soaps and alteration of the duodenal oxidation state that reduces calcium absorption. In vivo research has confirmed the reverse correlation between body weight and bone density and volume [37,38]. Evidently, expanded bone marrow adipocytes impose lipotoxic effects on osteoblasts and convert the normal milieu of the bone marrow to a pro-inflammatory microenvironment that disturbs bone metabolism. Furthermore, expanded adipose tissue actively releases pro-inflammatory cytokines, such as TNF-α, which promote osteoclast differentiation [39].
Recognizing the high-fat, Western-style diet (HFWD) and obesity as risk factors for osteopenia/osteoporosis implies that a large proportion of the world population is at increased risk given that the obesity prevalence has nearly tripled over the past few decades. This rapidly growing public health issue calls for an effective treatment with a significant ability to protect bones from the negative effects of obesity. In vivo evidence suggests that long-term treatment with LithoLexal® may protect trabecular bone from the pro-resorptive effect of an HFWD and excessive adipose tissue accumulation [20,40]. Aslam et al. supplemented the HFWD of C57BL/6 mice with LithoLexal® for 15 months. The 3D micro-computed tomography (micro-CT) scan of long bones at the endpoint revealed that female mice on the HFWD had lost a significant amount of bone minerals and trabeculae compared to animals on a low-fat diet. Deteriorative changes were observed in both cortical and trabecular areas of the femora and tibiae. However, supplementation with LithoLexal® effectively counteracted the impact of the HFWD and prevented the loss of bone tissue (Figure 3). Quantitative measurements substantiated these qualitative observations and indicated significant differences in bone mineral content/density, thickness, and cross-sectional area of cortical and trabecular bone regions between treated and untreated subjects. Bone strength and biomechanical properties were evaluated by four-point bending test. LithoLexal® supplementation enhanced bone yield load, failure load, and stiffness compared to both normal and HFWD control groups. Interestingly, LithoLexal® not only overcame the negative consequences of the HFWD in female mice but also preserved bone tissue and strength to a greater degree than the regular low-fat diet did [20].
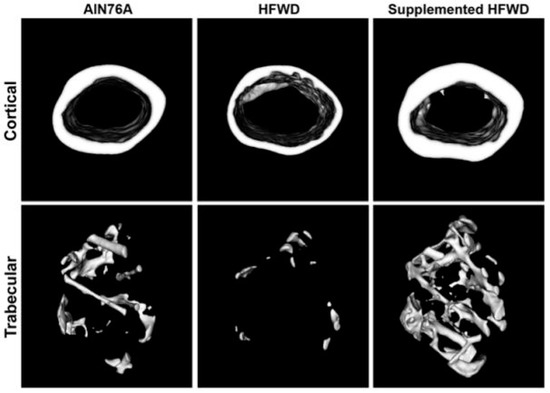
Figure 3.
Micro-CT cross-sectional images of mid-diaphysis (cortical) and distal metaphysis (trabecular) regions of femora from female mice on low-fat (AIN76A), high-fat (HFWD) and high-fat supplemented with LithoLexal® diets (adopted from Aslam et al.) [20].
A subsequent in vivo study with a follow-up period of 18 months corroborated the above findings. Repeated measurements of BMD with micro-CT showed that LithoLexal® had significant anabolic effects on bones in animals fed either a low- or high-fat diet, although the positive effects were more pronounced on trabecular bone and in the HFWD group [40]. In contrast, monomineralic supplementation with a calcium salt plus vitamin D had no significant effects on the BMD of spinal and femoral bones of HFWD-fed mice in another study [41]. In parallel to preserving BMD, LithoLexal® also mitigated the loss of cortical area and trabecular number/thickness compared to untreated groups. Notably, skeletal enhancement by LithoLexal® reached a significant level in femora and vertebrae after just 5 months of treatment. In agreement with the antecedent study by Aslam et al. [20], biomechanical testing unveiled considerable increases of 32% and 30% in the stiffness of femoral and vertebral bones, respectively. A noteworthy finding was that LithoLexal® caused a dramatic increase in the level of strontium (up to 10-fold) in bones relative to mice on unsupplemented diets [40]. The evidence for the efficacy and bioactivity of LithoLexal® is also detailed in a review by Carson and Clarke under its previous trade name, Aquamin® [8]. It may thus be concluded that LithoLexal® Bone supplementation can counterbalance the catabolic effects of a HFWD and obesity on both long and axial bones by diminishing the rate of demineralization and loss of trabecular and cortical bone structures.
6. Pharmacological Advantages of DMAT over Conventional Monomineralic Supplements
As argued earlier, conventional monomineralic supplements come short of realizing the full potential of mineral adjunctive therapy for the prevention and treatment of bone resorption disorders. This gap can potentially be bridged by replacing monomineralic adjunctive therapies (e.g., calcium carbonate or citrate) with the available multimineral DMAT LithoLexal® Bone, containing key osteogenic bioactives. Describing the pharmacological advantages of this treatment in detail requires a separate review; nonetheless, the main headlines are listed as follows.
Anti-inflammatory properties: While conventional monomineralic calcium supplements fail to produce a significant impact on serum levels of inflammatory biomarkers [42,43], LithoLexal® has been documented through laboratory and clinical research to possess anti-inflammatory effects exerted at different levels of the immune response cascade (see Section 3).
The effect of essential trace elements: Besides exerting positive effects on inflammatory pathways and oxidative stress, several trace elements, such as magnesium, copper, and zinc, are known beyond doubt to play important roles in bone development, metabolism, and mineralization [44]. Since the level of these trace minerals is frequently low in high-risk groups, it can be argued that part of the accelerated pace of bone loss in senile/postmenopausal osteoporosis may be rooted in the clinical/subclinical deficiency of certain trace biominerals [45]. This is further supported by the add-on anabolic effects of certain trace minerals on bones [46,47]. The superior osteogenic capacity of LithoLexal® Bone compared to monomineralic supplements (described in Section 4) may, indeed, be underlain by its unique combination of trace elements.
Porous and particulate physical configuration: A pharmacokinetic advantage of LithoLexal® Bone is its short disintegration time, porous microstructure, and four times higher surface area compared to the rocky and impermeable structure of limestone-based preparations. Its substantially smaller particle size and higher ionizability [48] imply that LithoLexal® Bone has increased absorbability and oral bioavailability. This is supported by an experiment on healthy women which showed that the smaller particle size has a greater impact on bioavailability than that of solubility [49].
7. Summary and Conclusions
Based on recent evidence, in addition to calcium, adequate intake of several trace elements is critical for normal bone metabolism and remodeling. Therefore, ensuring adequate intake of all essential bone building minerals is an imperative part of the clinical management of osteopenia/osteoporosis. Alarmingly, individuals at increased risk of developing osteoporosis are also more likely to suffer from dietary insufficiency and malabsorption of calcium and trace elements, making adequate supplementation a necessity. Yet the majority of conventional bone adjunctive therapies are not developed to meet this clinical need. Beyond this, monomineralic supplements are not supported by evidence to mitigate low-grade inflammation and the secretion of pro-inflammatory cytokines, which has emerged as a core pathology in osteoporotic patients.
A recently released product branded as LithoLexal®, harvested from the calcareous cytoskeleton of Lithothamnion sp., can serve as a DMAT for osteoporosis. This treatment with a naturally particulate and porous physical structure has a characteristic multimolecular composition, which provides a range of bioactivities beyond that of each one of its components. LithoLexal® has been documented in vitro and in vivo to possess anti-inflammatory properties. This includes a significant ability to downregulate the pro-resorptive cytokines TNF-α and IL-1β and inhibit the activity of NF-κB. Accordingly, LithoLexal® Bone is referred to as a DMAT that modifies a number of underlying pathological processes of osteoporosis. It is suggested that the disease-modifying effects of LithoLexal® are driven by an undetermined cluster of trace minerals derived from sea water. Several of its trace minerals have recognized osteogenic, anti-inflammatory, and antioxidative activities. However, further targeted mechanistic and clinical research is required to determine which combination of elements is behind each biological activity of LithoLexal® Bone.
Controlled in vivo and clinical research supports the efficacy of LithoLexal® Bone, particularly in preventing inflammation-induced bone resorption, i.e., postmenopausal and high-fat diet-induced bone loss. Long-term treatment with LithoLexal® has shown efficacy in preventing accelerated bone loss after menopause and preserving the cortical and trabecular microstructure of long bones. Best results were achieved when treatment was initiated at an early stage after menopause. Based on head-to-head in vivo studies, treatment with LithoLexal® has a superior PTH-suppressive, osteogenic, and anti-resorptive efficacy compared with commonly used monomineralic calcium salts. It is important to note that findings from in vitro and in vivo research need to be verified by controlled clinical trials in human subjects.
In conclusion, the available data suggest additional pharmacodynamic and clinical benefits for LithoLexal® Bone over conventional calcium/vitamin D supplementation for preserving bone density and microstructure. It, therefore, appears reasonable to recommend this plant-based treatment as a primary adjunctive therapy for the prevention and treatment of osteopenia and osteoporosis with or without anti-resorptive medications in both men and women. LithoLexal® Bone is expected to provide disease-modifying properties, i.e., cytokine suppression, which contribute to a more potent anti-resorptive and anabolic effect on bone compared to traditional calcium supplements. Further research is warranted to better clarify the clinical potential/application of this extract and other natural sources of bone minerals.
Author Contributions
Conceptualization, E.F.E. and D.M.O.; methodology, D.M.O.; validation, Z.N., A.Y. and R.M.; resources, D.M.O.; writing—original draft preparation, E.F.E.; writing—review and editing, D.M.O., Z.N., A.Y., R.M. and E.F.E.; supervision, E.F.E.; funding acquisition, D.M.O. All authors have read and agreed to the published version of the manuscript.
Funding
No financial support was received by the authors for preparing this article. Article processing charges were covered by Nordic Medical Ltd., London, UK.
Institutional Review Board Statement
The human study by O’Gorman and colleagues was approved by University College Cork Review Board (#SC00204).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the studies reviewed in this paper.
Data Availability Statement
The unpublished data cited in this review are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shen, Y.; Huang, X.; Wu, J.; Lin, X.; Zhou, X.; Zhu, Z.; Pan, X.; Xu, J.; Qiao, J.; Zhang, T.; et al. The Global Burden of Osteoporosis, Low Bone Mass, and Its Related Fracture in 204 Countries and Territories, 1990–2019. Front. Endocrinol. 2022, 13, 882241. [Google Scholar] [CrossRef]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.Y.; Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoarthritis (ESCEO). Executive summary of European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Aging Clin. Exp. Res. 2019, 31, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Kumssa, D.B.; Joy, E.J.; Ander, E.L.; Watts, M.J.; Young, S.D.; Walker, S.; Broadley, M.R. Dietary calcium and zinc deficiency risks are decreasing but remain prevalent. Sci. Rep. 2015, 5, 10974. [Google Scholar] [CrossRef] [PubMed]
- Bullamore, J.R.; Wilkinson, R.; Gallagher, J.C.; Nordin, B.E.; Marshall, D.H. Effect of age on calcium absorption. Lancet 1970, 2, 535–537. [Google Scholar] [CrossRef]
- Lamy, O.; Burckhardt, P. Calcium revisited: Part II calcium supplements and their effects. Bonekey Rep. 2014, 3, 579. [Google Scholar] [CrossRef]
- Tai, V.; Leung, W.; Grey, A.; Reid, I.R.; Bolland, M.J. Calcium intake and bone mineral density: Systematic review and meta-analysis. BMJ 2015, 351, h4183. [Google Scholar] [CrossRef]
- Nakamura, Y.; Suzuki, T.; Kamimura, M.; Murakami, K.; Ikegami, S.; Uchiyama, S.; Kato, H. Vitamin D and calcium are required at the time of denosumab administration during osteoporosis treatment. Bone Res. 2017, 5, 17021. [Google Scholar] [CrossRef] [PubMed]
- Carson, M.A.; Clarke, S.A. Bioactive Compounds from Marine Organisms: Potential for Bone Growth and Healing. Mar. Drugs 2018, 16, 340. [Google Scholar] [CrossRef] [PubMed]
- Kobrin, S.M.; Goldstein, S.J.; Shangraw, R.F.; Raja, R.M. Variable efficacy of calcium carbonate tablets. Am. J. Kidney Dis. 1989, 14, 461–465. [Google Scholar] [CrossRef]
- Heller, H.J.; Greer, L.G.; Haynes, S.D.; Poindexter, J.R.; Pak, C.Y. Pharmacokinetic and pharmacodynamic comparison of two calcium supplements in postmenopausal women. J. Clin. Pharmacol. 2000, 40, 1237–1244. [Google Scholar] [CrossRef]
- Hanzlik, R.P.; Fowler, S.C.; Fisher, D.H. Relative bioavailability of calcium from calcium formate, calcium citrate, and calcium carbonate. J. Pharmacol. Exp. Ther. 2005, 313, 1217–1222. [Google Scholar] [CrossRef]
- Brennan, O.; Sweeney, J.; O’Meara, B.; Widaa, A.; Bonnier, F.; Byrne, H.J.; O’Gorman, D.M.; O’Brien, F.J. A Natural, Calcium-Rich Marine Multi-mineral Complex Preserves Bone Structure, Composition and Strength in an Ovariectomised Rat Model of Osteoporosis. Calcif. Tissue Int. 2017, 101, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Zenk, J.L.; Frestedt, J.L.; Kuskowski, M.A. Effect of Calcium Derived from Lithothamnion sp. on Markers of Calcium Metabolism in Premenopausal Women. J. Med. Food 2018, 21, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Attili, D.; Jenkins, B.; Aslam, M.N.; Dame, M.K.; Varani, J. Growth control in colon epithelial cells: Gadolinium enhances calcium-mediated growth regulation. Biol. Trace Elem. Res. 2012, 150, 467–476. [Google Scholar] [CrossRef]
- Aslam, M.N.; Bergin, I.; Naik, M.; Paruchuri, T.; Hampton, A.; Rehman, M.; Dame, M.K.; Rush, H.; Varani, J. A multimineral natural product from red marine algae reduces colon polyp formation in C57BL/6 mice. Nutr. Cancer 2012, 64, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Aviello, G.; Amu, S.; Saunders, S.P.; Fallon, P.G. A mineral extract from red algae ameliorates chronic spontaneous colitis in IL-10 deficient mice in a mouse strain dependent manner. Phytother. Res. 2014, 28, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Hampton, A.L.; Aslam, M.N.; Naik, M.K.; Bergin, I.L.; Allen, R.M.; Craig, R.A.; Kunkel, S.L.; Veerapaneni, I.; Paruchuri, T.; Patterson, K.A.; et al. Ulcerative Dermatitis in C57BL/6NCrl Mice on a Low-Fat or High-Fat Diet with or without a Mineralized Red-Algae Supplement. J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 487–496. [Google Scholar]
- Eriksen, E.F.; Lech, O.; Nakama, G.Y.; O’Gorman, D.M. Disease-Modifying Adjunctive Therapy (DMAT) in Osteoarthritis-The Biological Effects of a Multi-Mineral Complex, LithoLexal® Joint-A Review. Clin Pract. 2021, 11, 901–913. [Google Scholar] [CrossRef]
- Lacativa, P.G.; Farias, M.L. Osteoporosis and inflammation. Arq. Bras. Endocrinol. Metabol. 2010, 54, 123–132. [Google Scholar] [CrossRef]
- Aslam, M.N.; Kreider, J.M.; Paruchuri, T.; Bhagavathula, N.; DaSilva, M.; Zernicke, R.F.; Goldstein, S.A.; Varani, J. A mineral-rich extract from the red marine algae Lithothamnion calcareum preserves bone structure and function in female mice on a Western-style diet. Calcif. Tissue Int. 2010, 86, 313–324. [Google Scholar] [CrossRef]
- O’Gorman, D.M.; Tierney, C.M.; Brennan, O.; O’Brien, F.J. The marine-derived, multi-mineral formula, Aquamin, enhances mineralisation of osteoblast cells In Vitro. Phytother. Res. 2012, 26, 375–380. [Google Scholar] [CrossRef]
- Brennan, O.; Stenson, B.; Widaa, A.; DM, O.G.; FJ, O.B. Incorporation of the natural marine multi-mineral dietary supplement Aquamin enhances osteogenesis and improves the mechanical properties of a collagen-based bone graft substitute. J. Mech. Behav. Biomed. Mater. 2015, 47, 114–123. [Google Scholar] [CrossRef]
- Widaa, A.; Brennan, O.; O’Gorman, D.M.; O’Brien, F.J. The osteogenic potential of the marine-derived multi-mineral formula aquamin is enhanced by the presence of vitamin D. Phytother. Res. 2014, 28, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Redlich, K.; Smolen, J.S. Inflammatory bone loss: Pathogenesis and therapeutic intervention. Nat. Rev. Drug Discov. 2012, 11, 234–250. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.; O’Gorman, D.M.; Nolan, Y.M. Evidence that the marine-derived multi-mineral Aquamin has anti-inflammatory effects on cortical glial-enriched cultures. Phytother. Res. 2011, 25, 765–767. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.T.M.C.; Doolan, A.M.; Molloy, M.G.; Dinan, T.G.; O’Gorman, D.M.; Nally, K. The Marine-derived, Multi-mineral formula, AquaPT Reduces TNF-a Levels in Osteoarthritis Patients. J. Nutr. Health Food Sci. 2014, 2, 1–3. [Google Scholar]
- Abu-Amer, Y. NF-κB signaling and bone resorption. Osteoporos. Int. 2013, 24, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, D.M.; O’Carroll, C.; Carmody, R.J. Evidence that marine-derived, multi-mineral, Aquamin inhibits the NF-κB signaling pathway In Vitro. Phytother. Res. 2012, 26, 630–632. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, R.; Brown, C.; Puscheck, E.; Friedrich, E.; Slatopolsky, E.; Maggio, D.; McCracken, R.; Avioli, L.V. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc. Natl. Acad. Sci. USA 1991, 88, 5134–5138. [Google Scholar] [CrossRef]
- Khosla, S.; Atkinson, E.J.; Melton, L.J., 3rd; Riggs, B.L. Effects of age and estrogen status on serum parathyroid hormone levels and biochemical markers of bone turnover in women: A population-based study. J. Clin. Endocrinol. Metab. 1997, 82, 1522–1527. [Google Scholar]
- Clarke, B.L.; Khosla, S. Physiology of bone loss. Radiol. Clin. N. Am. 2010, 48, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Shevde, N.K.; Bendixen, A.C.; Dienger, K.M.; Pike, J.W. Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. Proc. Natl. Acad. Sci. USA 2000, 97, 7829–7834. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R. Immune regulation of osteoclast function in postmenopausal osteoporosis: A critical interdisciplinary perspective. Int. J. Med. Sci. 2012, 9, 825–832. [Google Scholar] [CrossRef]
- Lee, H.G.; Lee, T.H.; Kim, J.H.; Seok, J.W.; Lee, S.H.; Kim, Y.H.; Kim, J.E.; Chung, M.J.; Yeo, M.H. The effects of a mineral supplement (Aquamin F®) and its combination with multi-species lactic acid bacteria (lab) on bone accretion in an ovariectomized rat model. J. Exp. Biomed. Sci. 2010, 16, 213–220. [Google Scholar]
- Bae, Y.J.; Kim, M.H. Calcium and Magnesium Supplementation Improves Serum OPG/RANKL in Calcium-Deficient Ovariectomized Rats. Calcif. Tissue Int. 2010, 87, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Slevin, M.M.; Allsopp, P.J.; Magee, P.J.; Bonham, M.P.; Naughton, V.R.; Strain, J.J.; Duffy, M.E.; Wallace, J.M.; Mc Sorley, E.M. Supplementation with calcium and short-chain fructo-oligosaccharides affects markers of bone turnover but not bone mineral density in postmenopausal women. J. Nutr. 2014, 144, 297–304. [Google Scholar] [CrossRef]
- Patsch, J.M.; Kiefer, F.W.; Varga, P.; Pail, P.; Rauner, M.; Stupphann, D.; Resch, H.; Moser, D.; Zysset, P.K.; Stulnig, T.M.; et al. Increased bone resorption and impaired bone microarchitecture in short-term and extended high-fat diet-induced obesity. Metabolism 2011, 60, 243–249. [Google Scholar] [CrossRef]
- Wang, Y.; Dellatore, P.; Douard, V.; Qin, L.; Watford, M.; Ferraris, R.P.; Lin, T.; Shapses, S.A. High fat diet enriched with saturated, but not monounsaturated fatty acids adversely affects femur, and both diets increase calcium absorption in older female mice. Nutr. Res. 2016, 36, 742–750. [Google Scholar] [CrossRef]
- Tian, L.; Yu, X. Fat, Sugar, and Bone Health: A Complex Relationship. Nutrients 2017, 9, 506. [Google Scholar] [CrossRef]
- Aslam, M.N.; Bergin, I.; Jepsen, K.; Kreider, J.M.; Graf, K.H.; Naik, M.; Goldstein, S.A.; Varani, J. Preservation of bone structure and function by Lithothamnion sp. derived minerals. Biol. Trace Elem. Res. 2013, 156, 210–220. [Google Scholar] [CrossRef]
- Bastie, C.C.; Gaffney-Stomberg, E.; Lee, T.W.; Dhima, E.; Pessin, J.E.; Augenlicht, L.H. Dietary cholecalciferol and calcium levels in a Western-style defined rodent diet alter energy metabolism and inflammatory responses in mice. J. Nutr. 2012, 142, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Pittas, A.G.; Harris, S.S.; Stark, P.C.; Dawson-Hughes, B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care 2007, 30, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Gannage-Yared, M.H.; Azoury, M.; Mansour, I.; Baddoura, R.; Halaby, G.; Naaman, R. Effects of a short-term calcium and vitamin D treatment on serum cytokines, bone markers, insulin and lipid concentrations in healthy post-menopausal women. J. Endocrinol. Investig. 2003, 26, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C. The role of nutrients in bone health, from A to Z. Crit. Rev. Food Sci. Nutr. 2006, 46, 621–628. [Google Scholar] [CrossRef]
- Gur, A.; Çolpan, L.; Nas, K.; Çevik, R.; Saraç, J.; Erdogan, F.; Düz, M.Z. The role of trace minerals in the pathogenesis of postmenopausal osteoporosis and a new effect of calcitonin. J. Bone Miner. Metab. 2002, 20, 39–43. [Google Scholar] [PubMed]
- Strause, L.; Saltman, P.; Smith, K.T.; Bracker, M.; Andon, M.B. Spinal bone loss in postmenopausal women supplemented with calcium and trace minerals. J. Nutr. 1994, 124, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Saltman, P.D.; Strause, L.G. The role of trace minerals in osteoporosis. J. Am. Coll. Nutr. 1993, 12, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Assoumani, M.B. Aquamin, a natural calcium supplement derived from seaweed. Agro Food Ind. Hi-Tech 1997, 8, 45–47. [Google Scholar]
- Wang, H.; Bua, P.; Capodice, J. A comparative study of calcium absorption following a single serving administration of calcium carbonate powder versus calcium citrate tablets in healthy premenopausal women. Food Nutr. Res. 2014, 58, 23229. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).