1. Introduction
Cholestyramine is a bile acid sequestrant that has been widely used since the 1960s for lowering cholesterol levels [
1]. It exerts its action by enhancing the intestinal excretion of bile acids, thereby stimulating their de novo synthesis. During this process, the body utilizes circulating cholesterol to replenish the bile acid pool, resulting in a reduction of plasma cholesterol concentrations [
2].
The enterohepatic circulation plays a crucial role in thyroid hormone homeostasis. Triiodothyronine (T3) and thyroxine (T4) are metabolized in the liver, conjugated to glucuronides and sulfates, and then secreted into bile. These conjugated hormones reach the intestine, where bacterial enzymes deconjugate them, allowing free hormones to be reabsorbed into the circulation.
In 1986, Nicoloff demonstrated that the conjugation of T4 to glucuronides and sulfates is enhanced in thyrotoxic conditions, leading to increased biliary, urinary, and fecal excretion of both conjugated and free T4 [
3]. Building on this study, Solomon et al. demonstrated that cholestyramine, by binding iodothyronines and bile acids from the enterohepatic circulation and promoting their fecal excretion, would result in a more rapid decrease in thyroid hormone levels, particularly T4 [
4]. Cholestyramine has additionally been shown to directly inhibit the intestinal absorption of exogenous levothyroxine by forming a complex with T4 in the stomach, thereby reducing its intestinal uptake and increasing its fecal excretion; in vitro studies demonstrated that 50 mg of cholestyramine could bind at least 3000 µg of LT4 [
5]. While these findings led to the recommendation that LT4 and cholestyramine should be administered 4–6 h apart, an important implication is that cholestyramine may also serve as a potential antidote in cases of LT4 overdose or misuse [
6].
This review aims to summarize current knowledge on the use of cholestyramine in the management of hyperthyroidism and thyrotoxicosis across various clinical settings. Our analysis is based on clinical guidelines, clinical studies, and the broader literature.
Before this outline, a brief introduction will be provided on the different types of hyperthyroidism and thyrotoxicosis, in order to better understand the various types and causes of elevated thyroid hormone levels, and to gain a clearer insight into the different uses and approaches that can be adopted with cholestyramine.
Hyperthyroidism and Thyrotoxicosis
Hyperthyroidism and thyrotoxicosis are distinct terms, each describing a separate aspect of thyroid function or dysfunction. Hyperthyroidism refers specifically to conditions in which there is an increased synthesis and secretion of thyroid hormones by the thyroid gland. Thyrotoxicosis, on the other hand, is a broader term describing the clinical state resulting from excessive circulating thyroid hormones, regardless of their source.
While hyperthyroidism is most commonly caused by Graves’ disease (GD, 60–80% of cases), toxic multinodular goiter (15–20%), and autonomously functioning thyroid adenomas (<5%), thyrotoxicosis can also result from destructive thyroiditis or non-thyroidal causes, such as exogenous thyroid hormone intake. Subacute thyroiditis is a relatively common inflammatory thyroid disorder characterized by thyroid pain and tenderness, leading to destructive thyrotoxicosis for the release of preformed hormones from damaged thyroid tissue [
7]; other destructive thyroid disorders causing thyrotoxicosis are silent and postpartum thyroiditis. Amiodarone-induced thyrotoxicosis (AIT) manifests in two distinct forms with different pathophysiological mechanisms and treatment approaches. Type 1 AIT (AIT1) occurs in patients with preexisting thyroid autonomy, where excessive iodine from amiodarone enhances thyroid hormone synthesis, similar to the Jod-Basedow phenomenon. In contrast, type 2 AIT (AIT2) is a destructive thyroiditis caused by the cytotoxic effects of amiodarone and its metabolites, leading to uncontrolled hormone release from damaged thyroid follicles.
A severe and potentially fatal complication of thyrotoxicosis is thyroid storm, an endocrine emergency potentially fatal. It can be precipitated by factors such as thyroid surgery, anesthesia, severe illness, or radioiodine therapy in predisposed individuals. Clinically, thyroid storm is characterized by severe hyperthermia, hemodynamic instability, tachyarrhythmias, heart failure, gastrointestinal symptoms, jaundice, neuropsychiatric disturbances, seizures, and coma, requiring urgent medical intervention.
The management of hyperthyroidism focuses on reducing thyroid hormone synthesis, while thyrotoxicosis treatment aims to control symptoms and limit hormone effects, as antithyroid drugs are ineffective in cases without active hormone production.
To treat hyperthyroidism, methimazole (MMI) is the first-line treatment, inhibiting thyroid hormone synthesis. Propylthiouracil (PTU) is an alternative, mainly used in the first trimester of pregnancy, due to the lower risk of congenital malformation than MMI, and in thyroid storm, due to its additional peripheral inhibition of T4-to-T3 conversion.
In thyrotoxicosis without hyperthyroidism, thionamides are not effective (except for PTU’s peripheral effect). Instead, glucocorticoids (e.g., prednisone, dexamethasone) are used to reduce inflammation and suppress T4-to-T3 conversion, particularly in destructive thyroiditis as in subacute thyroiditis and type 2 amiodarone-induced thyrotoxicosis (AIT2).
In specific cases, perchlorate may be considered to block iodine uptake, such as in iodine-induced hyperthyroidism (Jod-Basedow effect) or type 1 amiodarone-induced thyrotoxicosis (AIT1).
Beta-blockers are frequently used as adjunctive therapy to manage adrenergic symptoms such as tachycardia, palpitations, and tremors. Propranolol is often preferred because, at high doses, it also partially inhibits the peripheral conversion of T4 to T3.
Cholestyramine has been suggested as an adjunct therapy for thyrotoxicosis, but its routine use remains uncertain due to limited clinical evidence and ambiguous guideline recommendations.
2. Methods
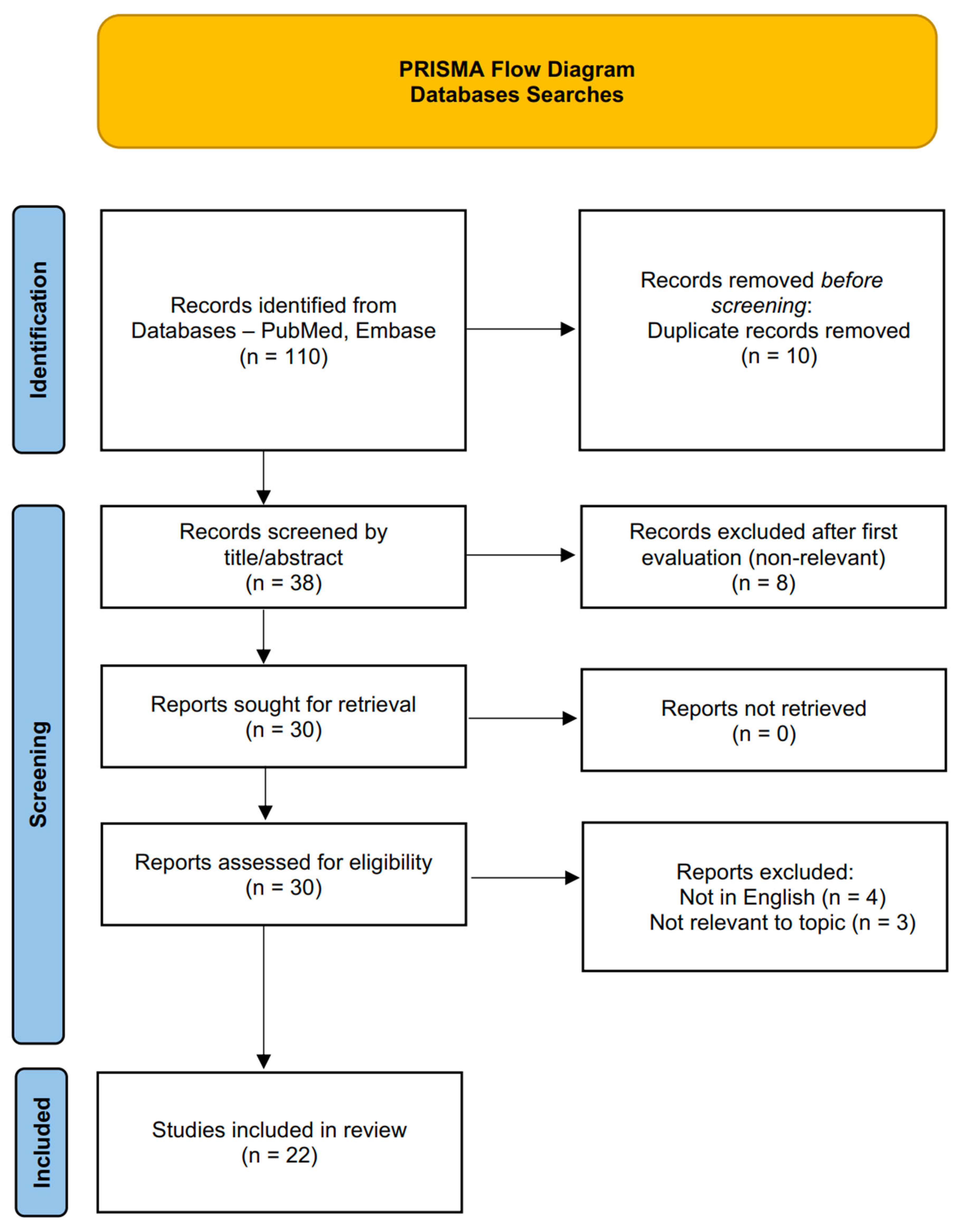
A targeted literature review search was conducted and revised by two authors, using PubMed and Embase databases, up to March 2025. The string search included the keywords cholestyramine AND (hyperthyroidism OR thyrotoxicosis OR thyroxine OR triiodothyronine). Some case reports and case series were identified in the references of the articles found. Results in languages other than English and abstracts not published in peer-reviewed journals were excluded.
The selection process, i.e., inclusion and exclusion criteria, is depicted in the “Preferred Reporting Items for Systematic reviews and Meta-Analyses” (PRISMA) flow diagram (
Figure 1).
In the next section, we summarize the international guidelines on the use of cholestyramine in treating hyperthyroidism and thyrotoxicosis. Following this, we review the clinical evidence, including randomized trials and case studies which assess the effectiveness of cholestyramine in various forms of hyperthyroidism, highlighting case reports involving cholestyramine’s use in Graves’ disease, levothyroxine overdose, amiodarone-induced hyperthyroidism, and thyroid storm. Finally, we discuss the challenges associated with cholestyramine, such as gastrointestinal side effects and drug interactions, which may impact patient adherence and its clinical application.
The flow diagram was constructed using the PRISMA 2020 template: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71.
3. Guideline Recommendations on the Use of Cholestyramine in Hyperthyroidism and Thyrotoxicosis
While not a first-line treatment, cholestyramine has been incorporated into specific clinical scenarios outlined by most recent American Thyroid Association (ATA) guidelines.
3.1. American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis [6]
The 2016 ATA guidelines recommend the use of cholestyramine in cases where achieving euthyroid status in a patient with Graves’ disease (GD) prior to thyroidectomy is not feasible, when thyroidectomy is urgently required, or when the patient has a contraindication to antithyroid drugs. In such instances, the patient should be appropriately managed with beta-adrenergic blockade, potassium iodide, glucocorticoids, and, when necessary, cholestyramine during the immediate preoperative period (Recommendation 26). Furthermore, cholestyramine is referenced under “Other medical treatments for hyperthyroidism during pregnancy” (Recommendation 88), though its use should be approached with caution due to its potential interference with maternal vitamin absorption. However, since it is not absorbed in the gut, it is considered safe for the fetus. Lastly, cholestyramine is also indicated as a treatment option for thyrotoxicosis factitia (resulting from either intentional or unintentional ingestion of thyroid hormone), particularly in cases of severe thyrotoxicosis, as outlined in the section “How should other causes of thyrotoxicosis be managed?” (X7).
3.2. Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum [8]
The 2017 ATA guidelines mention the use of cholestyramine during pregnancy in combination with iodine inhibitors when thionamides are contraindicated or not tolerated (Recommendation 44, question 55).
None of the recent European Thyroid Association (ETA) guidelines on hyperthyroidism and thyrotoxicosis, including “2022 European Thyroid Association Guideline for the management of pediatric Graves’ disease” [
9], “2021 European Thyroid Association Guidelines for the Management of Iodine-Based Contrast Media-Induced Thyroid Dysfunction” [
10], “2018 European Thyroid Association Guideline for the Management of Graves’ Hyperthyroidism” [
11], and “2018 European Thyroid Association (ETA) Guidelines for the Management of Amiodarone-Associated Thyroid Dysfunction” [
12] mention the use of cholestyramine as an adjunctive treatment.
4. Evidences on the Use of Cholestyramine in Hyperthyroidism and Thyrotoxicosis
Few interventional studies have investigated the use of cholestyramine in the treatment of hyperthyroidism and thyrotoxicosis. The results of the available clinical trials are summarized in
Table 1.
A double-blind, placebo-controlled crossover trial conducted by Solomon et al. in 1993 [
4] evaluated the use of cholestyramine as an adjunctive treatment for thyrotoxicosis. Since thionamides may take weeks to reach their full therapeutic effect, patients often remain symptomatic due to the continued presence of circulating thyroid hormones. The study hypothesized that cholestyramine could enhance hormone clearance by binding iodothyronines and bile acids, thereby increasing their fecal excretion. The trial included 15 patients (14 with GD and 1 with a toxic adenoma), who were administered either cholestyramine (4 g, four times daily) or a placebo, alongside atenolol and MMI, in alternating two-week phases with a one-week washout period. The results showed that cholestyramine significantly accelerated the reduction of T3 and T4 levels compared to the placebo, with the most pronounced effect observed early in the treatment. However, thyroid-stimulating hormone and thyrotrophin-binding inhibitory antibodies levels remained unaffected. These findings suggest that cholestyramine is a safe and effective adjunctive treatment for the early management of thyrotoxicosis, though it has no immunomodulatory role.
A 1996 randomized controlled trial [
13] investigated whether the addition of cholestyramine to antithyroid drugs (ATD) and propranolol could accelerate the achievement of euthyroidism in 30 patients with hyperthyroid Graves’ disease. The patients were divided into three treatment groups: (I) ATD, propranolol, and cholestyramine for 1 month; (II) ATD and propranolol for 1 month; and (III) ATD, propranolol, and cholestyramine for 2 weeks, followed by 2 weeks of ATD and propranolol alone. Group I, which received cholestyramine for the full month, exhibited the most significant reduction in thyroid hormone levels. In contrast, Group III, which initially received cholestyramine, showed a slower response after discontinuing the drug, with their results ultimately resembling those of Group II. The treatment was generally well tolerated, with no significant adverse effects observed. These findings support cholestyramine as a safe and effective adjunct in the early management of hyperthyroidism.
Tsai et al., in 2005 [
14], conducted a randomized clinical trial to assess the efficacy of combining PTU with cholestyramine in the treatment of Graves’ hyperthyroidism. Thirty patients were randomly assigned to either the combination treatment group (PTU, propranolol, and cholestyramine) or the control group (PTU and propranolol). After 2 and 4 weeks of treatment, the combination group exhibited significantly greater reductions in T3 and T4 levels compared to the control group. However, no significant differences were observed in anti-TSH receptor antibody levels between the two groups. The study suggests that the addition of cholestyramine to PTU therapy can enhance the reduction of thyroid hormone levels in thyrotoxic patients, offering a potentially more effective treatment strategy, with no effect on thyroid antibodies.
Three years later, a randomized, double-blind, placebo-controlled trial [
15] evaluated the efficacy of low doses of cholestyramine in the treatment of hyperthyroid GD. Forty-five patients were randomly assigned to one of three groups: Cholestyramine 2 g twice daily (Group I), Cholestyramine 1 g twice daily (Group II), or placebo (Group III), with all groups also receiving MMI and propranolol. Over the course of four weeks, thyroid hormone levels decreased more rapidly in the cholestyramine groups compared to the placebo group, although all groups achieved euthyroidism by the end of the study. Notably, all patients in Group I reached a euthyroid state earlier than those in the other groups, emphasizing the effectiveness of cholestyramine in accelerating the reduction of thyroid hormone levels.
Since 2008, to our knowledge, no additional randomized studies have been conducted to assess the effectiveness of cholestyramine in treating hyperthyroidism.
A 2016 retrospective study at Seoul St. Mary’s Hospital [
16] evaluated the efficacy and safety of adding cholestyramine to high-dose MMI therapy in severe thyrotoxicosis. Five patients received MMI (≥30 mg daily) and cholestyramine (4 g three times daily), and were compared to 12 patients treated with MMI alone matched for age, gender, initial T4 level, and MMI dose. The combination therapy group showed a significantly faster decline in serum free T4 and T3 levels compared to the MMI-only group. Importantly, no significant adverse effects were observed in the cholestyramine group.
In most recent years, publications have primarily been limited to clinical reports. Therefore, we have compiled these reports and series, categorized by the underlying causes of hyperthyroidism or thyrotoxicosis, in order to evaluate the effect and use of cholestyramine in various clinical scenarios.
4.1. Graves’ Disease
In 2008, a case report [
17] described a patient with GD who was unresponsive to MMI and propranolol, yet demonstrated rapid improvement in thyroid hormone levels within one week after the addition of cholestyramine. In 2015, Yang et al. [
18] reported a case of a 40-year-old woman with severe GD who was resistant to thionamides and beta-blockers and had contraindications for corticosteroids. After two weeks of high-dose cholestyramine treatment, thyroid hormone levels were markedly reduced, allowing the patient to successfully undergo thyroidectomy.
These reports, along with the previous trials presented, suggest that cholestyramine may be effective for managing refractory GD when traditional treatments fail. However, most studies on cholestyramine have been conducted over short periods, so its long-term effects remain unclear.
4.2. Treatment of Levothyroxine Abuse
In 1993, a study [
19] examined the use of bile acid sequestrants, such as cholestyramine, to lower thyroid hormone levels in two patients with iatrogenic hyperthyroidism due to excessive levothyroxine intake. The patients were treated with 4 g of cholestyramine, administered four times daily. A euthyroid state was achieved within 72 to 96 h, whereas in three similar patients who did not receive cholestyramine treatment, it took nearly eight days for serum thyroid hormone levels to return to the normal range.
In 2002, de Luis et al. [
20] reported a case of a 26-year-old woman who ingested 5 mg of levothyroxine in a suicide attempt. Upon examination, she appeared healthy but developed distal tremor and sweating on the first day, along with elevated T4 levels and suppressed TSH. The patient was treated with cholestyramine, 4 g every 8 h, while vital signs were monitored every 6 h and thyroid hormone levels were checked daily. By the sixth day, her T4 levels had normalized.
These cases highlight cholestyramine as an effective and rapid treatment for levothyroxine overdose, efficiently normalizing thyroid hormone levels. Minimal to no side effects were observed, further supporting its safety and practicality in acute toxicity management.
4.3. Treatment of Amiodarone-Induced Hyperthyroidism
In 2021, Rummaan et al. [
21] reported the case of a 49-year-old man with atrial fibrillation who developed AIT2 due to excessive thyroid hormone release from amiodarone-induced destructive thyroiditis. Despite treatment with high doses of carbimazole and prednisolone, the patient’s thyrotoxicosis persisted. After introducing cholestyramine as adjunct therapy, significant clinical and biochemical improvements were observed, with thyroid hormone levels normalizing within one week.
Although cholestyramine is not currently mentioned in the guidelines for treating AIT, likely due to the absence of interventional trials and the publication of the available case report after the production of these guidelines, this case highlights cholestyramine’s potential as an effective treatment for refractory AIT2, to be considered when standard therapies fail or in unstable patients when a rapid decrease in T4 levels is required. Cholestyramine could also be considered for AIT1 hyperthyroidism, in conjunction with thionamides, when a rapid reduction in T4 levels is required, similar to its application in GD.
4.4. Treatment of Thyroid Storm
Thyroid storm is a rare but potentially life-threatening condition, and only a limited number of studies have assessed the efficacy and safety of specific pharmacological treatments for it, yielding unclear results [
22]. Most of the literature consists of anecdotal reports [
23,
24,
25]. High-dose thionamides remain the first-line treatment, while other medications may serve as adjunctive options for patients requiring more intensive therapy.
In 2021, Sullivan et al. [
26] reported a case of a 24-year-old woman with a history of GD who developed thyroid storm following COVID-19 infection. The patient was treated with PTU, propranolol, hydrocortisone, and cholestyramine, resulting in significant improvement in symptoms and vital signs, with resolution of the acute phase within 36 h.
Another paper [
27] reported the case of a 25-year-old woman admitted with sepsis and subacute thyroiditis, who later developed thyroid storm. The patient was managed in the ICU and showed a positive response to a combination of steroids, propranolol, cholestyramine, and PTU, ultimately achieving a euthyroid state upon discharge.
These cases highlight cholestyramine’s potential effectiveness as an adjunct to thionamides and steroids for thyroid storm.
4.5. Treatment of Hyperthyroidisms and Thyrotoxicosis in Pregnancy
According to ATA Guidelines [
5], thionamides are the first-line treatments for overt hyperthyroidism during pregnancy, administered at the lowest effective dose to minimize fetal risks. PTU is recommended during the first trimester due to its lower teratogenic risk, while MMI is preferred for therapy initiation after the first trimester. Beta-adrenergic blockers can be used to control hypermetabolic symptoms until euthyroidism is achieved with ATD, although their use carries risks such as fetal bradycardia and intrauterine growth restriction. Cholestyramine is considered safe during pregnancy as it does not cross the placenta and is commonly used to relieve pruritus in intrahepatic cholestasis of pregnancy. Its potential use as adjunctive therapy in thyrotoxicosis may, therefore, be considered. However, its use should be carefully managed, as it may cause gastrointestinal side effects (nausea, vomiting, diarrhea) and reduce the absorption of essential fat-soluble vitamins and other medications.
To date, no clinical studies or case reports have documented the use of cholestyramine for the treatment of thyrotoxicosis during pregnancy, though its potential benefit is also noted in the 2017 ATA guidelines [
8]. It should be, therefore, considered for short-term use in cases of severe thyrotoxicosis not adequately responsive to thionamide.
4.6. Preparation for Thyroidectomy
In 2015, a case [
28] was reported of a 52-year-old woman with a history of an enlarging goiter who developed thyrotoxicosis, which worsened after a contrast-enhanced CT scan. She also experienced obstructive symptoms. Despite a three-week course of high-dose dexamethasone, carbimazole, and propranolol, the thyroroxic state did not improve. However, after cholestyramine was added to her treatment, her T4 levels dropped by 30% within five days and normalized after 12 days. She later underwent a total thyroidectomy to address both the hyperthyroidism and the obstructive symptoms. Similarly, in 2024, another case report [
29] highlighted the improvement in thyroid function following a four-week course of adjunct cholestyramine in a 14-year-old girl with severe GD. Despite 18 months of maximal conventional therapy, including antithyroid drugs, beta-blockers, and multiple courses of steroids, the patient remained hyperthyroid. Following cholestyramine treatment, her thyroid function normalized, and she successfully underwent thyroidectomy without complications.
Whenever possible, thyrotoxic patients scheduled for thyroidectomy should be rendered euthyroid with MMI prior to surgery. In most patients with GD, preoperative administration of potassium iodide or Lugol’s solution is recommended. This treatment helps reduce thyroid blood flow, vascularity, and intraoperative blood loss during the procedure. For emergency surgeries, as outlined in the 2016 ATA guidelines, the use of corticosteroids and cholestyramine can be considered [
5].
4.7. Other Causes of Thyrotoxicosis
A case reported by Lin et al. in 2013 [
30] involved a 44-year-old Korean woman undergoing pegylated interferon and ribavirin therapy for chronic hepatitis C, which led to exacerbate severe thyrotoxicosis. Due to the patient’s pancytopenia and hypotension, which contraindicated the use of standard antithyroid medications and beta-blockers, she was treated with cholestyramine at a dose of 4 g three times daily. Remarkably, the patient showed rapid clinical improvement, with thyroid function normalizing after 2.5 weeks of treatment. This case highlights the potential of cholestyramine as an effective treatment in patients with complex contraindications to traditional therapies. Similarly, a 2022 report by Iqbal et al. described the successful use of cholestyramine in the management of subacute thyroiditis during the COVID-19 pandemic [
31].
These experiences further emphasizes that cholestyramine could be also used in case of iatrogenic thyrotoxicosis and subacute thyroiditis, especially when conventional treatments are contraindicated or ineffective.
5. Challenges in the Use of Cholestyramine for Hyperthyroidism Management
Cholestyramine may be associated with gastrointestinal disturbances, including constipation, bloating, and abdominal discomfort. Additionally, it can interfere with the absorption of other medications, such as anticoagulants, digoxin, and thiazide diuretics. To mitigate these interactions, it is recommended to administer these drugs at least 1 h before or 4–6 h after cholestyramine intake. Furthermore, cholestyramine can be difficult for some patients to take, as it is only available in powder form. Its use has declined since the introduction of statins, which have largely replaced it as a lipid-lowering therapy. As a result, fewer formulations are available, and the current use in clinical practice is limited.
The unpalatable taste of cholestyramine indeed poses a challenge for patient compliance. To improve palatability, it is often mixed with fruit juices [
32]. The typical dosing regimen involves administering 4 g of cholestyramine orally up to four times daily. However, the frequency and duration of treatment should be tailored to the individual patient’s needs and response to therapy.
6. Conclusions
Cholestyramine, while primarily recognized for its lipid-lowering properties, has emerged as a valuable adjunct in the management of hyperthyroidism and thyrotoxicosis. As discussed throughout this review, its mechanism of binding thyroid hormones in the gastrointestinal tract offers a complementary approach to conventional therapies, particularly in cases where rapid symptom control is desired or standard treatments are contraindicated. Notably, its role in managing severe thyrotoxicosis during pregnancy is of particular interest, as cholestyramine may help reduce the need for high doses of thionamides and promote a more rapid restoration of euthyroidism.
The main limitations of cholestyramine use are (i) the uncertainty related to its long-term efficacy and (ii) the scarcity of supporting literature and guidelines.
Regarding the first point, while short- to medium-term results from the studies conducted so far are promising, the long-term effectiveness of this therapy remains unclear. The reduction in thyroid hormone levels induced by cholestyramine may be temporary due to several factors: (i) In subclinical or mild hyperthyroidism, the compensatory mechanisms of TSH secretion may be reactivated when pituitary suppression is alleviated, thereby reducing cholestyramine’s effectiveness; (ii) The body may adapt to bile acid sequestration, compensating for the enhanced loss, leading to a rebound in circulating thyroid hormone levels after an initial decrease; (iii) Cholestyramine does not exert immunomodulatory effects, meaning it cannot address the underlying autoimmune mechanisms in conditions like GD, and it does not impact TSH-receptor antibody [
4] levels. Consequently, while cholestyramine may temporarily effectively reduce thyroid hormone levels, it may not offer a long-term solution for hyperthyroidism, but further studies are needed to determine its sustained efficacy and potential role in long-term management.
With regard of evidence-based use of cholestyramine, scientific literature is limited, with only four randomized controlled trials available—all involving small patient populations and with the most recent published in 2008. Moreover, no randomized trials or case reports to date have explored its use during pregnancy. These gaps highlight the need for further well-designed studies to clarify the sustained efficacy and safety of cholestyramine, particularly in long-term management and in specific populations such as pregnant patients. Given the limited number of studies evaluating its efficacy and long-term safety, cholestyramine should be regarded primarily as an adjunctive therapy rather than a standalone treatment. Its use may be appropriate in cases where standard therapies are contraindicated or poorly tolerated due to adverse effects.
Despite these limitations, cholestyramine remains a valuable short-term solution for managing hyperthyroidism in specific clinical scenarios where traditional therapies, including thionamides, fail or are contraindicated, or in cases of that require rapid lowering of thyroid hormones to stabilize the patient. Its well-established safety profile makes it an attractive option in cases of severe thyrotoxicosis, in preparation for thyroidectomy, or during pregnancy. However, its gastrointestinal side effects and potential drug interactions necessitate careful management to ensure safe and optimal use.
Ultimately, further comprehensive research is essential to clearly define the role of cholestyramine in the management of thyroid disorders and support its integration into evidence-based clinical guidelines. Well-designed randomized controlled trials should specifically address high-impact clinical scenarios such as thyrotoxicosis during pregnancy, complicated Graves’ disease, subacute thyroiditis, and preoperative preparation in urgent thyroidectomy cases.