Severe Hypoglycemia and Pituitary Stalk Interruption Syndrome in a 5-Year-Old Boy with Coexistent Hyperprolinaemia: A Case Report and Literature Review
Abstract
1. Introduction
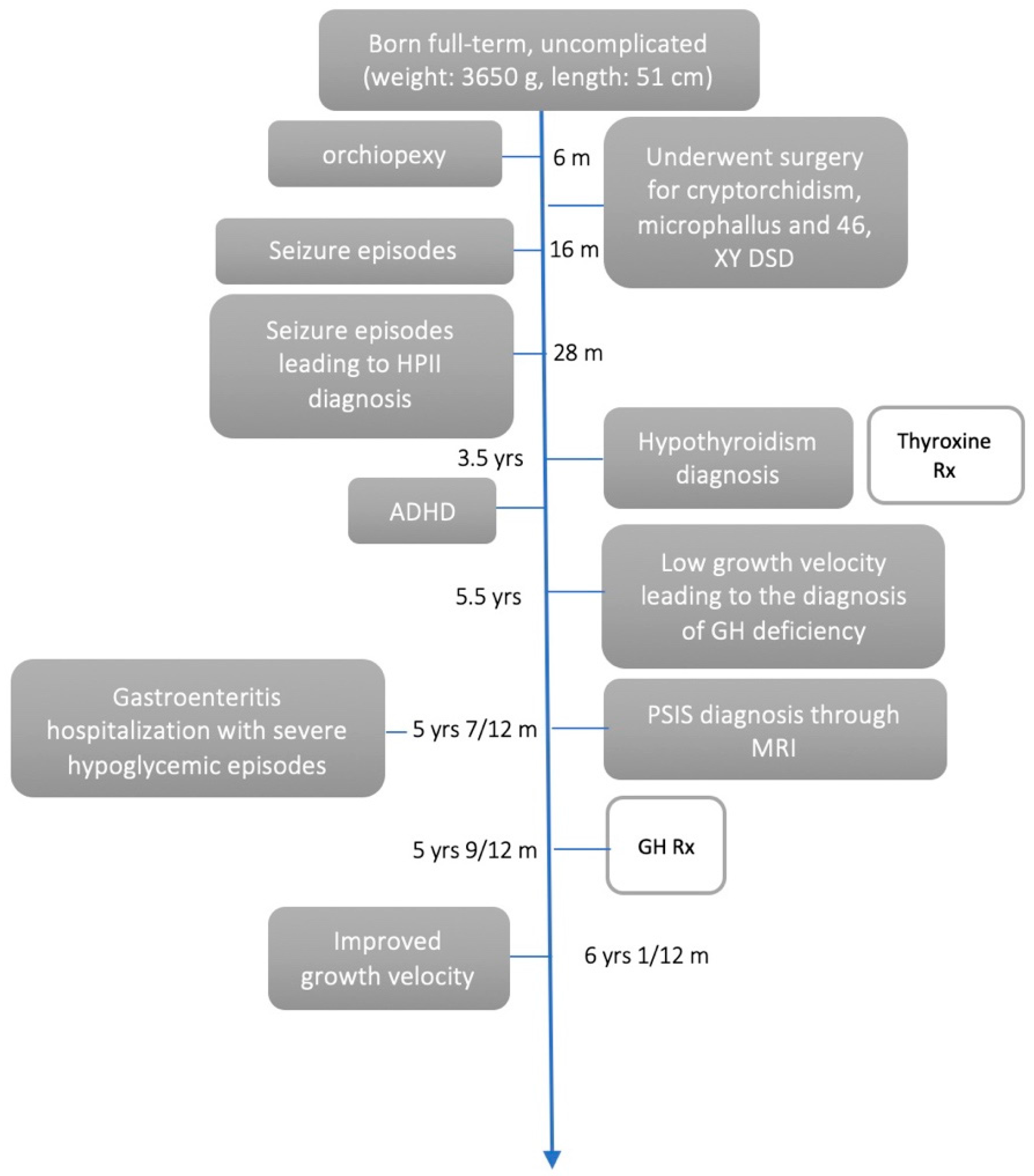
2. Case Presentation
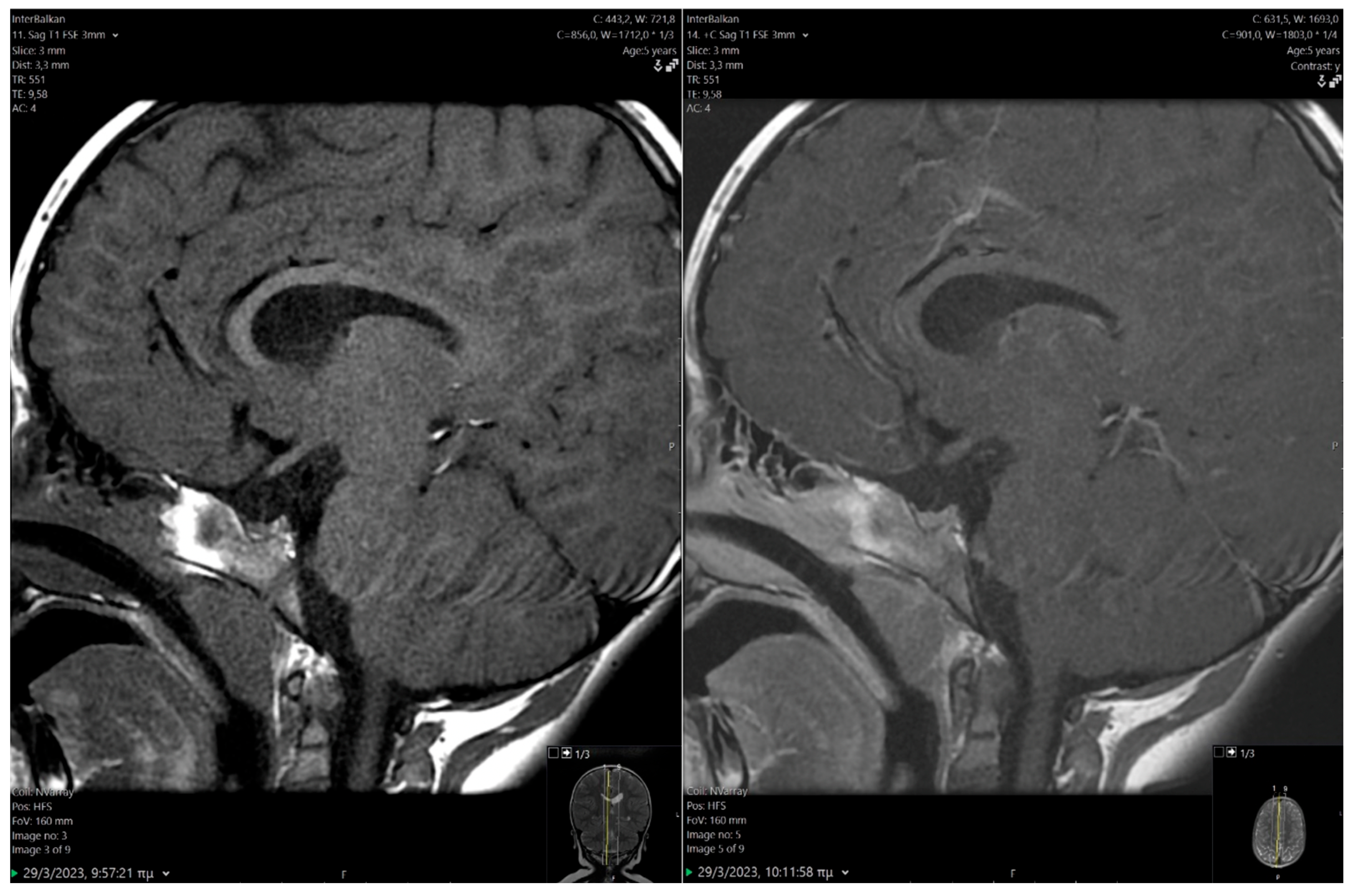
2.1. Clinical Findings/Diagnostic Assessment
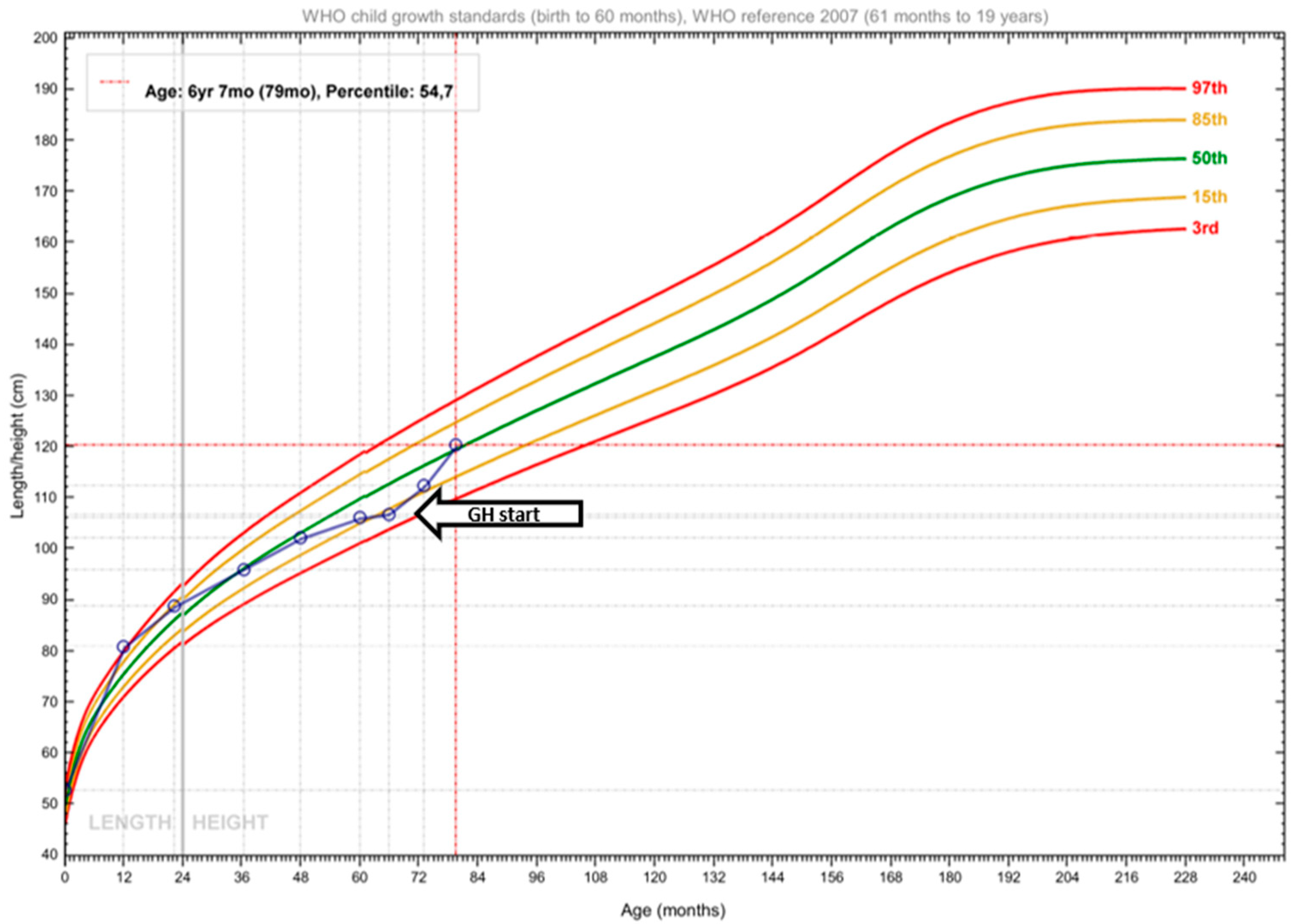
2.2. Therapeutic Intervention
2.3. Outcome and Follow-Up (10 Months)
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADHD | Attention deficit hyperactivity disorder |
| CNS | Central nervous system |
| GH | Growth hormone |
| GHD | Growth hormone deficiency |
| HAz | Height-for-age z-score |
| HPI | Hyperprolinemia type I |
| HPII | Hyperprolinemia type II |
| IGF-1 | Insulin-like growth factor-1 |
| MRI | Magnetic resonance imaging |
| P5C | Pyrroline-5-carboxylate |
| PSIS | Pituitary Stalk Interruption Syndrome |
| rhGH | Recombinant human growth hormone |
References
- DeArmond, P.D.; Dietzen, D.J.; Pyle-Eilola, A.L. Amino acids disorders. In Biomarkers in Inborn Errors of Metabolism; Elsevier: Amsterdam, The Netherlands, 2017; pp. 25–64. [Google Scholar]
- Phang, J.M.; Hu, C.A.; Valle, D. Disorders of Proline and Hydroxyproline Metabolism. In The Online Metabolic and Molecular Bases of Inherited Disease; Valle, D.L., Antonarakis, S., Ballabio, A., Beaudet, A.L., Mitchell, G.A., Eds.; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Mitsubuchi, H.; Nakamura, K.; Matsumoto, S.; Endo, F. Inborn Errors of Proline Metabolism. J. Nutr. 2008, 138, 2016S–2020S. [Google Scholar] [CrossRef] [PubMed]
- Farrant, R.D.; Walker, V.; Mills, G.A.; Mellor, J.M.; Langley, G.J. Pyridoxal Phosphate De-activation by Pyrroline-5-carboxylic Acid. J. Biol. Chem. 2001, 276, 15107–15116. [Google Scholar] [CrossRef] [PubMed]
- Mitsubuchi, H.; Nakamura, K.; Matsumoto, S.; Endo, F. Biochemical and clinical features of hereditary hyperprolinemia. Pediatr. Int. 2014, 56, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Motte, J.; Fisse, A.L.; Grüter, T.; Schneider, R.; Breuer, T.; Lücke, T.; Krueger, S.; Nguyen, H.P.; Gold, R.; Ayzenberg, I.; et al. Novel variants in a patient with late-onset hyperprolinemia type II: Diagnostic key for status epilepticus and lactic acidosis. BMC Neurol. 2019, 19, 345. [Google Scholar] [CrossRef]
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D. The CARE guidelines: Consensus-based clinical case reporting guideline development. J. Med. Case Rep. 2013, 7, 223. [Google Scholar] [CrossRef]
- de Onis, M. 4.1 The WHO Child Growth Standards. World Rev. Nutr. Diet 2015, 113, 278–294. [Google Scholar]
- Schafer, I.A.; Scriver, C.R.; Efron, M.L. Familial Hyperprolinemia, Cerebral Dysfunction and Renal Anomalies Occurring in a Family with Hereditary Nephropathy and Deafness. N. Engl. J. Med. 1962, 267, 51–60. [Google Scholar] [CrossRef]
- Mollica, F.; Pavone, L.; Antener, L. Familial Hyperprolinemia without Mental Retardation and Hereditary Nephropathy. Congr. Neuro-Genet. Neuro-Ophthalmol. 1970, 6, 144–145. [Google Scholar]
- Potter, J.L.; Waickman, F.J. Hyperprolinemia I: Study of a large family. J. Pediatr. 1973, 83, 635–638. [Google Scholar] [CrossRef]
- Walker, V.; Mills, G.A.; Peters, S.A.; Merton, W.L. Fits, pyridoxine, and hyperprolinaemia type II. Arch. Dis. Child. 2000, 82, 236–237. [Google Scholar] [CrossRef]
- Applegarth, D.A.; Ingram, P.; Hingston, J.; Hardwick, D.F. Hyperprolinemia Type II. Clin. Biochem. 1974, 7, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Shivananda, C.R.; Kumar, P. Type I hyperprolinemia. Indian J. Pediatr. 2000, 67, 541–543. [Google Scholar] [CrossRef] [PubMed]
- Rosa, G.D.; Pustorino, G.; Spano, M.; Campion, D.; Calabrò, M.; Aguennouz, M.; Caccamo, D.; Legallic, S.; Sgro, D.L.; Bonsignore, M.; et al. Type I hyperprolinemia and proline dehydrogenase (PRODH) mutations in four Italian children with epilepsy and mental retardation. Psychiatr. Genet. 2008, 18, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Flynn, M.P.; Martin, M.C.; Moore, P.T.; Stafford, J.A.; Fleming, G.A.; Phang, J.M. Type II hyperprolinaemia in a pedigree of Irish travellers (nomads). Arch. Dis. Child. 1989, 64, 1699–1707. [Google Scholar] [CrossRef]
- Hama, R.; Kido, J.; Sugawara, K.; Nakamura, T.; Nakamura, K. Hyperprolinemia type I caused by homozygous p.T466M mutation in PRODH. Hum. Genome Var. 2021, 8, 28. [Google Scholar] [CrossRef]
- Harries, J.T.; Piesowicz, A.T.; Seakins, J.W.T.; Francis, D.E.M.; Wolff, O.H. Low Proline Diet in Type 1 Hyperprolinaemia. Arch. Dis. Child. 1971, 46, 72–81. [Google Scholar] [CrossRef][Green Version]
- Humbertclaude, V.; Rivier, F.; Roubertie, A.; Echenne, B.; Bellet, H.; Vallat, C.; Morin, D. Is Hyperprolinemia Type I Actually a Benign Trait? Report of a Case With Severe Neurologic Involvement and Vigabatrin Intolerance. J. Child Neurol. 2001, 16, 622–623. [Google Scholar] [CrossRef]
- Kaur, R.; Paria, P.; Saini, A.G.; Suthar, R.; Bhatia, V.; Attri, S.V. Metabolic epilepsy in hyperprolinemia type II due to a novel nonsense ALDH4A1 gene variant. Metab. Brain Dis. 2021, 36, 1413–1417. [Google Scholar] [CrossRef]
- Namavar, Y.; Duineveld, D.J.; Both, G.I.A.; Fiksinski, A.M.; Vorstman, J.A.S.; Verhoeven-Duif, N.M.; Zinkstok, J.R. Psychiatric phenotypes associated with hyperprolinemia: A systematic review. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2021, 186, 289–317. [Google Scholar] [CrossRef]
- Oyanagi, K.; Tsuchiyama, A.; Itakura, Y.; Tamura, Y.; Nakao, T.; Fujita, S.; Shiono, H. Clinical, biochemical and enzymatic studies in type I hyperprolinemia associated with chromosomal abnormality. Tohoku J. Exp. Med. 1987, 151, 465–475. [Google Scholar] [CrossRef]
- Pavone, L.; Mollica, F.; Levy, H.L. Asymptomatic type II hyperprolinaemia associated with hyperglycinaemia in three sibs. Arch. Dis. Child. 1975, 50, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Raux, G.; Bumsel, E.; Hecketsweiler, B.; van Amelsvoort, T.; Zinkstok, J.; Manouvrier-Hanu, S.; Fantini, C.; Brévière, G.M.M.; Di Rosa, G.; Pustorino, G.; et al. Involvement of hyperprolinemia in cognitive and psychiatric features of the 22q11 deletion syndrome. Hum. Mol. Genet. 2007, 16, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Steinlin, M.; Boltshauser, E.; Steinmann, B.; Wichmann, W.; Niemeyer, G. Hyperprolinaemia type I and white matter disease: Coincidence or causal relationship? Eur. J. Pediatr. 1989, 149, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Wajner, M.; Wannmacher, C.M.; Purkiss, P. High urinary excretion of N-(pyrrole-2-carboxyl) glycine in type II hyperprolinemia. Clin. Genet. 1990, 37, 485–489. [Google Scholar] [CrossRef]
- Ferreira, A.G.K.; da Cunha, A.A.; Scherer, E.B.; Machado, F.R.; da Cunha, M.J.; Braga, A.; Mussulini, B.H.; Moreira, J.D.; Wofchuk, S.; Souza, D.O.; et al. Evidence that Hyperprolinemia Alters Glutamatergic Homeostasis in Rat Brain: Neuroprotector Effect of Guanosine. Neurochem. Res. 2012, 37, 205–213. [Google Scholar] [CrossRef]
- Ferreira, A.G.K.; Scherer, E.B.; da Cunha, A.A.; Manfredini, V.; Biancini, G.B.; Vanzin, C.S.; Vargas, C.R.; Wyse, A.T.S. Hyperprolinemia induces DNA, protein and lipid damage in blood of rats: Antioxidant protection. Int. J. Biochem. Cell Biol. 2014, 54, 20–25. [Google Scholar] [CrossRef]
- van de Ven, S.; Gardeitchik, T.; Kouwenberg, D.; Kluijtmans, L.; Wevers, R.; Morava, E. Long-term clinical outcome, therapy and mild mitochondrial dysfunction in hyperprolinemia. J. Inherit. Metab. Dis. 2014, 37, 383–390. [Google Scholar] [CrossRef]
- Ersoy, M.; Yılmaz, S.; Ceylaner, S. Antioxidant Therapy in a Patient with Hyperprolinemia Type 1 Presenting with Mild Neuromotor Retardation and Speech Disturbance. Indian J. Pediatr. 2021, 88, 601. [Google Scholar] [CrossRef]
- Goyer, R.A.; Mitchell, B.J.; Leonard, D.L. Dietary reduction of hyperprolinemia. Transl. Res. 1969, 73, 819–824. [Google Scholar]
- Ishikawa, Y.; Kameda, K.; Okabe, M.; Imai, T.; Nagaoka, M.; Minami, R. A case of type I hyperprolinemia associated with photogenic epilepsy. No To Hattatsu 1991, 23, 81–86. [Google Scholar]
- Feng, X.; Wang, X.; Zhou, L.; Pang, S.; Tang, H. The impact of glucose on mitochondria and life span is determined by the integrity of proline catabolism in Caenorhabditis elegans. J. Biol. Chem. 2023, 299, 102881. [Google Scholar] [CrossRef]
- Ferreira, A.G.K.; Da Cunha, A.A.; MacHado, F.R.; Pederzolli, C.D.; Dalazen, G.R.; De Assis, A.M.; Lamers, M.L.; Dos Santos, M.F.; Dutra-Filho, C.S.; Wyse, A.T.S. Experimental hyperprolinemia induces mild oxidative stress, metabolic changes, and tissue adaptation in rat liver. J. Cell. Biochem. 2012, 113, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.G.K.; Lima, D.D.; Delwing, D.; MacKedanz, V.; Tagliari, B.; Kolling, J.; Schuck, P.F.; Wajner, M.; Wyse, A.T.S. Proline impairs energy metabolism in cerebral cortex of young rats. Metab. Brain Dis. 2010, 25, 161–168. [Google Scholar] [CrossRef]
- Azcoitia, I.; Perez-Martin, M.; Salazar, V.; Castillo, C.; Ariznavarreta, C.; Garcia-Segura, L.M.; Tresguerres, J.A.F. Growth hormone prevents neuronal loss in the aged rat hippocampus. Neurobiol. Aging 2005, 26, 697–703. [Google Scholar] [CrossRef]
- Nyberg, F.; Hallberg, M. Growth hormone and cognitive function. Nat. Rev. Endocrinol. 2013, 9, 357–365. [Google Scholar] [CrossRef]
- Popa, M.; Florea, I.; Simionescu, L.; Dimitriu, V. Release of growth hormone, prolactin, LH, FSH and IRI in serum through orally administered 1-proline in high dosage. Endocrinologie 1077, 15, 267–270. [Google Scholar]
- Young, J.A.; Duran-Ortiz, S.; Bell, S.; Funk, K.; Tian, Y.; Liu, Q.; Patterson, A.D.; List, E.O.; Berryman, D.E.; Kopchick, J.J. Growth Hormone Alters Circulating Levels of Glycine and Hydroxyproline in Mice. Metabolites 2023, 13, 191. [Google Scholar] [CrossRef]
- Agha, M.; Sallam, M.S.M.; Abougabal, A.M.; Abdelgawad, M.S. Pituitary stalk interruption syndrome (PSIS): Do not miss this diagnosis. Egypt. J. Radiol. Nucl. Med. 2022, 53, 192. [Google Scholar] [CrossRef]
- Vijayanand, P.; Mahadevan, S.; Shivbalan, S.; Reddy, N.; Ramdoss, N. Pituitary Stalk Interruption Syndrome (PSIS). Indian J. Pediatr. 2007, 74, 874–875. [Google Scholar] [CrossRef]
- Pinto, G.; Netchine, I.; Sobrier, M.L.; Brunelle, F.; Souberbielle, J.C.; Brauner, R. Pituitary Stalk Interruption Syndrome: A Clinical-Biological-Genetic Assessment of Its Pathogenesis. J. Clin. Endocrinol. Metab. 1997, 82, 3450–3454. [Google Scholar] [CrossRef]
- Triulzi, F.; Scotti, G.; di Natale, B.; Pellini, C.; Lukezic, M.; Scognamiglio, M.; Chiumello, G. Evidence of a congenital midline brain anomaly in pituitary dwarfs: A magnetic resonance imaging study in 101 patients. Pediatrics 1994, 93, 409–416. [Google Scholar] [PubMed]
- Hamilton, J.; Blaser, S.; Daneman, D. MR imaging in idiopathic growth hormone deficiency. AJNR Am. J. Neuroradiol. 1998, 19, 1609–1615. [Google Scholar]
- Feingold, K.R.; Anawalt, B.; Blackman, M.R.; Boyce, A.; Chrousos, G. Endotext [Internet]; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
| Hormones | Concentrations | Concentrations | Reference Range |
|---|---|---|---|
| IGF-1 (ng/mL) | 11 | ↓ | 37–184 |
| FSH (mU/mL) | 2.1 | ↔ | 0.4–3.8 |
| LH (mU/mL) | <0.1 | ↔ | <0.33 |
| Testosterone (ng/dL) | <2.5 | ↔ | <35.7 |
| TSH (μU/mL) | 3.05 | ↔ | 0.61–4.43 |
| fT4 (ng/dL) | 1.22 | ↔ | 1.01 1.63 |
| ACTH (pg/mL) | 33.2 | ↔ | 7.2–63.6 |
| Cortisol (μg/dL) | 19.5 | ↔ | 5.3–22.5 |
| Ferritin (ng/mL) | 55.9 | ↔ | 13.7–78.8 |
| 25(OH)D (ng/mL) | 28 | ↔ | 20–100 |
| Total IgA (mg/dL) | 64 | ↔ | 21–291 |
| Time (min) | Glucose (mg/dL) | Cortisol (μg/dL) | Growth Hormone (ng/mL) |
|---|---|---|---|
| 0 | 96 | 17.3 | 0.71 |
| 30 | 167 | 16.6 | 0.62 |
| 60 | 153 | 16.2 | 1.00 |
| 90 | 84 | 21.9 | 1.05 |
| 120 | 62 | 24.9 | 0.78 |
| 150 | 60 | 32.0 | 0.49 |
| 180 | 70 | 25.1 | 0.35 |
| First Author | Origin | Study Design | Sample | Clinical Characteristics |
|---|---|---|---|---|
| Applegarth [13] | Canada | Case report | N = 1, boy with HPII (5-year old) | Seizures, abnormal EEG. |
| Shivananda [14] | India | Case report | N = 1, infant girl with HPI (2.5-month old) | Cluster attacks of myoclonic jerks. |
| Di Rosa [15] | Italy | Case series | N = 4, unrelated children with HPI (2–14-year-olds) | Epilepsy, intellectual disability, and behavioral disturbances. |
| Flynn [16] | Ireland | Cross-sectional | N = 312, subjects from 71 families, including patients with HPII (N = 63) | Seizures were reported in 17 patients, 10 exhibited mental handicap (IQ < 70). |
| Hama [17] | Japan | Case report | N = 1, boy with HPI (8-year old) | Preference for carbohydrate-rich foods and tendency toward neurodevelopmental disorders, including autism, ADHD, and learning disorders. |
| Harries [18] | UK | Case report | N = 1, infant with HPI (7-month old) | Delayed neurological development, abnormalities of the renal tract, EEG, and bones, as well as malabsorption. |
| Humbertcalude [19] | France | Case report | N = 1, infant boy with HPI (9-months old) | Generalized tonic–clonic seizures with fever. |
| Kaur [20] | India | Case report | N = 1, infant girl with HPII (11-months old) | Recurrent seizures, lethargy, and regression of milestones. |
| Navamar [21] | Netherlands | Systematic review | NR | High prevalence of developmental delay, intellectual disability, autism, and psychosis spectrum disorders. |
| Oyanagi [22] | Japan | Case report | N = 1, infant girl with HPI (11-month-old) | Mental and motor disabilities. |
| Pavone [23] | Italy | Case series | N = 3, siblings with HPII | Marked hyperprolinemia and hyperglycemia; otherwise, asymptomatic. |
| Raux [24] | France | Cross-sectional | N = 92, patients with VCFS, including children (N = 8) with HPI (2–14-year olds) | Intellectual disability generalized tonic–clonic seizures and autistic features. Higher serum proline concentrations were associated with lower IQ in patients harboring the 22q11 deletion. |
| Steinlin [25] | Switzerland | Case report | N = 1, boy with HPI (10-year old) | Intellectual disability, cerebral palsy, epilepsy, and nystagmus. |
| Wajner [26] | Brazil | Case report | N = 1, boy with HPII (5-year old) | Mild developmental delay, recurrent seizures of the grand mal type. |
| Walker [12] | UK | Case report | N = 1, girl with HPII (18-months old) | Vitamin B6 deficiency and convulsions. |
| First Author | Origin | Study Design | Population | Intervention Type | Intervention Duration | Results |
|---|---|---|---|---|---|---|
| Ersoy [30] | Turkey | Case report | N = 1, girl with HPI (4-year old) | Antioxidant therapy with 100 mg/d co-enzyme Q10 and B complex (B1 + B2 + B6 + B12 vitamins), 500 mg/day of vitamin C, and 500 mg/day L-carnitine | 6-month intervention, followed by a 1-month wash-out and then revision of intervention | During the intervention, proline concentrations fell within the reference range. During washout, proline concentrations gradually increased, speech impairment increased, and fine motor skills were impaired. At 69 months of treatment, IQ and speech disturbance improved significantly. |
| Goyer [31] | Japan | Case report | N = 1, young girl | Low proline and low protein diet NOD, with a mixture of essential amino acids as the sole protein source | 1 month | Plasma urea and proline were reduced to normal concentrations, and prolinuria lessened. The addition of proline to the diet increased plasma proline. The diet was palatable and well tolerated. |
| Harries [18] | UK | Case report | N = 1, infant with HPI (7-month old) | Restriction of dietary proline NOD | 18 months | Fall of plasma proline concentrations within the reference range, satisfactory growth, improved mental development, and the EEG, renal, skeletal, and intestinal abnormalities disappeared. |
| Ishikawa [32] | Japan | Case report | N = 1, girl with HPI (9-year old) | Restriction of proline and protein intake NOD | Growth was satisfactory, but mental development failed to improve (progressive speech and motor disabilities). | |
| Oyanagi [22] | Japan | Case report | N = 1, infant girl with HPI (11-month old) | Low proline dietary therapy NOD | Since 12 months of age | Serum proline concentrations decreased, reaching the reference range, and growth was satisfactory, but mental development did not improve. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theodosiadi, A.; Toulia, I.; Grammatikopoulou, M.G.; Adamidou, F.; Chourmouzi, D.; Antachopoulos, C.; Evangeliou, A.E.; Goulis, D.G.; Tsiroukidou, K. Severe Hypoglycemia and Pituitary Stalk Interruption Syndrome in a 5-Year-Old Boy with Coexistent Hyperprolinaemia: A Case Report and Literature Review. Endocrines 2025, 6, 20. https://doi.org/10.3390/endocrines6020020
Theodosiadi A, Toulia I, Grammatikopoulou MG, Adamidou F, Chourmouzi D, Antachopoulos C, Evangeliou AE, Goulis DG, Tsiroukidou K. Severe Hypoglycemia and Pituitary Stalk Interruption Syndrome in a 5-Year-Old Boy with Coexistent Hyperprolinaemia: A Case Report and Literature Review. Endocrines. 2025; 6(2):20. https://doi.org/10.3390/endocrines6020020
Chicago/Turabian StyleTheodosiadi, Aikaterini, Ilektra Toulia, Maria G. Grammatikopoulou, Fotini Adamidou, Danai Chourmouzi, Charalampos Antachopoulos, Athanasios E. Evangeliou, Dimitrios G. Goulis, and Kyriaki Tsiroukidou. 2025. "Severe Hypoglycemia and Pituitary Stalk Interruption Syndrome in a 5-Year-Old Boy with Coexistent Hyperprolinaemia: A Case Report and Literature Review" Endocrines 6, no. 2: 20. https://doi.org/10.3390/endocrines6020020
APA StyleTheodosiadi, A., Toulia, I., Grammatikopoulou, M. G., Adamidou, F., Chourmouzi, D., Antachopoulos, C., Evangeliou, A. E., Goulis, D. G., & Tsiroukidou, K. (2025). Severe Hypoglycemia and Pituitary Stalk Interruption Syndrome in a 5-Year-Old Boy with Coexistent Hyperprolinaemia: A Case Report and Literature Review. Endocrines, 6(2), 20. https://doi.org/10.3390/endocrines6020020







