Magnetic Particle Imaging in Oncology: Advances and Prospects for Tumor Progression Monitoring and Targeted Therapy
Abstract
1. Introduction
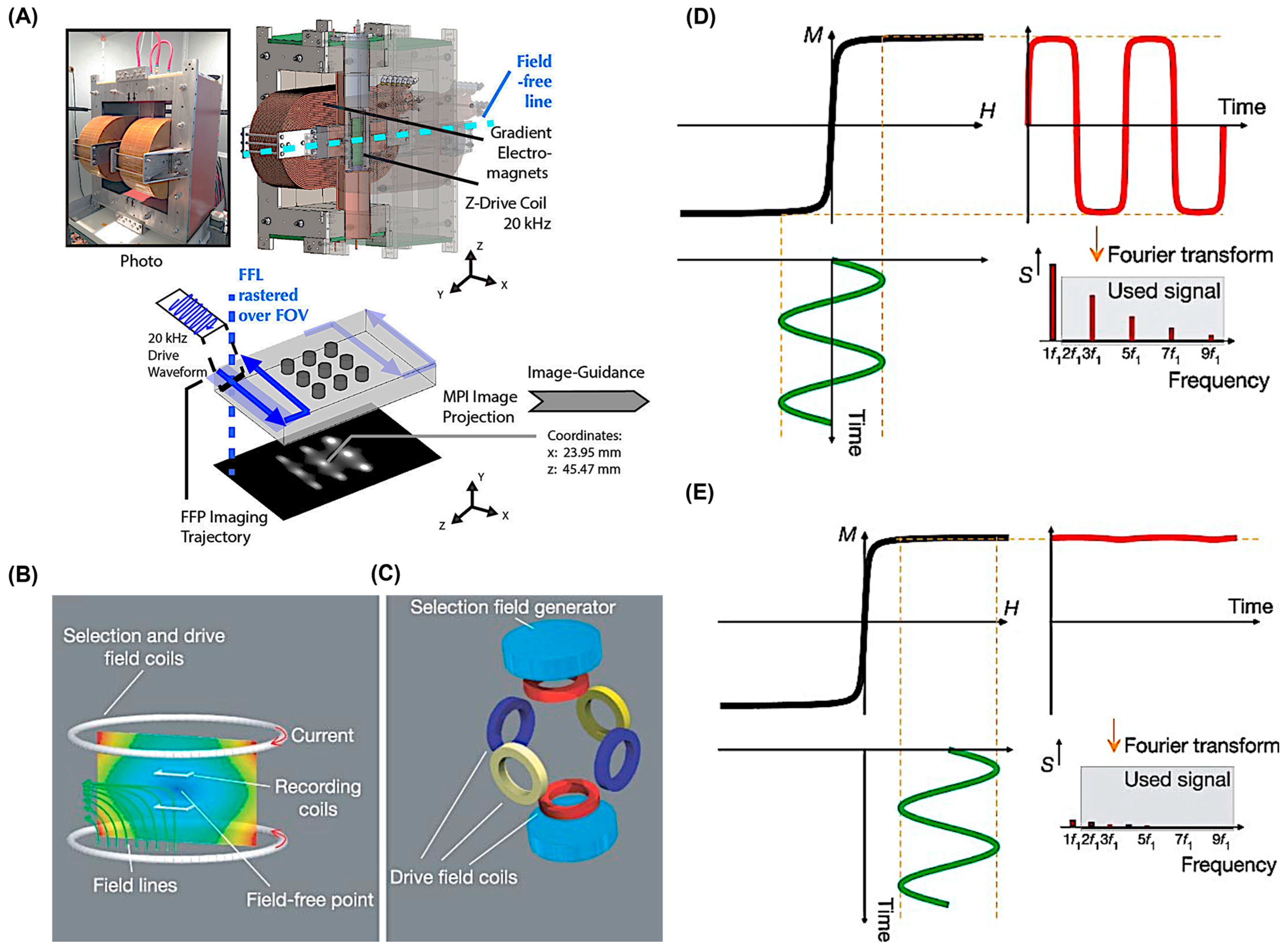
2. Brief Overview of Magnetic Particle Imaging (MPI)
3. Resolution, Sensitivity, and Contrast Using MPI
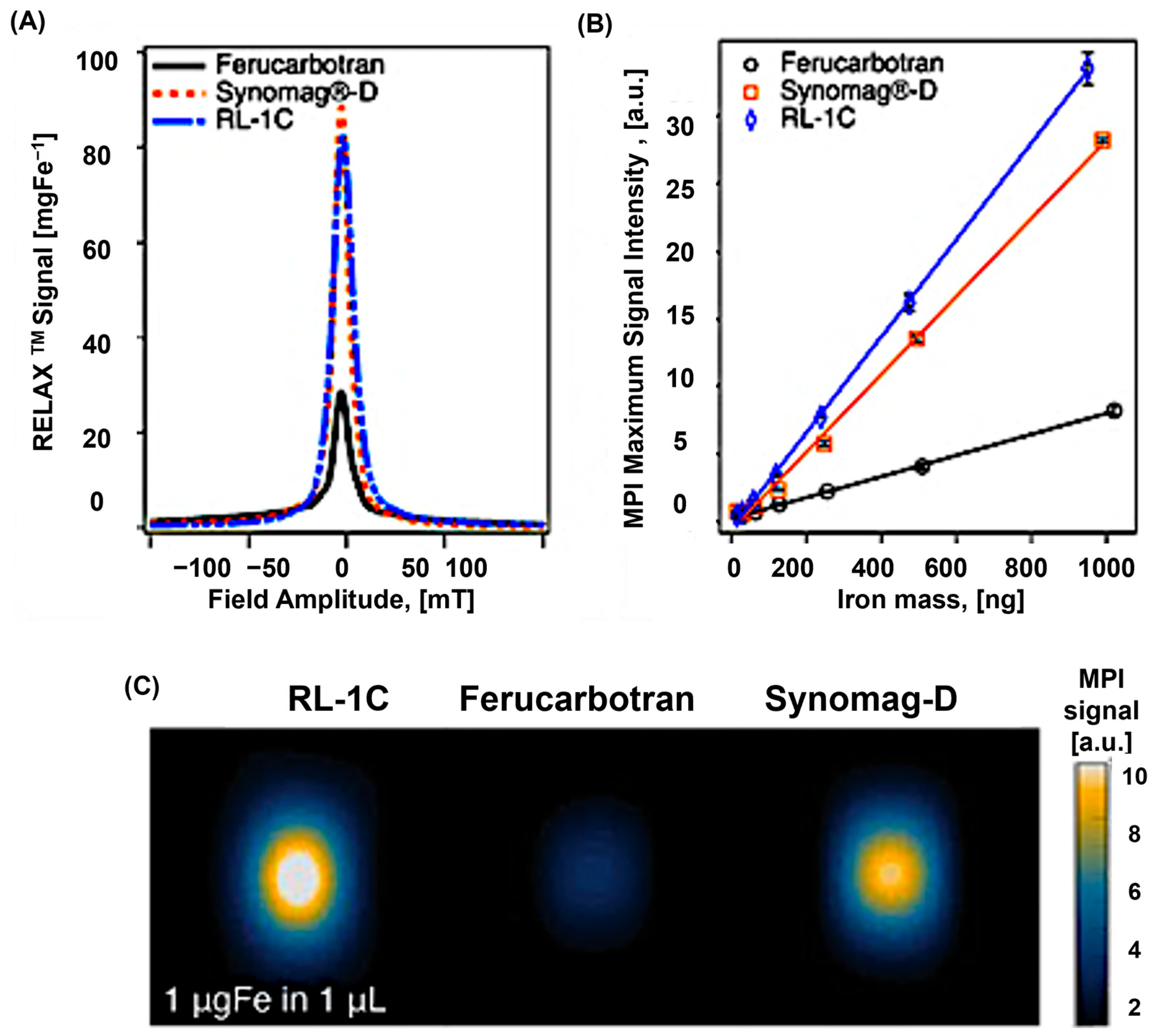
4. Commercial Tracers for MPI
5. Tracer Characteristics and Their Correlation with MPI Performance
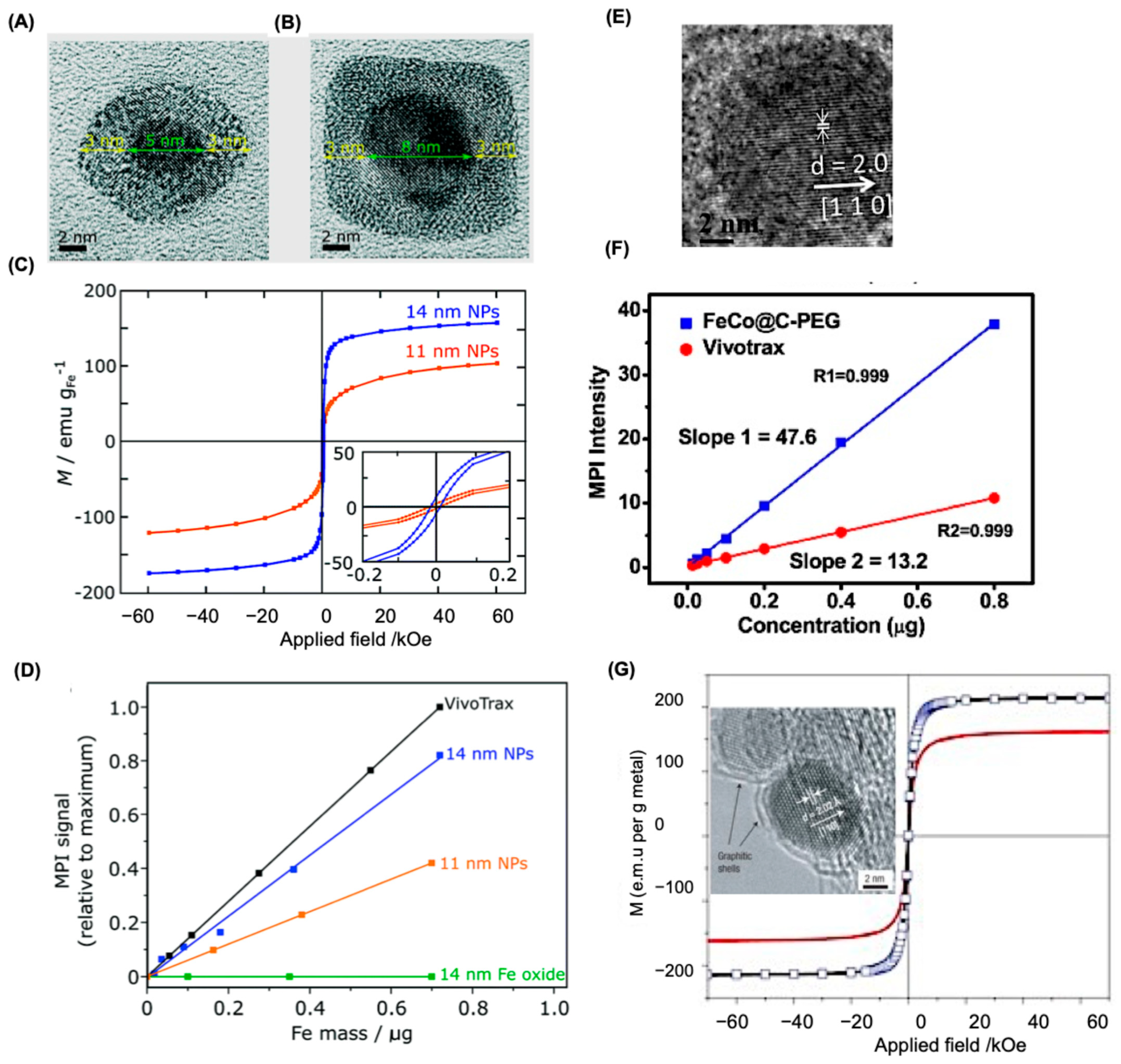
5.1. Size Correlation of SPIONS
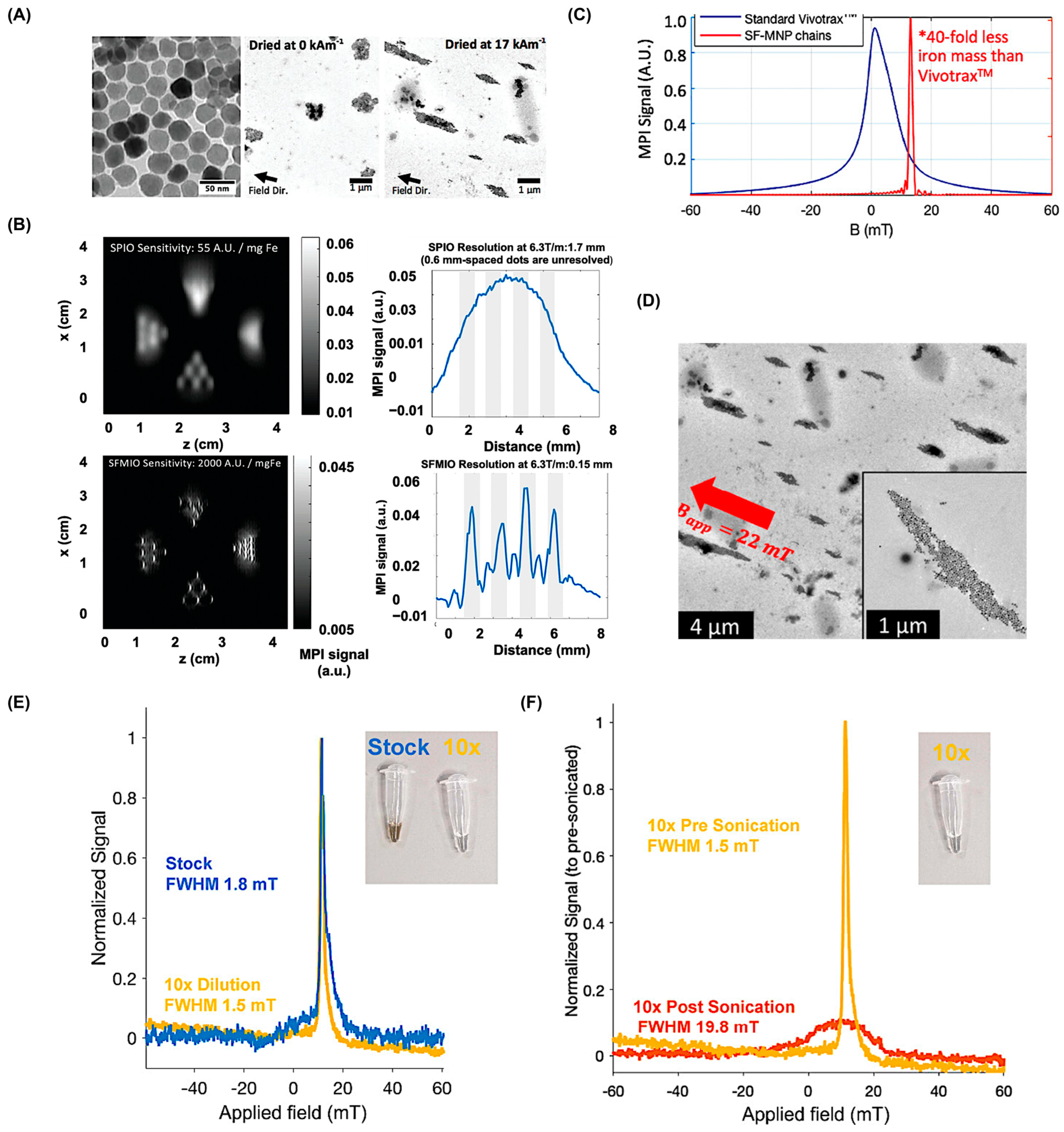
5.2. Shape Characteristics of SPIONS
5.3. Role of Surface Coatings on SPIONS
5.4. Alternatives to SPIONs as MPI Tracers
6. Application of MPI in Oncology
6.1. Cell Tracking
6.1.1. Tumor Cell Tracking
6.1.2. Immune Cell Tracking and Therapy
6.1.3. Imaging and Tracking Inflammatory Cells
6.2. In Vivo Tumor Imaging
6.2.1. Breast Cancer Imaging
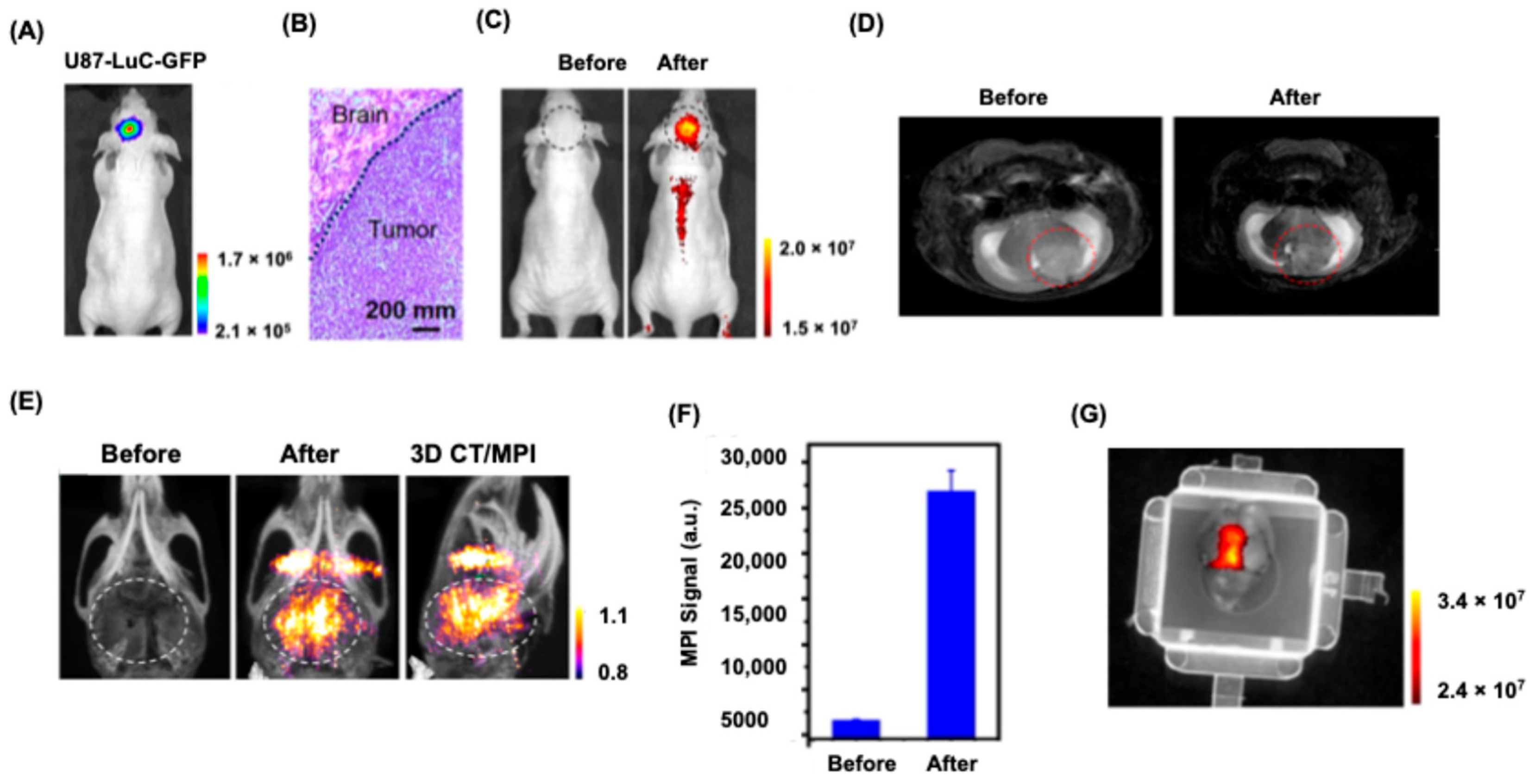
6.2.2. Brain Tumor Imaging
6.3. Theranostic Applications
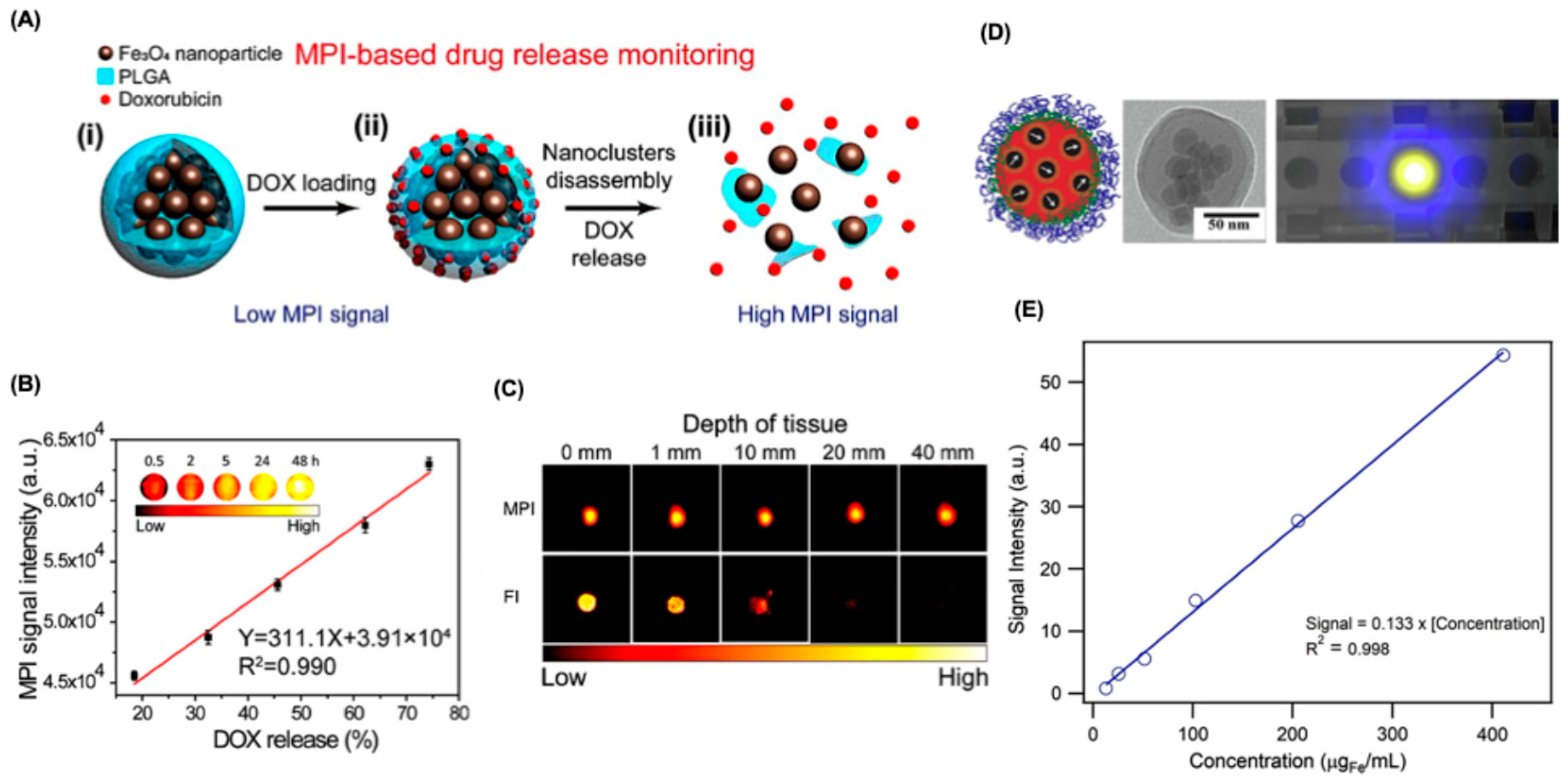
6.3.1. Drug Delivery Tracking
6.3.2. Magnetic Fluid Hyperthermia
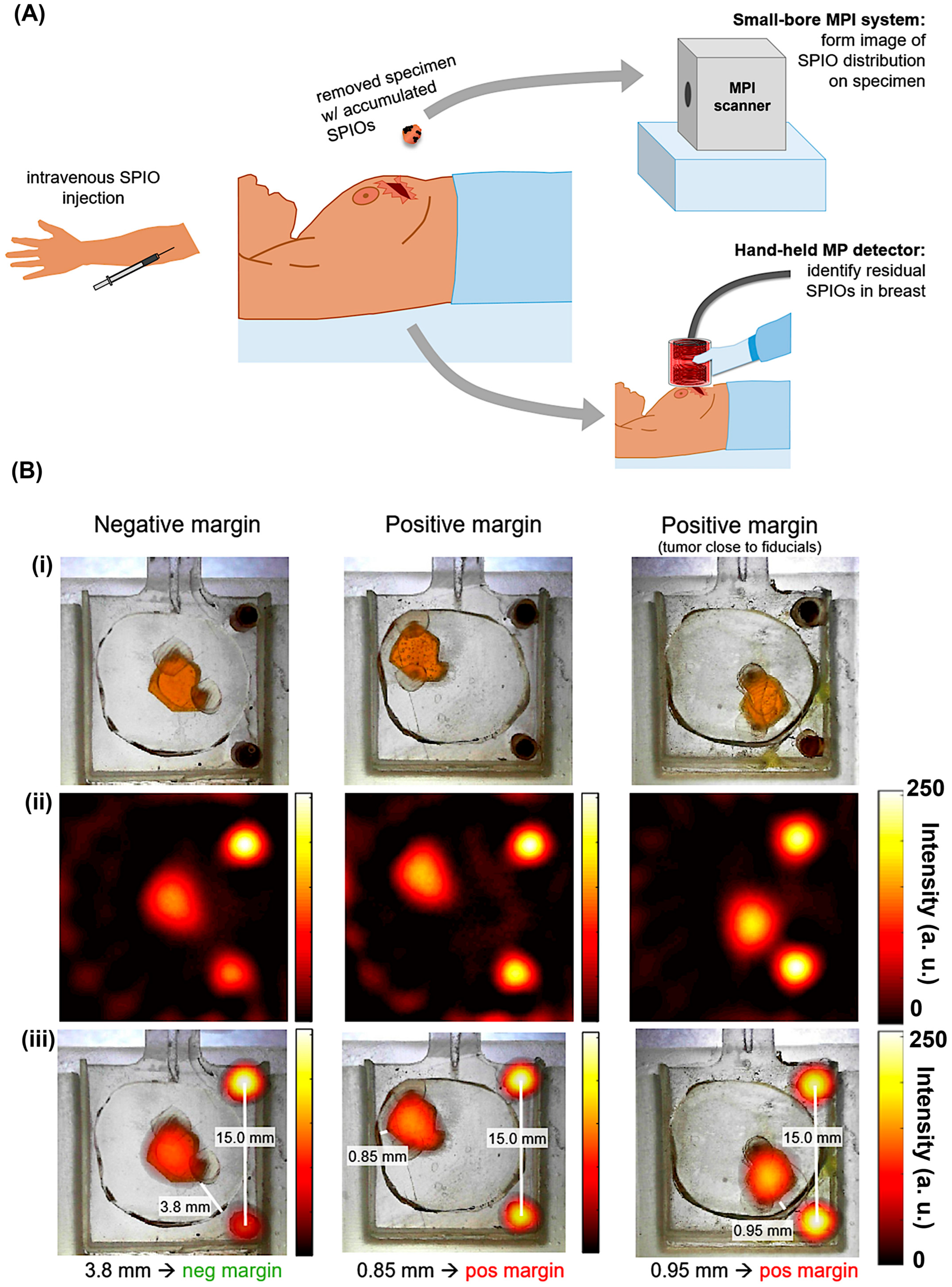
6.3.3. Navigation for Intraoperative
6.3.4. Combinatorial Therapies
7. Machine Learning Assisted MPI
8. Clinical Translation
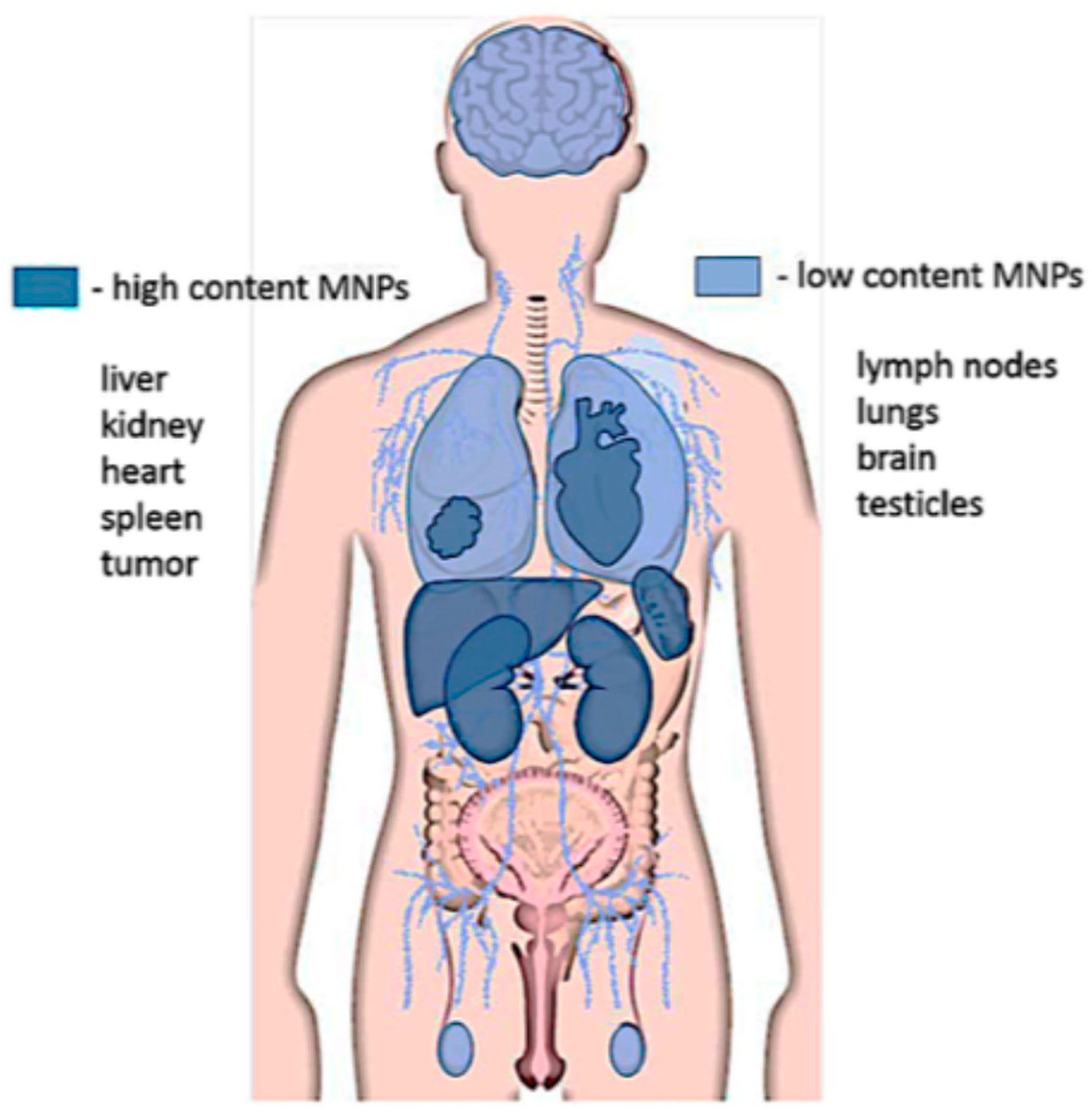
9. Biosafety and Toxicity of SPIONS and MPI
10. Conclusions and Perspective
Funding
Acknowledgments
Conflicts of Interest
References
- Hussain, S.; Mubeen, I.; Ullah, N.; Shah, S.S.U.D.; Khan, B.A.; Zahoor, M.; Ullah, R.; Khan, F.A.; Sultan, M.A. Modern Diagnostic Imaging Technique Applications and Risk Factors in the Medical Field: A Review. Biomed. Res. Int. 2022, 2022, 5164970. [Google Scholar] [CrossRef]
- Rong, J.; Liu, Y. Advances in Medical Imaging Techniques. BMC Methods 2024, 1, 10. [Google Scholar] [CrossRef]
- Christensen-Jeffries, K.; Couture, O.; Dayton, P.A.; Eldar, Y.C.; Hynynen, K.; Kiessling, F.; O’Reilly, M.; Pinton, G.F.; Schmitz, G.; Tang, M.-X.; et al. Super-Resolution Ultrasound Imaging. Ultrasound Med. Biol. 2020, 46, 865–891. [Google Scholar] [CrossRef]
- Moran, C.M.; Thomson, A.J.W. Preclinical Ultrasound Imaging—A Review of Techniques and Imaging Applications. Front. Phys. 2020, 8, 124. [Google Scholar] [CrossRef]
- Trotter, J.; Pantel, A.R.; Teo, B.-K.K.; Escorcia, F.E.; Li, T.; Pryma, D.A.; Taunk, N.K. Positron Emission Tomography (PET)/Computed Tomography (CT) Imaging in Radiation Therapy Treatment Planning: A Review of PET Imaging Tracers and Methods to Incorporate PET/CT. Adv. Radiat. Oncol. 2023, 8, 101212. [Google Scholar] [CrossRef] [PubMed]
- Moonen, C.T.W.; Van Zijl, P.C.M.; Frank, J.A.; Le Bihan, D.; Becker, E.D. Functional Magnetic Resonance Imaging in Medicine and Physiology. Science 1990, 250, 53–61. [Google Scholar] [CrossRef] [PubMed]
- La Fougère, C.; Rominger, A.; Förster, S.; Geisler, J.; Bartenstein, P. PET and SPECT in Epilepsy: A Critical Review. Epilepsy Behav. 2009, 15, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Panagiotopoulos, N.; Vogt, F.; Barkhausen, J.; Buzug, T.M.; Duschka, R.L.; Lüdtke-Buzug, K.; Ahlborg, M.; Bringout, G.; Debbeler, C.; Gräser, M.; et al. Magnetic Particle Imaging: Current Developments and Future Directions. Int. J. Nanomed. 2015, 10, 3097. [Google Scholar] [CrossRef]
- Yang, X.; Shao, G.; Zhang, Y.; Wang, W.; Qi, Y.; Han, S.; Li, H. Applications of Magnetic Particle Imaging in Biomedicine: Advancements and Prospects. Front. Physiol. 2022, 13, 898426. [Google Scholar] [CrossRef] [PubMed]
- Gleich, B.; Weizenecker, J. Tomographic Imaging Using the Nonlinear Response of Magnetic Particles. Nature 2005, 435, 1214–1217. [Google Scholar] [CrossRef]
- Ludwig, F.; Remmer, H.; Kuhlmann, C.; Wawrzik, T.; Arami, H.; Ferguson, R.M.; Krishnan, K.M. Self-Consistent Magnetic Properties of Magnetite Tracers Optimized for Magnetic Particle Imaging Measured by Ac Susceptometry, Magnetorelaxometry and Magnetic Particle Spectroscopy. J. Magn. Magn. Mater. 2014, 360, 169–173. [Google Scholar] [CrossRef]
- Xie, X.; Zhai, J.; Zhou, X.; Guo, Z.; Lo, P.; Zhu, G.; Chan, K.W.Y.; Yang, M. Magnetic Particle Imaging: From Tracer Design to Biomedical Applications in Vasculature Abnormality. Adv. Mater. 2024, 36, 2306450. [Google Scholar] [CrossRef]
- Lu, C.; Han, L.; Wang, J.; Wan, J.; Song, G.; Rao, J. Engineering of Magnetic Nanoparticles as Magnetic Particle Imaging Tracers. Chem. Soc. Rev. 2021, 50, 8102–8146. [Google Scholar] [CrossRef]
- Song, G.; Chen, M.; Zhang, Y.; Cui, L.; Qu, H.; Zheng, X.; Wintermark, M.; Liu, Z.; Rao, J. Janus Iron Oxides @ Semiconducting Polymer Nanoparticle Tracer for Cell Tracking by Magnetic Particle Imaging. Nano Lett. 2018, 18, 182–189. [Google Scholar] [CrossRef]
- Song, G.; Zheng, X.; Wang, Y.; Xia, X.; Chu, S.; Rao, J. A Magneto-Optical Nanoplatform for Multimodality Imaging of Tumors in Mice. ACS Nano 2019, 13, 7750–7758. [Google Scholar] [CrossRef]
- Chandrasekharan, P.; Tay, Z.W.; Hensley, D.; Zhou, X.Y.; Fung, B.K.; Colson, C.; Lu, Y.; Fellows, B.D.; Huynh, Q.; Saayujya, C.; et al. Using Magnetic Particle Imaging Systems to Localize and Guide Magnetic Hyperthermia Treatment: Tracers, Hardware, and Future Medical Applications. Theranostics 2020, 10, 2965–2981. [Google Scholar] [CrossRef] [PubMed]
- Talebloo, N.; Gudi, M.; Robertson, N.; Wang, P. Magnetic Particle Imaging: Current Applications in Biomedical Research. J. Magn. Reson. Imaging 2020, 51, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Tay, Z.W.; Chandrasekharan, P.; Chiu-Lam, A.; Hensley, D.W.; Dhavalikar, R.; Zhou, X.Y.; Yu, E.Y.; Goodwill, P.W.; Zheng, B.; Rinaldi, C.; et al. Magnetic Particle Imaging-Guided Heating in Vivo Using Gradient Fields for Arbitrary Localization of Magnetic Hyperthermia Therapy. ACS Nano 2018, 12, 3699–3713. [Google Scholar] [CrossRef]
- Liao, Y.; Li, L.; Ge, D.; He, N.; Guo, P.; Hu, J.; Xu, C.; Gao, Y.; Zhang, Z.; Wang, Q.; et al. Improving the Resolution of Single-Harmonic MPI Using Perpendicular Signal Transformation. Int. J. Magn. Part. Imaging 2024, 10. [Google Scholar] [CrossRef]
- Shen, Q.; Yu, C. Advances in Superparamagnetic Iron Oxide Nanoparticles Modified with Branched Polyethyleneimine for Multimodal Imaging. Front. Bioeng. Biotechnol. 2024, 11, 1323316. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, Y.; Yang, F.; Hui, H.; Tian, J. MPI Reconstruction Based on System Matrix Using a Field Free Line. Int. J. Magn. Part. Imaging 2023, 9 (Suppl. 1). [Google Scholar] [CrossRef]
- Chandrasekharan, P.; Tay, Z.W.; Zhou, X.Y.; Yu, E.; Orendorff, R.; Hensley, D.; Huynh, Q.; Fung, K.L.B.; VanHook, C.C.; Goodwill, P.; et al. A Perspective on a Rapid and Radiation-Free Tracer Imaging Modality, Magnetic Particle Imaging, with Promise for Clinical Translation. Br. J. Radiol. 2018, 91, 20180326. [Google Scholar] [CrossRef]
- Goodwill, P.W.; Conolly, S.M. Multidimensional X-Space Magnetic Particle Imaging. IEEE Trans. Med. Imaging 2011, 30, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Goodwill, P.W.; Saritas, E.U.; Croft, L.R.; Kim, T.N.; Krishnan, K.M.; Schaffer, D.V.; Conolly, S.M. X-Space MPI: Magnetic Nanoparticles for Safe Medical Imaging. Adv. Mater. 2012, 24, 3870–3877. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, R.M.; Khandhar, A.P.; Krishnan, K.M. Tracer Design for Magnetic Particle Imaging (Invited). J. Appl. Phys. 2012, 111, 07B318. [Google Scholar] [CrossRef] [PubMed]
- Duong, H.T.K.; Abdibastami, A.; Gloag, L.; Barrera, L.; Gooding, J.J.; Tilley, R.D. A Guide to the Design of Magnetic Particle Imaging Tracers for Biomedical Applications. Nanoscale 2022, 14, 13890–13914. [Google Scholar] [CrossRef]
- Deissler, R.J.; Wu, Y.; Martens, M.A. Dependence of Brownian and Néel Relaxation Times on Magnetic Field Strength. Med. Phys. 2014, 41, 012301. [Google Scholar] [CrossRef]
- Ota, S.; Takemura, Y. Characterization of Néel and Brownian Relaxations Isolated from Complex Dynamics Influenced by Dipole Interactions in Magnetic Nanoparticles. J. Phys. Chem. C 2019, 123, 28859–28866. [Google Scholar] [CrossRef]
- Rahmer, J.; Weizenecker, J.; Gleich, B.; Borgert, J. Signal Encoding in Magnetic Particle Imaging: Properties of the System Function. BMC Med. Imaging 2009, 9, 4. [Google Scholar] [CrossRef]
- Goodwill, P.W.; Conolly, S.M. The X-Space Formulation of the Magnetic Particle Imaging Process: 1-D Signal, Resolution, Bandwidth, SNR, SAR, and Magnetostimulation. IEEE Trans. Med. Imaging 2010, 29, 1851–1859. [Google Scholar] [CrossRef]
- Arami, H.; Teeman, E.; Troksa, A.; Bradshaw, H.; Saatchi, K.; Tomitaka, A.; Gambhir, S.S.; Häfeli, U.O.; Liggitt, D.; Krishnan, K.M. Tomographic Magnetic Particle Imaging of Cancer Targeted Nanoparticles. Nanoscale 2017, 9, 18723–18730. [Google Scholar] [CrossRef]
- Knopp, T.; Biederer, S.; Sattel, T.F.; Erbe, M.; Buzug, T.M. Prediction of the Spatial Resolution of Magnetic Particle Imaging Using the Modulation Transfer Function of the Imaging Process. IEEE Trans. Med. Imaging 2011, 30, 1284–1292. [Google Scholar] [CrossRef]
- Moses, W.W. Fundamental Limits of Spatial Resolution in PET. Nucl. Instrum. Methods Phys. Res. A 2011, 648, S236–S240. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.L.; Willowson, K.P. An Evidence-Based Review of Quantitative SPECT Imaging and Potential Clinical Applications. J. Nucl. Med. 2013, 54, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Vazin, T.; Goodwill, P.W.; Conway, A.; Verma, A.; Ulku Saritas, E.; Schaffer, D.; Conolly, S.M. Magnetic Particle Imaging Tracks the Long-Term Fate of in Vivo Neural Cell Implants with High Image Contrast. Sci. Rep. 2015, 5, 14055. [Google Scholar] [CrossRef]
- Saritas, E.U.; Goodwill, P.W.; Croft, L.R.; Konkle, J.J.; Lu, K.; Zheng, B.; Conolly, S.M. Magnetic Particle Imaging (MPI) for NMR and MRI Researchers. J. Magn. Reson. 2013, 229, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.C.; Zhang, Y.; Steinberg, G.; Qu, H.; Huang, S.; Cheng, M.; Bliss, T.; Du, F.; Rao, J.; Song, G.; et al. A Review of Magnetic Particle Imaging and Perspectives on Neuroimaging. Am. J. Neuroradiol. 2019, 40, 206–212. [Google Scholar] [CrossRef]
- Goodwill, P.W.; Tamrazian, A.; Croft, L.R.; Lu, C.D.; Johnson, E.M.; Pidaparthi, R.; Ferguson, R.M.; Khandhar, A.P.; Krishnan, K.M.; Conolly, S.M. Ferrohydrodynamic Relaxometry for Magnetic Particle Imaging. Appl. Phys. Lett. 2011, 98, 262502. [Google Scholar] [CrossRef]
- Biederer, S.; Knopp, T.; Sattel, T.F.; Lüdtke-Buzug, K.; Gleich, B.; Weizenecker, J.; Borgert, J.; Buzug, T.M. Magnetization Response Spectroscopy of Superparamagnetic Nanoparticles for Magnetic Particle Imaging. J. Phys. D Appl. Phys. 2009, 42, 205007. [Google Scholar] [CrossRef]
- Tay, Z.W.; Goodwill, P.W.; Hensley, D.W.; Taylor, L.A.; Zheng, B.; Conolly, S.M. A High-Throughput, Arbitrary-Waveform, MPI Spectrometer and Relaxometer for Comprehensive Magnetic Particle Optimization and Characterization. Sci. Rep. 2016, 6, 34180. [Google Scholar] [CrossRef]
- Liu, S.; Chiu-Lam, A.; Rivera-Rodriguez, A.; DeGroff, R.; Savliwala, S.; Sarna, N.; Rinaldi-Ramos, C.M. Long Circulating Tracer Tailored for Magnetic Particle Imaging. Nanotheranostics 2021, 5, 348–361. [Google Scholar] [CrossRef]
- Irfan, M.; Dogan, N.; Sapmaz, T.; Bingolbali, A. Development of MPI Relaxometer for Characterization of Superparamagnetic Nanoparticles. J. Magn. Magn. Mater. 2021, 536, 168082. [Google Scholar] [CrossRef]
- Reimer, P.; Balzer, T. Ferucarbotran (Resovist): A New Clinically Approved RES-Specific Contrast Agent for Contrast-Enhanced MRI of the Liver: Properties, Clinical Development, and Applications. Eur. Radiol. 2003, 13, 1266–1276. [Google Scholar] [CrossRef]
- Eberbeck, D.; Wiekhorst, F.; Wagner, S.; Trahms, L. How the Size Distribution of Magnetic Nanoparticles Determines Their Magnetic Particle Imaging Performance. Appl. Phys. Lett. 2011, 98, 182502. [Google Scholar] [CrossRef]
- Avugadda, S.K.; Wickramasinghe, S.; Niculaes, D.; Ju, M.; Lak, A.; Silvestri, N.; Nitti, S.; Roy, I.; Samia, A.C.S.; Pellegrino, T. Uncovering the Magnetic Particle Imaging and Magnetic Resonance Imaging Features of Iron Oxide Nanocube Clusters. Nanomaterials 2020, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Good, H.J.; Sehl, O.C.; Gevaert, J.J.; Yu, B.; Berih, M.A.; Montero, S.A.; Rinaldi-Ramos, C.M.; Foster, P.J. Inter-User Comparison for Quantification of Superparamagnetic Iron Oxides with Magnetic Particle Imaging across Two Institutions Highlights a Need for Standardized Approaches. Mol. Imaging Biol. 2023, 25, 954–967. [Google Scholar] [CrossRef]
- Gevaert, J.; Van Beek, K.; Sehl, O.C.; Foster, P.J. VivoTrax+ Improves the Detection of Cancer Cells with Magnetic Particle Imaging. Int. J. Magn. Part. Imaging 2022, 8, 2210001. [Google Scholar] [CrossRef]
- Nejadnik, H.; Pandit, P.; Lenkov, O.; Lahiji, A.P.; Yerneni, K.; Daldrup-Link, H.E. Ferumoxytol Can Be Used for Quantitative Magnetic Particle Imaging of Transplanted Stem Cells. Mol. Imaging Biol. 2019, 21, 465–472. [Google Scholar] [CrossRef]
- Vogel, P.; Kampf, T.; Rückert, M.; Grüttner, C.; Kowalski, A.; Teller, H.; Behr, V. Synomag®: The New High-Performance Tracer for Magnetic Particle Imaging. Int. J. Magn. Part. Imaging 2021, 7, 2103003. [Google Scholar] [CrossRef]
- Ferguson, R.M.; Khandhar, A.P.; Kemp, S.J.; Arami, H.; Saritas, E.U.; Croft, L.R.; Konkle, J.; Goodwill, P.W.; Halkola, A.; Rahmer, J.; et al. Magnetic Particle Imaging With Tailored Iron Oxide Nanoparticle Tracers. IEEE Trans. Med. Imaging 2015, 34, 1077–1084. [Google Scholar] [CrossRef]
- Lawaczeck, R.; Bauer, H.; Frenzel, T.; Hasegawa, M.; Ito, Y.; Kito, K.; Miwa, N.; Tsutsui, H.; Vogler, H.; Weinmann, H.-J. Magnetic Iron Oxide Particles Coated with Carboxydextran for Parenteral Administration and Liver Contrasting: Pre-Clinical Profile of SH U555A. Acta Radiol. 1997, 38, 584–597. [Google Scholar] [CrossRef]
- Balakrishnan, V.S.; Rao, M.; Kausz, A.T.; Brenner, L.; Pereira, B.J.G.; Frigo, T.B.; Lewis, J.M. Physicochemical Properties of Ferumoxytol, a New Intravenous Iron Preparation. Eur. J. Clin. Investig. 2009, 39, 489–496. [Google Scholar] [CrossRef]
- Rezaei, B.; Tay, Z.W.; Mostufa, S.; Manzari, O.N.; Azizi, E.; Ciannella, S.; Moni, H.-E.-J.; Li, C.; Zeng, M.; Gómez-Pastora, J.; et al. Magnetic Nanoparticles for Magnetic Particle Imaging (MPI): Design and Applications. Nanoscale 2024, 16, 11802–11824. [Google Scholar] [CrossRef]
- Lu, K.; Goodwill, P.; Zheng, B.; Conolly, S. Reshaping the 2D MPI PSF to Be Isotropic and Sharp Using Vector Acquisition and Equalization. In Proceedings of the 2015 5th International Workshop on Magnetic Particle Imaging (IWMPI), Istanbul, Turkey, 26–28 March 2015; IEEE: New York, NY, USA, 2015; p. 1. [Google Scholar]
- Ziemian, S.; Löwa, N.; Kosch, O.; Bajj, D.; Wiekhorst, F.; Schütz, G. Optimization of Iron Oxide Tracer Synthesis for Magnetic Particle Imaging. Nanomaterials 2018, 8, 180. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, X.; Liao, H.; Liang, Z.; Li, F.; Tian, J.; Ling, D. Artificially Engineered Cubic Iron Oxide Nanoparticle as a High-Performance Magnetic Particle Imaging Tracer for Stem Cell Tracking. ACS Nano 2020, 14, 2053–2062. [Google Scholar] [CrossRef]
- Tay, Z.W.; Hensley, D.W.; Vreeland, E.C.; Zheng, B.; Conolly, S.M. The Relaxation Wall: Experimental Limits to Improving MPI Spatial Resolution by Increasing Nanoparticle Core Size. Biomed. Phys. Eng. Express 2017, 3, 035003. [Google Scholar] [CrossRef] [PubMed]
- Sehl, O.C.; Gevaert, J.J.; Melo, K.P.; Knier, N.N.; Foster, P.J. A Perspective on Cell Tracking with Magnetic Particle Imaging. Tomography 2020, 6, 315–324. [Google Scholar] [CrossRef]
- Nigam, S.; Mohapatra, J.; Makela, A.V.; Hayat, H.; Rodriguez, J.M.; Sun, A.; Kenyon, E.; Redman, N.A.; Spence, D.; Jabin, G.; et al. Shape Anisotropy-Governed High-Performance Nanomagnetosol for In Vivo Magnetic Particle Imaging of Lungs. Small 2024, 20, 2305300. [Google Scholar] [CrossRef]
- Juhong, A.; Li, B.; Liu, Y.; Yang, C.; Yao, C.; Agnew, D.W.; Lei, Y.L.; Luker, G.D.; Bumpers, H.; Huang, X.; et al. Multihead Attention U-Net for Magnetic Particle Imaging–Computed Tomography Image Segmentation. Adv. Intell. Syst. 2024, 6, 2400007. [Google Scholar] [CrossRef]
- Palchoudhury, S.; Xu, Y.; Goodwin, J.; Bao, Y. Synthesis of Iron Oxide Nanoworms. J. Appl. Phys. 2011, 109, 07E314. [Google Scholar] [CrossRef]
- Park, J.; Von Maltzahn, G.; Zhang, L.; Schwartz, M.P.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Magnetic Iron Oxide Nanoworms for Tumor Targeting and Imaging. Adv. Mater. 2008, 20, 1630–1635. [Google Scholar] [CrossRef]
- Yang, C.-W.; Liu, K.; Yao, C.-Y.; Li, B.; Juhong, A.; Qiu, Z.; Huang, X. Indocyanine Green-Conjugated Superparamagnetic Iron Oxide Nanoworm for Multimodality Breast Cancer Imaging. ACS Appl. Nano Mater. 2022, 5, 18912–18920. [Google Scholar] [CrossRef] [PubMed]
- Tay, Z.W.; Savliwala, S.; Hensley, D.W.; Fung, K.L.B.; Colson, C.; Fellows, B.D.; Zhou, X.; Huynh, Q.; Lu, Y.; Zheng, B.; et al. Superferromagnetic Nanoparticles Enable Order-of-Magnitude Resolution & Sensitivity Gain in Magnetic Particle Imaging. Small Methods 2021, 5, 2100796. [Google Scholar] [CrossRef]
- Fung, K.L.B.; Colson, C.; Bryan, J.; Saayujya, C.; Mokkarala-Lopez, J.; Hartley, A.; Yousuf, K.; Kuo, R.; Lu, Y.; Fellows, B.D.; et al. First Superferromagnetic Remanence Characterization and Scan Optimization for Super-Resolution Magnetic Particle Imaging. Nano Lett. 2023, 23, 1717–1725. [Google Scholar] [CrossRef]
- Zhao, Z.; Rinaldi, C. Computational Predictions of Enhanced Magnetic Particle Imaging Performance by Magnetic Nanoparticle Chains. Phys. Med. Biol. 2020, 65, 185013. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.P.P.; Nafiujjaman, M.; Makela, A.V.; Hadrick, K.; Hill, M.L.; Lee, M.; Kim, T. Development of Iron Oxide Nanochains as a Sensitive Magnetic Particle Imaging Tracer for Cancer Detection. ACS Appl. Mater. Interfaces 2025, 17, 20859–20871. [Google Scholar] [CrossRef]
- Tomitaka, A.; Ota, S.; Nishimoto, K.; Arami, H.; Takemura, Y.; Nair, M. Dynamic Magnetic Characterization and Magnetic Particle Imaging Enhancement of Magnetic-Gold Core–Shell Nanoparticles. Nanoscale 2019, 11, 6489–6496. [Google Scholar] [CrossRef]
- Horvat, S.; Vogel, P.; Kampf, T.; Brandl, A.; Alshamsan, A.; Alhadlaq, H.A.; Ahamed, M.; Albrecht, K.; Behr, V.C.; Beilhack, A.; et al. Crosslinked Coating Improves the Signal-to-Noise Ratio of Iron Oxide Nanoparticles in Magnetic Particle Imaging (MPI). Chem. NanoMat. 2020, 6, 755–758. [Google Scholar] [CrossRef]
- Moor, L.; Scheibler, S.; Gerken, L.; Scheffler, K.; Thieben, F.; Knopp, T.; Herrmann, I.K.; Starsich, F.H.L. Particle Interactions and Their Effect on Magnetic Particle Spectroscopy and Imaging. Nanoscale 2022, 14, 7163–7173. [Google Scholar] [CrossRef]
- Azizi, E.; Rezaei, B.; Mostufa, S.; Liang, S.; Wang, Y.A.; Chugh, V.K.; Wang, J.-P.; Li, C.; Gómez-Pastora, J.; He, R.; et al. Effect of Tracer Size Distribution on Magnetic Particle Imaging Performance. Phys. Scr. 2025, 100, 025529. [Google Scholar] [CrossRef]
- Shasha, C.; Teeman, E.; Krishnan, K.M. Nanoparticle Core Size Optimization for Magnetic Particle Imaging. Biomed. Phys. Eng. Express 2019, 5, 055010. [Google Scholar] [CrossRef]
- Gloag, L.; Mehdipour, M.; Ulanova, M.; Mariandry, K.; Nichol, M.A.; Hernández-Castillo, D.J.; Gaudet, J.; Qiao, R.; Zhang, J.; Nelson, M.; et al. Zero Valent Iron Core–Iron Oxide Shell Nanoparticles as Small Magnetic Particle Imaging Tracers. Chem. Commun. 2020, 56, 3504–3507. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.S.; Lee, J.H.; Sun, X.; Suzuki, Y.; Mann, D.; Liu, Z.; Terashima, M.; Yang, P.C.; McConnell, M.V.; Nishimura, D.G.; et al. FeCo/Graphitic-Shell Nanocrystals as Advanced Magnetic-Resonance-Imaging and near-Infrared Agents. Nat. Mater. 2006, 5, 971–976. [Google Scholar] [CrossRef]
- Song, G.; Kenney, M.; Chen, Y.-S.; Zheng, X.; Deng, Y.; Chen, Z.; Wang, S.X.; Gambhir, S.S.; Dai, H.; Rao, J. Carbon-Coated FeCo Nanoparticles as Sensitive Magnetic-Particle-Imaging Tracers with Photothermal and Magnetothermal Properties. Nat. Biomed. Eng. 2020, 4, 325–334. [Google Scholar] [CrossRef]
- Liu, J.; Wu, K.; Wang, J.-P. Magnetic Properties of Cubic FeCo Nanoparticles with Anisotropic Long Chain Structure. AIP Adv. 2016, 6, 056126. [Google Scholar] [CrossRef]
- Wu, X.W.; Guslienko, K.Y.; Chantrell, R.W.; Weller, D. Magnetic Anisotropy and Thermal Stability Study on FePt Nanoparticle Assembly. Appl. Phys. Lett. 2003, 82, 3475–3477. [Google Scholar] [CrossRef]
- Yu, J.; Yang, C.; Li, J.; Ding, Y.; Zhang, L.; Yousaf, M.Z.; Lin, J.; Pang, R.; Wei, L.; Xu, L.; et al. Multifunctional Fe5C2 Nanoparticles: A Targeted Theranostic Platform for Magnetic Resonance Imaging and Photoacoustic Tomography-Guided Photothermal Therapy. Adv. Mater. 2014, 26, 4114–4120. [Google Scholar] [CrossRef]
- Silvestri, N.; Gavilán, H.; Guardia, P.; Brescia, R.; Fernandes, S.; Samia, A.C.S.; Teran, F.J.; Pellegrino, T. Di- and Tri-Component Spinel Ferrite Nanocubes: Synthesis and Their Comparative Characterization for Theranostic Applications. Nanoscale 2021, 13, 13665–13680. [Google Scholar] [CrossRef]
- Bauer, L.M.; Situ, S.F.; Griswold, M.A.; Samia, A.C.S. High-Performance Iron Oxide Nanoparticles for Magnetic Particle Imaging—Guided Hyperthermia (hMPI). Nanoscale 2016, 8, 12162–12169. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Han, X.; Du, Y.; Li, Y.; Li, Y.; Li, J.; Tian, J.; Wu, A. Mixed Metal Metal–Organic Frameworks Derived Carbon Supporting ZnFe2O4/C for High-Performance Magnetic Particle Imaging. Nano Lett. 2021, 21, 2730–2737. [Google Scholar] [CrossRef] [PubMed]
- Kratz, H.; Mohtashamdolatshahi, A.; Eberbeck, D.; Kosch, O.; Wiekhorst, F.; Taupitz, M.; Hamm, B.; Stolzenburg, N.; Schnorr, J. Tailored Magnetic Multicore Nanoparticles for Use as Blood Pool MPI Tracers. Nanomaterials 2021, 11, 1532. [Google Scholar] [CrossRef]
- Kratz, H.; Taupitz, M.; Ariza De Schellenberger, A.; Kosch, O.; Eberbeck, D.; Wagner, S.; Trahms, L.; Hamm, B.; Schnorr, J. Novel Magnetic Multicore Nanoparticles Designed for MPI and Other Biomedical Applications: From Synthesis to First in Vivo Studies. PLoS ONE 2018, 13, e0190214. [Google Scholar] [CrossRef]
- Makela, A.V.; Schott, M.A.; Madsen, C.S.; Greeson, E.M.; Contag, C.H. Magnetic Particle Imaging of Magnetotactic Bacteria as Living Contrast Agents Is Improved by Altering Magnetosome Arrangement. Nano Lett. 2022, 22, 4630–4639. [Google Scholar] [CrossRef]
- Zhu, X.; Li, J.; Peng, P.; Hosseini Nassab, N.; Smith, B.R. Quantitative Drug Release Monitoring in Tumors of Living Subjects by Magnetic Particle Imaging Nanocomposite. Nano Lett. 2019, 19, 6725–6733. [Google Scholar] [CrossRef]
- Antonelli, A.; Szwargulski, P.; Scarpa, E.-S.; Thieben, F.; Cordula, G.; Ambrosi, G.; Guidi, L.; Ludewig, P.; Knopp, T.; Magnani, M. Development of Long Circulating Magnetic Particle Imaging Tracers: Use of Novel Magnetic Nanoparticles and Entrapment Into Human Erythrocytes. Nanomedicine 2020, 15, 739–753. [Google Scholar] [CrossRef]
- Hildebrand, S.; Löwa, N.; Paysen, H.; Fratila, R.M.; Reverte-Salisa, L.; Trakoolwilaiwan, T.; Niu, Z.; Kasparis, G.; Preuss, S.F.; Kosch, O.; et al. Quantification of Lipoprotein Uptake in Vivo Using Magnetic Particle Imaging and Spectroscopy. ACS Nano 2021, 15, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Harvell-Smith, S.; Tung, L.D.; Thanh, N.T.K. Magnetic Particle Imaging: Tracer Development and the Biomedical Applications of a Radiation-Free, Sensitive, and Quantitative Imaging Modality. Nanoscale 2022, 14, 3658–3697. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, A.; Fraederich, B.M.; Pries, R.; Wollenberg, B.; Lüdtke-Buzug, K.; Graefe, K. Biological Impact of Superparamagnetic Iron Oxide Nanoparticles for Magnetic Particle Imaging of Head and Neck Cancer Cells. Int. J. Nanomed. 2014, 9, 5025. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Arami, H.; Krishnan, K.M. Detection of Cancer-Specific Proteases Using Magnetic Relaxation of Peptide-Conjugated Nanoparticles in Biological Environment. Nano Lett. 2016, 16, 3668–3674. [Google Scholar] [CrossRef]
- Melo, K.P.; Makela, A.V.; Knier, N.N.; Hamilton, A.M.; Foster, P.J. Magnetic Microspheres Can Be Used for Magnetic Particle Imaging of Cancer Cells Arrested in the Mouse Brain. Magn. Reson. Med. 2022, 87, 312–322. [Google Scholar] [CrossRef]
- Parkins, K.M.; Melo, K.P.; Chen, Y.; Ronald, J.A.; Foster, P.J. Visualizing Tumour Self-Homing with Magnetic Particle Imaging. Nanoscale 2021, 13, 6016–6023. [Google Scholar] [CrossRef]
- Makela, A.V.; Schott, M.A.; Sehl, O.C.; Gevaert, J.J.; Foster, P.J.; Contag, C.H. Tracking the Fates of Iron-Labeled Tumor Cells in Vivo Using Magnetic Particle Imaging. Nanoscale Adv. 2022, 4, 3617–3623. [Google Scholar] [CrossRef]
- Knier, N.N.; Dubois, V.P.; Chen, Y.; Ronald, J.A.; Foster, P.J. A Method for the Efficient Iron-Labeling of Patient-Derived Xenograft Cells and Cellular Imaging Validation. J. Biol. Methods 2021, 8, 1. [Google Scholar] [CrossRef]
- Makela, A.V.; Gaudet, J.M.; Foster, P.J. Quantifying Tumor Associated Macrophages in Breast Cancer: A Comparison of Iron and Fluorine-Based MRI Cell Tracking. Sci. Rep. 2017, 7, 42109. [Google Scholar] [CrossRef] [PubMed]
- Makela, A.V.; Foster, P.J. Imaging Macrophage Distribution and Density in Mammary Tumors and Lung Metastases Using Fluorine-19 MRI Cell Tracking. Magn. Reson. Med. 2018, 80, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Makela, A.V.; Gaudet, J.M.; Schott, M.A.; Sehl, O.C.; Contag, C.H.; Foster, P.J. Magnetic Particle Imaging of Macrophages Associated with Cancer: Filling the Voids Left by Iron-Based Magnetic Resonance Imaging. Mol. Imaging Biol. 2020, 22, 958–968. [Google Scholar] [CrossRef]
- Gerosa, M.; Ren, G.; Zhang, Y.; Goodwill, P.; Mansfield, J.; Marzola, P.; Wintermark, M. Preliminary Results: Imaging of in Situ Labeled Tumor-Associated Macrophages with Magnetic Particle Imaging. Int. J. Magn. Part. Imaging 2020, 6 (Suppl. 1), 2009029. [Google Scholar] [CrossRef]
- Gaudet, J.; Mansfield, J.; Goodwill, P. Imaging Cancer Immunology: Tracking Immune Cells in Vivo with Magnetic Particle Imaging. J. Immunol. 2019, 202, 130.7. [Google Scholar] [CrossRef]
- Rivera-Rodriguez, A.; Hoang-Minh, L.B.; Chiu-Lam, A.; Sarna, N.; Marrero-Morales, L.; Mitchell, D.A.; Rinaldi-Ramos, C.M. Tracking Adoptive T Cell Immunotherapy Using Magnetic Particle Imaging. Nanotheranostics 2021, 5, 431–444. [Google Scholar] [CrossRef]
- Gevaert, J.J.; Fink, C.; Dikeakos, J.D.; Dekaban, G.A.; Foster, P.J. Magnetic Particle Imaging Is a Sensitive In Vivo Imaging Modality for the Detection of Dendritic Cell Migration. Mol. Imaging Biol. 2022, 24, 886–897. [Google Scholar] [CrossRef]
- Lefevre, S.; Ruimy, D.; Jehl, F.; Neuville, A.; Robert, P.; Sordet, C.; Ehlinger, M.; Dietemann, J.-L.; Bierry, G. Septic Arthritis: Monitoring with USPIO-Enhanced Macrophage MR Imaging. Radiology 2011, 258, 722–728. [Google Scholar] [CrossRef]
- Ruehm, S.G.; Corot, C.; Vogt, P.; Kolb, S.; Debatin, J.F. Magnetic Resonance Imaging of Atherosclerotic Plaque With Ultrasmall Superparamagnetic Particles of Iron Oxide in Hyperlipidemic Rabbits. Circulation 2001, 103, 415–422. [Google Scholar] [CrossRef]
- Tong, W.; Hui, H.; Shang, W.; Zhang, Y.; Tian, F.; Ma, Q.; Yang, X.; Tian, J.; Chen, Y. Highly Sensitive Magnetic Particle Imaging of Vulnerable Atherosclerotic Plaque with Active Myeloperoxidase-Targeted Nanoparticles. Theranostics 2021, 11, 506–521. [Google Scholar] [CrossRef]
- Chandrasekharan, P.; Fung, K.L.B.; Zhou, X.Y.; Cui, W.; Colson, C.; Mai, D.; Jeffris, K.; Huynh, Q.; Saayujya, C.; Kabuli, L.; et al. Non-Radioactive and Sensitive Tracking of Neutrophils towards Inflammation Using Antibody Functionalized Magnetic Particle Imaging Tracers. Nanotheranostics 2021, 5, 240–255. [Google Scholar] [CrossRef]
- Remmo, A.; Löwa, N.; Kosch, O.; Eberbeck, D.; Ludwig, A.; Kampen, L.; Grüttner, C.; Wiekhorst, F. Cell Tracking by Magnetic Particle Imaging: Methodology for Labeling THP-1 Monocytes with Magnetic Nanoparticles for Cellular Imaging. Cells 2022, 11, 2892. [Google Scholar] [CrossRef] [PubMed]
- Mangarova, D.B.; Brangsch, J.; Mohtashamdolatshahi, A.; Kosch, O.; Paysen, H.; Wiekhorst, F.; Klopfleisch, R.; Buchholz, R.; Karst, U.; Taupitz, M.; et al. Ex Vivo Magnetic Particle Imaging of Vascular Inflammation in Abdominal Aortic Aneurysm in a Murine Model. Sci. Rep. 2020, 10, 12410. [Google Scholar] [CrossRef]
- Alzahrani, E.O.; Asiri, A.; El-Dessoky, M.M.; Kuang, Y. Quiescence as an Explanation of Gompertzian Tumor Growth Revisited. Math. Biosci. 2014, 254, 76–82. [Google Scholar] [CrossRef]
- Lin, Z.; Ding, J.; Sun, G.; Li, D.; He, S.; Liang, X.; Huang, X.; Xie, J. Application of Paclitaxel-Loaded EGFR Peptide-Conjugated Magnetic Polymeric Liposomes for Liver Cancer Therapy. Curr. Med. Sci. 2020, 40, 145–154. [Google Scholar] [CrossRef]
- Yu, E.Y.; Bishop, M.; Zheng, B.; Ferguson, R.M.; Khandhar, A.P.; Kemp, S.J.; Krishnan, K.M.; Goodwill, P.W.; Conolly, S.M. Magnetic Particle Imaging: A Novel in Vivo Imaging Platform for Cancer Detection. Nano Lett. 2017, 17, 1648–1654. [Google Scholar] [CrossRef]
- Meng, L.; Ma, X.; Jiang, S.; Ji, G.; Han, W.; Xu, B.; Tian, J.; Tian, W. High-Efficiency Fluorescent and Magnetic Multimodal Probe for Long-Term Monitoring and Deep Penetration Imaging of Tumors. J. Mater. Chem. B 2019, 7, 5345–5351. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Shang, W.; Min, X.; Weinreb, J.; Li, Q.; Leapman, M.; Wang, L.; Tian, J. Sight and Switch off: Nerve Density Visualization for Interventions Targeting Nerves in Prostate Cancer. Sci. Adv. 2020, 6, eaax6040. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Gai, L.; Zhang, Q.; Kang, Y.; Liu, Z.; He, Y.; Liu, W.; Jiang, T.; Du, Z.; Du, S.; et al. Development of a Human-Size Magnetic Particle Imaging Device for Sentinel Lymph Node Biopsy of Breast Cancer. Front. Bioeng. Biotechnol. 2024, 12, 1327521. [Google Scholar] [CrossRef]
- Yang, C.-W.; Liu, K.; Yao, C.-Y.; Li, B.; Juhong, A.; Ullah, A.K.M.A.; Bumpers, H.; Qiu, Z.; Huang, X. Active Targeting Hyaluronan Conjugated Nanoprobe for Magnetic Particle Imaging and Near-Infrared Fluorescence Imaging of Breast Cancer and Lung Metastasis. ACS Appl. Mater. Interfaces 2024, 16, 27055–27064. [Google Scholar] [CrossRef]
- Tomitaka, A.; Arami, H.; Gandhi, S.; Krishnan, K.M. Lactoferrin Conjugated Iron Oxide Nanoparticles for Targeting Brain Glioma Cells in Magnetic Particle Imaging. Nanoscale 2015, 7, 16890–16898. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hui, H.; Shang, W.; Gao, P.; Zhou, Y.; Pang, W.; Woo, C.M.; Tian, J.; Lai, P. Deep Penetrating and Sensitive Targeted Magnetic Particle Imaging and Photothermal Therapy of Early-Stage Glioblastoma Based on a Biomimetic Nanoplatform. Adv. Sci. 2023, 10, 2300854. [Google Scholar] [CrossRef] [PubMed]
- Toomajian, V.A.; Tundo, A.; Ural, E.E.; Greeson, E.M.; Contag, C.H.; Makela, A.V. Magnetic Particle Imaging Reveals That Iron-Labeled Extracellular Vesicles Accumulate in Brains of Mice with Metastases. ACS Appl. Mater. Interfaces 2024, 16, 30860–30873. [Google Scholar] [CrossRef] [PubMed]
- Fontes De Paula Aguiar, M.; Bustamante Mamani, J.; Klei Felix, T.; Ferreira Dos Reis, R.; Rodrigues Da Silva, H.; Nucci, L.P.; Nucci-da-Silva, M.P.; Gamarra, L.F. Magnetic Targeting with Superparamagnetic Iron Oxide Nanoparticles for in Vivo Glioma. Nanotechnol. Rev. 2017, 6, 449–472. [Google Scholar] [CrossRef]
- Shakeri-Zadeh, A.; Bulte, J.W.M. Imaging-Guided Precision Hyperthermia with Magnetic Nanoparticles. Nat. Rev. Bioeng. 2024, 3, 245–260. [Google Scholar] [CrossRef]
- Salamon, J.; Dieckhoff, J.; Kaul, M.G.; Jung, C.; Adam, G.; Möddel, M.; Knopp, T.; Draack, S.; Ludwig, F.; Ittrich, H. Visualization of Spatial and Temporal Temperature Distributions with Magnetic Particle Imaging for Liver Tumor Ablation Therapy. Sci. Rep. 2020, 10, 7480. [Google Scholar] [CrossRef]
- Zhang, S.-S.; Li, R.-Q.; Chen, Z.; Wang, X.-Y.; Dumont, A.S.; Fan, X. Immune Cells: Potential Carriers or Agents for Drug Delivery to the Central Nervous System. Mil. Med. Res. 2024, 11, 19. [Google Scholar] [CrossRef]
- Shaghaghi, B.; Khoee, S.; Bonakdar, S. Preparation of Multifunctional Janus Nanoparticles on the Basis of SPIONs as Targeted Drug Delivery System. Int. J. Pharm. 2019, 559, 1–12. [Google Scholar] [CrossRef]
- Fuller, E.G.; Scheutz, G.M.; Jimenez, A.; Lewis, P.; Savliwala, S.; Liu, S.; Sumerlin, B.S.; Rinaldi, C. Theranostic Nanocarriers Combining High Drug Loading and Magnetic Particle Imaging. Int. J. Pharm. 2019, 572, 118796. [Google Scholar] [CrossRef]
- Tomitaka, A.; Arami, H.; Ahmadivand, A.; Pala, N.; McGoron, A.J.; Takemura, Y.; Febo, M.; Nair, M. Magneto-Plasmonic Nanostars for Image-Guided and NIR-Triggered Drug Delivery. Sci. Rep. 2020, 10, 10115. [Google Scholar] [CrossRef]
- Jia, G.; Han, Y.; An, Y.; Ding, Y.; He, C.; Wang, X.; Tang, Q. NRP-1 Targeted and Cargo-Loaded Exosomes Facilitate Simultaneous Imaging and Therapy of Glioma in Vitro and in Vivo. Biomaterials 2018, 178, 302–316. [Google Scholar] [CrossRef]
- Jung, K.O.; Jo, H.; Yu, J.H.; Gambhir, S.S.; Pratx, G. Development and MPI Tracking of Novel Hypoxia-Targeted Theranostic Exosomes. Biomaterials 2018, 177, 139–148. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, G.; Ma, X.; Zhang, Y.; Chen, S.; Tiwari, S.; Shi, K.; et al. Comprehensive Understanding of Magnetic Hyperthermia for Improving Antitumor Therapeutic Efficacy. Theranostics 2020, 10, 3793–3815. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, O.; Sajjamark, K.; Franke, J.; Wei, H.; Behrends, A.; Münkel, C.; Grüttner, C.; Levan, P.; Von Elverfeldt, D.; Graeser, M.; et al. In Situ Theranostic Platform Combining Highly Localized Magnetic Fluid Hyperthermia, Magnetic Particle Imaging, and Thermometry in 3D. Theranostics 2024, 14, 324–340. [Google Scholar] [CrossRef]
- Bleul, R.; Baki, A.; Freese, C.; Paysen, H.; Kosch, O.; Wiekhorst, F. Continuously Manufactured Single-Core Iron Oxide Nanoparticles for Cancer Theranostics as Valuable Contribution in Translational Research. Nanoscale Adv. 2020, 2, 4510–4521. [Google Scholar] [CrossRef]
- Du, Y.; Liu, X.; Liang, Q.; Liang, X.-J.; Tian, J. Optimization and Design of Magnetic Ferrite Nanoparticles with Uniform Tumor Distribution for Highly Sensitive MRI/MPI Performance and Improved Magnetic Hyperthermia Therapy. Nano Lett. 2019, 19, 3618–3626. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Scholz, R.; Wust, P.; Schirra, H.; Schiestel, T.; Schmidt, H.; Felix, R. Endocytosis of Dextran and Silan-Coated Magnetite Nanoparticles and the Effect of Intracellular Hyperthermia on Human Mammary Carcinoma Cells in Vitro. J. Magn. Magn. Mater. 1999, 194, 185–196. [Google Scholar] [CrossRef]
- Liu, X.L.; Fan, H.M.; Yi, J.B.; Yang, Y.; Choo, E.S.G.; Xue, J.M.; Fan, D.D.; Ding, J. Optimization of Surface Coating on Fe3O4 Nanoparticles for High Performance Magnetic Hyperthermia Agents. J. Mater. Chem. 2012, 22, 8235. [Google Scholar] [CrossRef]
- Khurshid, H.; Alonso, J.; Nemati, Z.; Phan, M.H.; Mukherjee, P.; Fdez-Gubieda, M.L.; Barandiarán, J.M.; Srikanth, H. Anisotropy Effects in Magnetic Hyperthermia: A Comparison between Spherical and Cubic Exchange-Coupled FeO/Fe3O4 Nanoparticles. J. Appl. Phys. 2015, 117, 17A337. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of Nanoparticle Delivery to Tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Guardia, P.; Di Corato, R.; Lartigue, L.; Wilhelm, C.; Espinosa, A.; Garcia-Hernandez, M.; Gazeau, F.; Manna, L.; Pellegrino, T. Water-Soluble Iron Oxide Nanocubes with High Values of Specific Absorption Rate for Cancer Cell Hyperthermia Treatment. ACS Nano 2012, 6, 3080–3091. [Google Scholar] [CrossRef]
- Liu, X.L.; Yang, Y.; Ng, C.T.; Zhao, L.Y.; Zhang, Y.; Bay, B.H.; Fan, H.M.; Ding, J. Magnetic Vortex Nanorings: A New Class of Hyperthermia Agent for Highly Efficient In Vivo Regression of Tumors. Adv. Mater. 2015, 27, 1939–1944. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Jang, J.; Choi, J.; Moon, S.H.; Noh, S.; Kim, J.; Kim, J.-G.; Kim, I.-S.; Park, K.I.; Cheon, J. Exchange-Coupled Magnetic Nanoparticles for Efficient Heat Induction. Nat. Nanotechnol. 2011, 6, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Boubeta, C.; Simeonidis, K.; Makridis, A.; Angelakeris, M.; Iglesias, O.; Guardia, P.; Cabot, A.; Yedra, L.; Estradé, S.; Peiró, F.; et al. Learning from Nature to Improve the Heat Generation of Iron-Oxide Nanoparticles for Magnetic Hyperthermia Applications. Sci. Rep. 2013, 3, 1652. [Google Scholar] [CrossRef] [PubMed]
- Serantes, D.; Simeonidis, K.; Angelakeris, M.; Chubykalo-Fesenko, O.; Marciello, M.; Morales, M.D.P.; Baldomir, D.; Martinez-Boubeta, C. Multiplying Magnetic Hyperthermia Response by Nanoparticle Assembling. J. Phys. Chem. C 2014, 118, 5927–5934. [Google Scholar] [CrossRef]
- Gandia, D.; Gandarias, L.; Rodrigo, I.; Robles-García, J.; Das, R.; Garaio, E.; García, J.Á.; Phan, M.; Srikanth, H.; Orue, I.; et al. Unlocking the Potential of Magnetotactic Bacteria as Magnetic Hyperthermia Agents. Small 2019, 15, 1902626. [Google Scholar] [CrossRef]
- Mason, E.E.; Mattingly, E.; Herb, K.; Śliwiak, M.; Franconi, S.; Cooley, C.Z.; Slanetz, P.J.; Wald, L.L. Concept for Using Magnetic Particle Imaging for Intraoperative Margin Analysis in Breast-Conserving Surgery. Sci. Rep. 2021, 11, 13456. [Google Scholar] [CrossRef]
- Azargoshasb, S.; Molenaar, L.; Rosiello, G.; Buckle, T.; Van Willigen, D.M.; Van De Loosdrecht, M.M.; Welling, M.M.; Alic, L.; Van Leeuwen, F.W.B.; Winter, A.; et al. Advancing Intraoperative Magnetic Tracing Using 3D Freehand Magnetic Particle Imaging. Int. J. Comput. Assist. Radiol. Surg. 2022, 17, 211–218. [Google Scholar] [CrossRef]
- Liu, J.F.; Neel, N.; Dang, P.; Lamb, M.; McKenna, J.; Rodgers, L.; Litt, B.; Cheng, Z.; Tsourkas, A.; Issadore, D. Radiofrequency-Triggered Drug Release from Nanoliposomes with Millimeter-Scale Resolution Using a Superimposed Static Gating Field. Small 2018, 14, 1802563. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, S.; Shimada, K.; Enmeiji, K.; Murase, K. Development of Magnetic Nanocarriers Based on Thermosensitive Liposomes and Their Visualization Using Magnetic Particle Imaging. Int. J. Nanomed. Nanosurg. 2016, 2. [Google Scholar] [CrossRef]
- Rost, N.C.V.; Sen, K.; Savliwala, S.; Singh, I.; Liu, S.; Unni, M.; Raniero, L.; Rinaldi, C. Magnetic Particle Imaging Performance of Liposomes Encapsulating Iron Oxide Nanoparticles. J. Magn. Magn. Mater. 2020, 504, 166675. [Google Scholar] [CrossRef]
- Fuller, E.G.; Sun, H.; Dhavalikar, R.D.; Unni, M.; Scheutz, G.M.; Sumerlin, B.S.; Rinaldi, C. Externally Triggered Heat and Drug Release from Magnetically Controlled Nanocarriers. ACS Appl. Polym. Mater. 2019, 1, 211–220. [Google Scholar] [CrossRef]
- Nigam, S.; Gjelaj, E.; Wang, R.; Wei, G.; Wang, P. Machine Learning and Deep Learning Applications in Magnetic Particle Imaging. Magn. Reson. Imaging 2025, 61, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Hayat, H.; Sun, A.; Hayat, H.; Liu, S.; Talebloo, N.; Pinger, C.; Bishop, J.O.; Gudi, M.; Dwan, B.F.; Ma, X.; et al. Artificial Intelligence Analysis of Magnetic Particle Imaging for Islet Transplantation in a Mouse Model. Mol. Imaging Biol. 2021, 23, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.; Hayat, H.; Liu, S.; Tull, E.; Bishop, J.O.; Dwan, B.F.; Gudi, M.; Talebloo, N.; Dizon, J.R.; Li, W.; et al. 3D in Vivo Magnetic Particle Imaging of Human Stem Cell-Derived Islet Organoid Transplantation Using a Machine Learning Algorithm. Front. Cell Dev. Biol. 2021, 9, 704483. [Google Scholar] [CrossRef]
- Dittmer, S.; Kluth, T.; Henriksen, M.T.R.; Maass, P. Deep Image Prior for 3D Magnetic Particle Imaging: A Quantitative Comparison of Regularization Techniques on Open MPI Dataset. arXiv 2020, arXiv:2007.01593. [Google Scholar] [CrossRef]
- Yin, L.; Guo, H.; Zhang, P.; Li, Y.; Hui, H.; Du, Y.; Tian, J. System Matrix Recovery Based on Deep Image Prior in Magnetic Particle Imaging. Phys. Med. Biol. 2023, 68, 035006. [Google Scholar] [CrossRef]
- Dittmer, S.; Kluth, T.; Baguer, D.O.; Maass, P. A Deep Prior Approach to Magnetic Particle Imaging. In Machine Learning for Medical Image Reconstruction; Deeba, F., Johnson, P., Würfl, T., Ye, J.C., Eds.; Lecture Notes in Computer Science; Springer International Publishing: Cham, Switzerland, 2020; Volume 12450, pp. 113–122. ISBN 978-3-030-61597-0. [Google Scholar]
- Gungor, A.; Askin, B.; Soydan, D.A.; Baris Top, C.; Cukur, T. Deep Learned Super Resolution of System Matrices for Magnetic Particle Imaging. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Guadalajara, Mexico, 1–5 November 2021; IEEE: New York, NY, USA; pp. 3749–3752. [Google Scholar]
- Malhotra, N.; Lee, J.-S.; Liman, R.A.D.; Ruallo, J.M.S.; Villaflores, O.B.; Ger, T.-R.; Hsiao, C.-D. Potential Toxicity of Iron Oxide Magnetic Nanoparticles: A Review. Molecules 2020, 25, 3159. [Google Scholar] [CrossRef]
- Singh, N.; Jenkins, G.J.S.; Asadi, R.; Doak, S.H. Potential Toxicity of Superparamagnetic Iron Oxide Nanoparticles (SPION). Nano Rev. 2010, 1, 5358. [Google Scholar] [CrossRef]
- Saritas, E.U.; Goodwill, P.W.; Zhang, G.Z.; Conolly, S.M. Magnetostimulation Limits in Magnetic Particle Imaging. IEEE Trans. Med. Imaging 2013, 32, 1600–1610. [Google Scholar] [CrossRef]
- Aggarwal, P.; Hall, J.B.; McLeland, C.B.; Dobrovolskaia, M.A.; McNeil, S.E. Nanoparticle Interaction with Plasma Proteins as It Relates to Particle Biodistribution, Biocompatibility and Therapeutic Efficacy. Adv. Drug Deliv. Rev. 2009, 61, 428–437. [Google Scholar] [CrossRef]
- Zamay, G.S.; Zamay, T.N.; Lukyanenko, K.A.; Kichkailo, A.S. Aptamers Increase Biocompatibility and Reduce the Toxicity of Magnetic Nanoparticles Used in Biomedicine. Biomedicines 2020, 8, 59. [Google Scholar] [CrossRef]
- Blesia, V.; Patel, V.B.; Al-Obaidi, H.; Renshaw, D.; Zariwala, M.G. Excessive Iron Induces Oxidative Stress Promoting Cellular Perturbations and Insulin Secretory Dysfunction in MIN6 Beta Cells. Cells 2021, 10, 1141. [Google Scholar] [CrossRef]
- Walkey, C.D.; Olsen, J.B.; Guo, H.; Emili, A.; Chan, W.C.W. Nanoparticle Size and Surface Chemistry Determine Serum Protein Adsorption and Macrophage Uptake. J. Am. Chem. Soc. 2012, 134, 2139–2147. [Google Scholar] [CrossRef]
- Pan, Y.; Du, X.; Zhao, F.; Xu, B. Magnetic Nanoparticles for the Manipulation of Proteins and Cells. Chem. Soc. Rev. 2012, 41, 2912. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Tarasevych, A.V.; Kukhar, V.P.; Boukherroub, R.; Szunerits, S. Recent Advances in Surface Chemistry Strategies for the Fabrication of Functional Iron Oxide Based Magnetic Nanoparticles. Nanoscale 2013, 5, 10729. [Google Scholar] [CrossRef] [PubMed]
- Manoj, G.M.; Shalini, M.; Thenmozhi, K.; Ponnusamy, V.K.; Hari, S. Recent Advancements in the Surface Modification and Functionalization of Magnetic Nanomaterials. Appl. Surf. Sci. Adv. 2024, 21, 100608. [Google Scholar] [CrossRef]
- Belyanina, I.V.; Zamay, T.N.; Zamay, G.S.; Zamay, S.S.; Kolovskaya, O.S.; Ivanchenko, T.I.; Denisenko, V.V.; Kirichenko, A.K.; Glazyrin, Y.E.; Garanzha, I.V.; et al. In Vivo Cancer Cells Elimination Guided by Aptamer-Functionalized Gold-Coated Magnetic Nanoparticles and Controlled with Low Frequency Alternating Magnetic Field. Theranostics 2017, 7, 3326–3337. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, H.; Salehiabar, M.; Fridoni, M.; Abdollahifar, M.-A.; Kheiri Manjili, H.; Davaran, S.; Danafar, H. New Insight about Biocompatibility and Biodegradability of Iron Oxide Magnetic Nanoparticles: Stereological and In Vivo MRI Monitor. Sci. Rep. 2019, 9, 7173. [Google Scholar] [CrossRef] [PubMed]
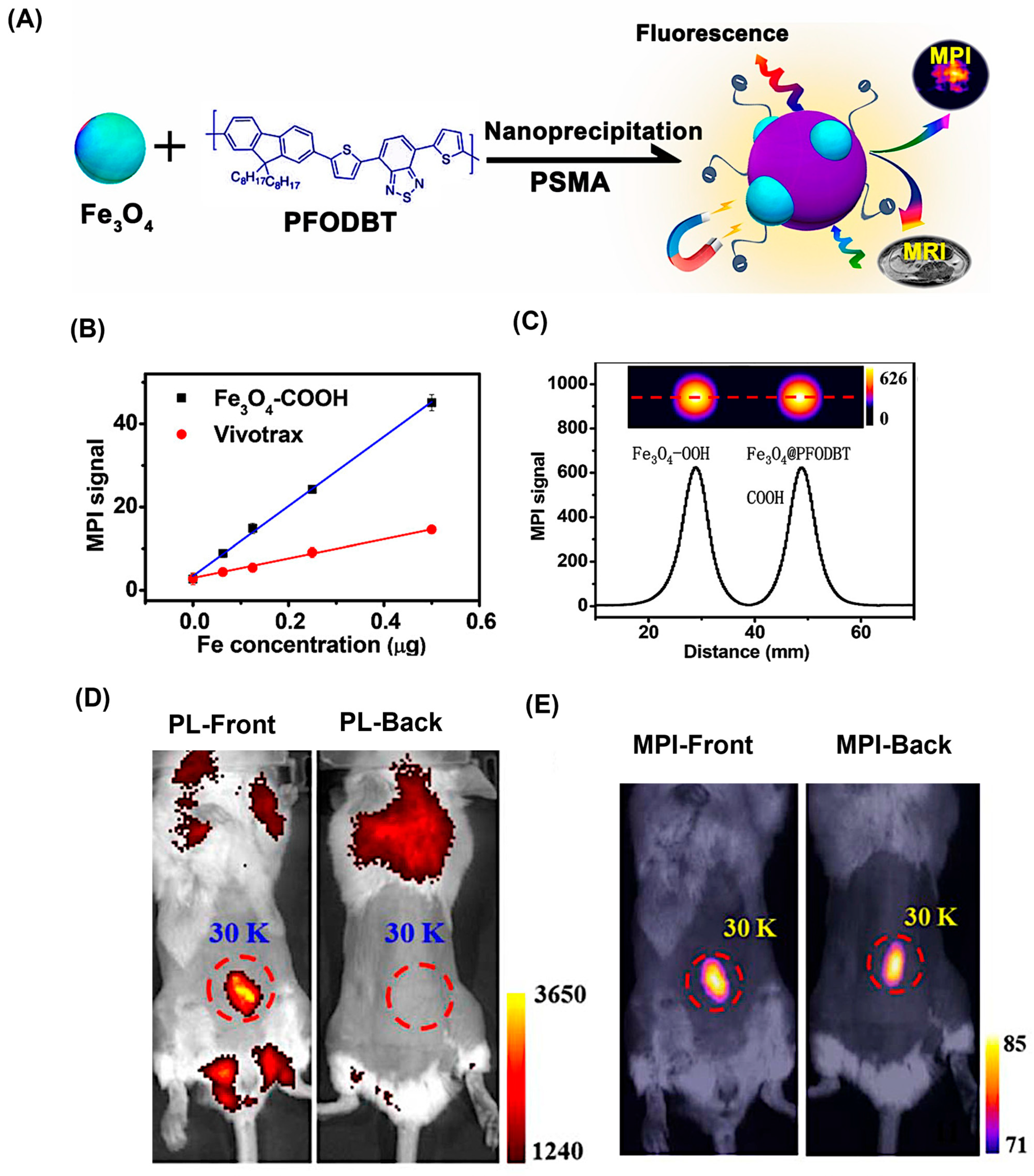
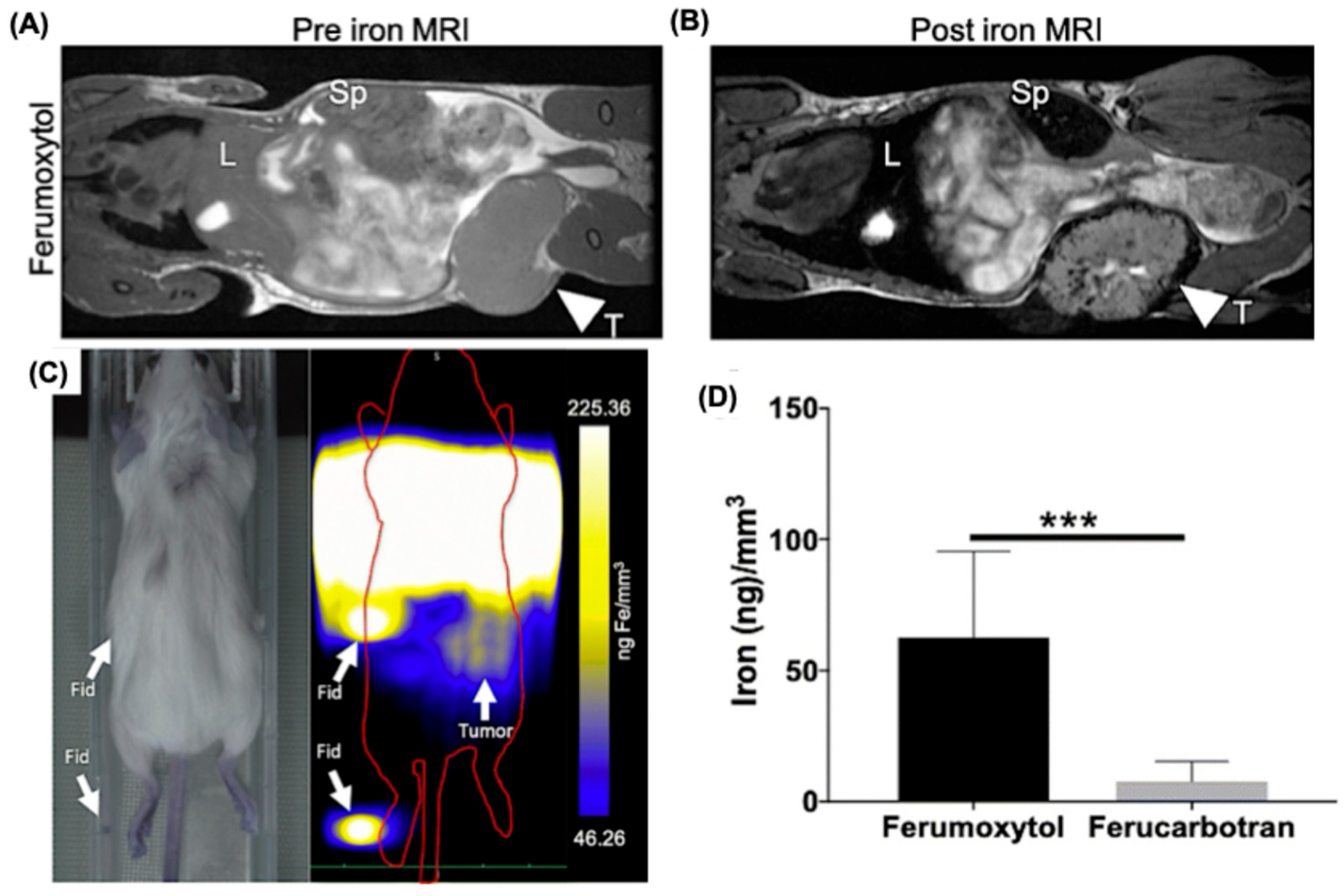

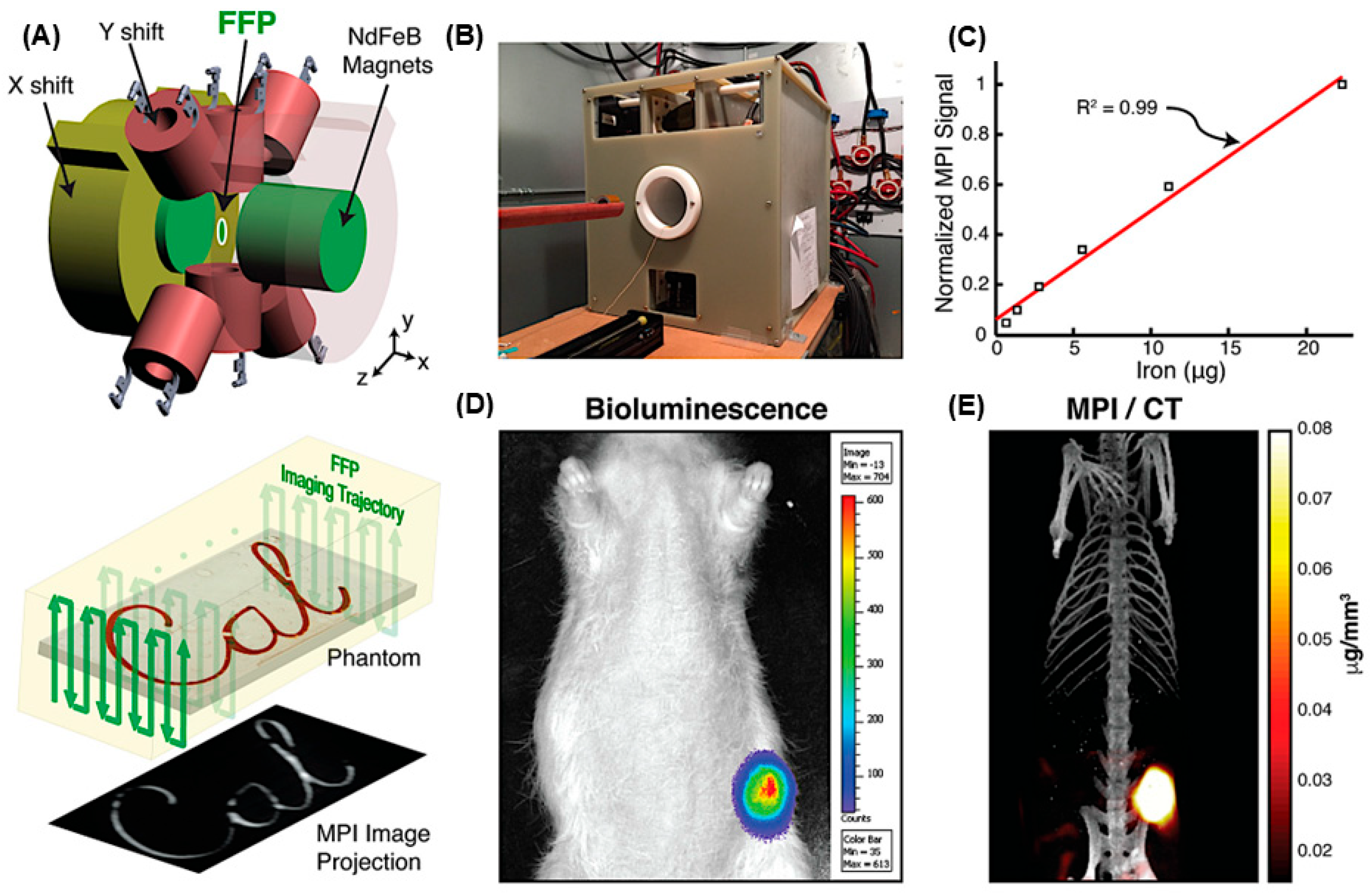
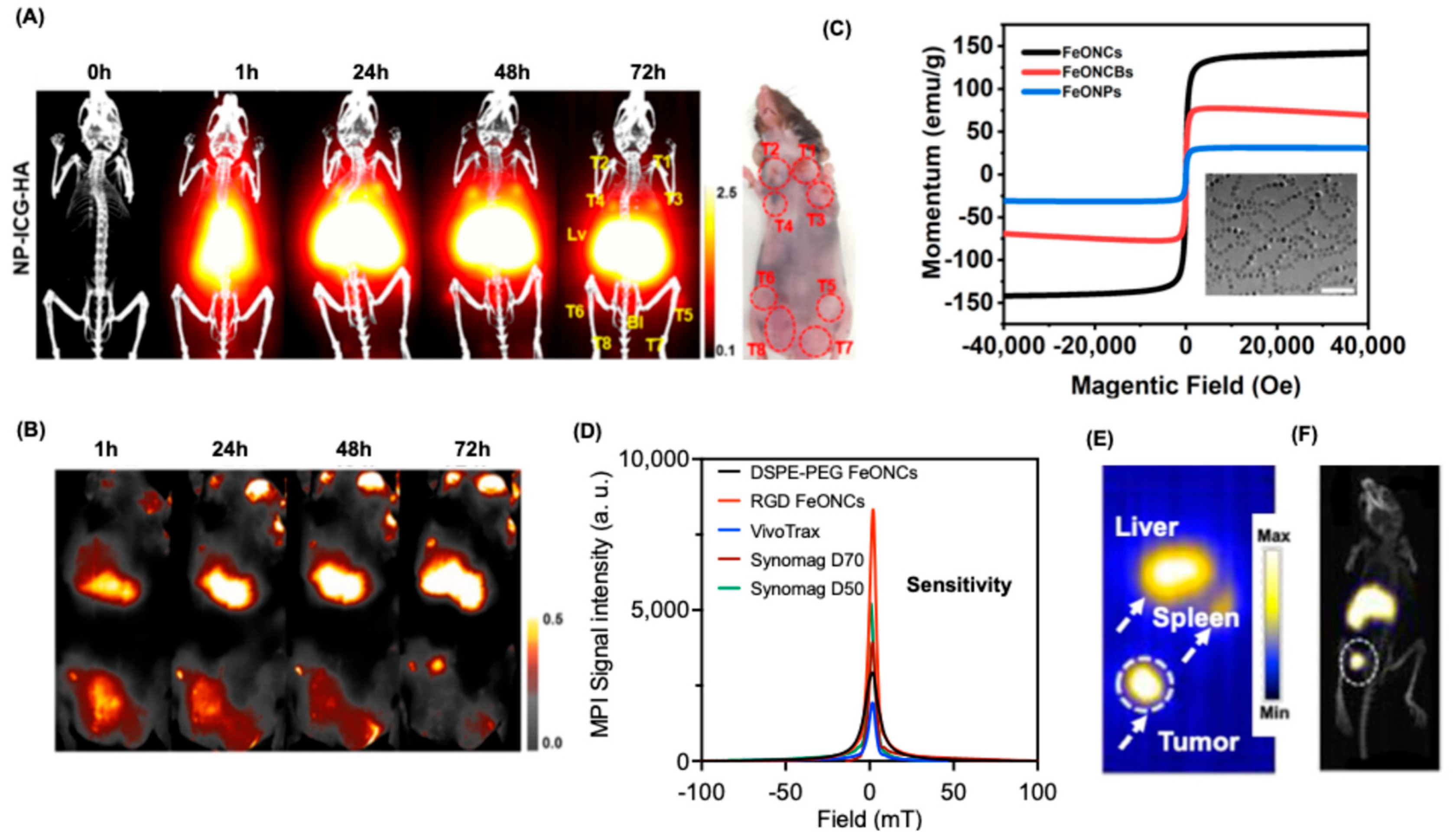

| Modality | Ultrasound | CT | MRI | PET | SPECT | MPI |
|---|---|---|---|---|---|---|
| Main clinical applications | Structural imaging | Structural imaging | Structural imaging | Tracer imaging | Tracer imaging | Tracer imaging |
| Spatial resolution | 1 mm | <1 mm | 1 mm | 4 mm | 3–10 mm | 1 mm |
| Temporal resolution | <1 s | Seconds | Seconds to hours | Minutes | Minutes | <1 s to minutes |
| Contrast agents/tracers | Microbubbles | Iodine | Gadolinium, iron oxide particles | Radioactive tracers | Radioactive tracers | Iron oxide particles |
| Sensitivity | Low | Low | Low | High | High | High |
| Patient risk | Heating and cavitation | Radiation | Heating and peripheral nerve stimulation | Radiation | Radiation | Heating and peripheral nerve stimulation |
| Cost | Low | Medium | High | High | Medium | Medium |
| Tracer | Manufacturer | Core Material | D (nm) | DH (nm) | FWHM | Sensitivity | System Used | Frequency and Gradient | Key Features | Application | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Resovist | Bayer Schering Pharma (Discontinued) | SPION coated with carboxydextran | 4.2–5 | 60–100 | 9.6 mT | 13.74 (mV/mgFe) | MPR | 20 kHz, 20 mT | First-generation commercial MPI tracer, iron-based, good biocompatibility | Liver imaging, preclinical studies | [22,51] |
| VivoTrax | Magnetic Insight | SPION coated with dextran | 4.2 | 62 | 11.4 mT | 8.83 (mV/mgFe) | MPR | 20 kHz, 20 mT | Optimized for preclinical MPI, high signal-to-noise ratio, good stability. | Preclinical research, cell tracking | [22] |
| VivoTrax+ | Magnetic Insight | SPIO dextran magnetite | 6 | 62 | 7.9 mT | 2.4x VivoTrax | MOMENTUM | n.r | Better resolution and sensitivity; Quantitative results for MPI. | Preclinical research | [47] |
| Ferucarbotran (Endorem) | Guerbet (Discontinued) | SPION coated with carboxydextran | 9.6 | 80–100 | 11.2 mT | 25.8 (a.u./mgFe) | MOMENTUM | 45 kHz, 16 mT, 5.7 T/m | Dextran-coated, used for MRI and studied for MPI. | Liver imaging, preclinical studies | [41] |
| Synomag-D | micromod Partikeltechnologie GmbH | Dextran maghemite | 5 nm | 50–80 | 9.2 mT | 87.8 (a.u./mgFe) | MOMENTUM | 45 kHz, 16 mT, 5.7 T/m | Dextran coating enhances biocompatibility, good colloidal stability. | Preclinical research, drug delivery | [41,49] |
| Feraheme (Ferumoxytol) | AMAG Pharmaceuticals | Magnetite coated with polyglucose sorbitol carboxymethylether | 3.25 | 17–31 | 39.5 mT | 2.12 (mV/mgFe) | MPR | 20 kHz, 20 mT | (MRI) results for up to three months after administration | Chronic kidney disease | [22,52] |
| PrecisionMRX | Imagion Biosystems | Magnetite with either mPEG or oleic acid or PEG-Carboxylic acid | 24.4 | 30–33 or 40–50 | 12.4 mT | 13.89 (mV/mgFe) | MPR | 20 kHz, 20 mT | Spherical morphology, narrow size dispersity, and high magnetic relaxivity | MRI, MPI, MFH, and drug delivery | [22] |
| Perimag | micromod Partikeltechnologie GmbH | dextran-coated SPIONS | 19 | 130 | 7.3 mT | 29.49 (mV/mgFe) | MPR | 20 kHz, 20 mT | Versatile, often used as a base for further functionalization | Preclinical, in vitro assays | [22] |
| MHT Agent | Characteristics | SAR (W/g) | Ref. |
|---|---|---|---|
| Iron oxide nanocubes | Size: 19 ± 3 nm; Msat = 80 emu/g; applied field: 29,000 A/m, 520 kHz | 2452 | [135] |
| Magnetic vortex nanorings | Inner/outer diameter: 42/70 nm; thickness: 50 nm; K1 = 1.35 × 105 erg/cm3; Msat = 77 emu/g; applied field: 64,000 A/m, 400 kHz | ~3000 | [136] |
| Core–shell ZnCoFe2O4@ZnMnFe2O4 nanoparticles | Size: 15 nm; K = 1.5 × 104 J/m3; Msat = 125 emu/g; applied field: 37,000 A/m, 500 kHz | 3886 | [137] |
| Magnetite nanoparticle chains | Particle size: 44 nm (σ = 0.17); Msat = 87 emu/g; 4.3-fold SAR increase due to dipolar chaining | – | [139] |
| Magnetotactic bacteria (M. gryphiswaldense) | Chain length: ~1 µm; particle size: 45 nm; Msat ≈ 90 emu/g; applied field: 28,000 A/m, 300 kHz | 2400 | [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, P.P.P. Magnetic Particle Imaging in Oncology: Advances and Prospects for Tumor Progression Monitoring and Targeted Therapy. J. Nanotheranostics 2025, 6, 32. https://doi.org/10.3390/jnt6040032
Kumar PPP. Magnetic Particle Imaging in Oncology: Advances and Prospects for Tumor Progression Monitoring and Targeted Therapy. Journal of Nanotheranostics. 2025; 6(4):32. https://doi.org/10.3390/jnt6040032
Chicago/Turabian StyleKumar, Panangattukara Prabhakaran Praveen. 2025. "Magnetic Particle Imaging in Oncology: Advances and Prospects for Tumor Progression Monitoring and Targeted Therapy" Journal of Nanotheranostics 6, no. 4: 32. https://doi.org/10.3390/jnt6040032
APA StyleKumar, P. P. P. (2025). Magnetic Particle Imaging in Oncology: Advances and Prospects for Tumor Progression Monitoring and Targeted Therapy. Journal of Nanotheranostics, 6(4), 32. https://doi.org/10.3390/jnt6040032







