Abstract
Background: Cystic artery bleeding (CAB) is a rare but potentially life-threatening condition. Its etiologies span iatrogenic trauma, inflammatory diseases, and trauma, often presenting variably as hemoperitoneum, upper gastrointestinal bleeding, or hemorrhagic shock. The clinical heterogeneity of CAB complicates its diagnosis, necessitating a high index of suspicion and reliance on imaging modalities, particularly computed tomography (CT), for accurate identification of bleeding sources and differentiation from other causes of abdominal pain. Methods: This pictorial essay highlights key imaging findings in CAB and pseudoaneurysms, emphasizing the role of ultrasound, CT, and digital subtraction angiography (DSA) in diagnosis and management planning. Additionally, a systematic review of transcatheter arterial embolization (TAE) is presented, consolidating data from 64 studies encompassing 90 patients. Results: The review evaluates patient demographics, etiologies, clinical presentations, and procedural outcomes, underscoring TAE’s high efficacy and safety as a first-line treatment. Conclusions: The findings reinforce the importance of early diagnosis and tailored intervention strategies to optimize outcomes in CAB management.
1. Introduction
Cystic artery bleeding (CAB) is an exceedingly rare occurrence, with fewer than one hundred cases reported in non-traumatic settings and only a handful associated with traumatic conditions [1,2]. This rarity poses a significant challenge for including CAB in the differential diagnosis of acute abdominal or gastrointestinal presentations.
The etiology of CAB encompasses a spectrum of factors, including iatrogenic trauma (notably during cholecystectomy), inflammatory conditions such as acute cholecystitis or pancreatitis, and post-traumatic injuries [1,3]. Its clinical presentation is highly heterogeneous, primarily influenced by the underlying cause and the severity of the hemorrhage. Symptoms can range from intraperitoneal bleeding to upper gastrointestinal hemorrhage, including hemobilia (defined as blood within the biliary tree) and, in severe cases, hemorrhagic shock [1].
Due to its diverse manifestations, CAB often eludes prompt recognition, necessitating high clinical suspicion. Imaging, particularly computed tomography (CT), plays a pivotal role in identifying the bleeding source, characterizing the hemorrhage, and differentiating CAB from other potential causes of abdominal pain or gastrointestinal bleeding [1].
Management strategies for CAB vary depending on the clinical context and the etiology of the bleeding. Surgical intervention and conservative therapy remain important options; however, transcatheter arterial embolization (TAE) has emerged as a cornerstone in the treatment of CAB, especially in cases arising from blunt trauma, laparoscopic cholecystectomy complications, duodenal ulcers, cholecystitis, gallstone erosion, or idiopathic origins [1,2].
This paper aims to comprehensively review the imaging findings associated with cystic artery bleeding and pseudoaneurysms, and to conduct a systematic analysis of the existing literature on interventional treatment strategies for these conditions.
1.1. Cystic Artery Vascular Anatomy
Anatomical variations, reported in 31.9% of individuals, include anomalous courses, variable lengths, and multiple cystic arteries, highlighting the complexity of this vascular anatomy.
The cystic artery typically arises from the right hepatic artery, traversing from the posterior to the cystic duct within the hepatobiliary triangle (Calot’s triangle). This region, bordered by the liver, common hepatic duct, and cystic duct, contains the cystic artery, lymphatics, and connective tissue [4,5].
In 79% to 89% of individuals, the cystic artery arises from the right hepatic artery. However, it may also originate from other branches of the celiac axis, including a replaced or accessory right hepatic artery, the gastroduodenal artery, the left hepatic artery, or the superior mesenteric artery (Figure 1) [5,6,7]. As the cystic artery approaches the gallbladder, it typically divides into two branches: a larger anterior superficial branch that courses beneath the serosal layer on the left side of the gallbladder, and a smaller posterior deep branch that runs between the gallbladder and the gallbladder fossa, forming a vascular network around the gallbladder [5,8].
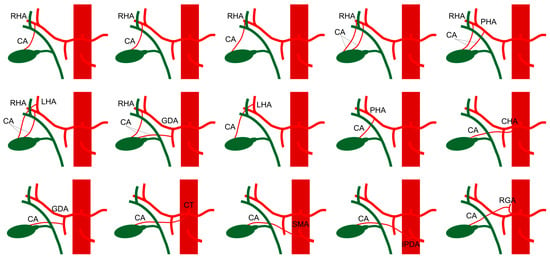
Figure 1.
Illustration of the most common variations of the cystic artery. Cystic artery (CA), right hepatic artery (RHA), left hepatic artery (LHA), proper hepatic artery (PHA), common hepatic artery (CHA), gastro-duodenal artery (GDA), superior mesenteric artery (SMA), celiac trunk (CT), inferior pancreatic-duodenal artery (IPDA), right gastric artery (RGA).
Anatomical variations, reported in 31.9% of individuals, include anomalous courses anterior to the common hepatic or bile ducts or inferior to the cystic duct. Additional variations include differences in the length of the cystic artery, with short or elongated courses, and the presence of multiple cystic arteries [5,9].
1.2. Etiology and Pathophysiology
CAB, including pseudoaneurysms (CAP), is a rare but clinically significant condition with multifactorial etiologies, including inflammatory, traumatic, and iatrogenic causes [10,11]. CAP results from localized arterial wall disruption, leading to rupture contained by the surrounding tissues [12].
Inflammatory processes, such as acute cholecystitis, weaken the arterial wall through erosion and thrombosis of the vasa vasorum, promoting pseudoaneurysm formation [12]. Chronic conditions, including gallstone-induced cholecystitis, exacerbate this damage, increasing the risk of rupture [8,10].
The cystic artery’s proximity to the gallbladder wall makes it particularly vulnerable in inflammatory states, often resulting in late-stage diagnosis [13].
Traumatic causes, accounting for 2% of blunt abdominal injuries, include shear forces that cause avulsion or laceration of the artery. Penetrating trauma, although less common, results in direct arterial damage and is often accompanied by liver or gallbladder injury (Figure 2, Figure 3 and Figure 4) [6].
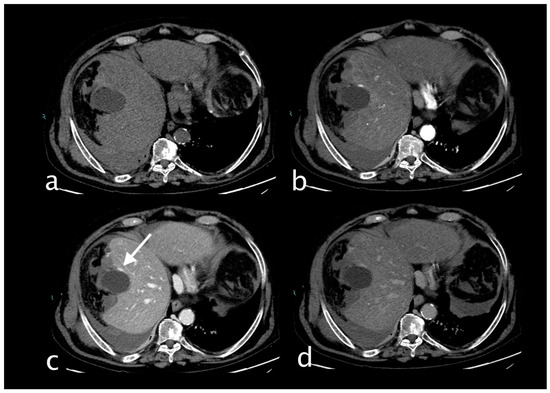
Figure 2.
Hemodynamically stable 78-year-old male who suffered motor vehicle accident (MVA) trauma (Hb 8 g/dL, n.v. 13–18; PCR 2.04 mg/dL, n.v. 0.0–0.5; WBC 7.66 103/mm3 n.v. 4.2–10.5). He underwent a CT with IV contrast ((a) non-contrast, (b) arterial, (c) venous, (d) delayed phase) that showed hepatic contusion and a conspicuous perihepatic and pericholecystic hematoma with cystic artery laceration and active bleeding ((c), white arrow), detected on the anterior gallbladder profile that increases in the portal (c) and delayed phases (d) particularly evident adjacent to a subcapsular (pre-existing) cyst. The patient underwent TAE successfully with super selective embolization of the anterior branch of the cystic artery (coils and PVA particles). The hepatic contusion was treated conservatively. Postoperatively, the patient was stable.
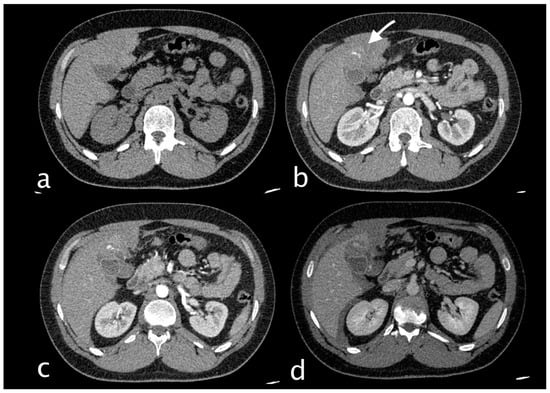
Figure 3.
Hemodynamically stable 30-year-old male who suffered MVA trauma (Hb 11.9 g/dL, n.v. 13–18; PCR 0.43 mg/dL, n.v. 0.0–0.5; WBC 15.19 103/mm3 n.v. 4.2–10.5). He underwent CT with IV contrast ((a) non-contrast, (b) arterial, (c) venous, (d) delayed phase) that showed subcapsular and pericholecystic hematoma, laceration with non-active bleeding of the fourth hepatic segment and cystic artery active bleeding ((b), white arrow) along the anterior gallbladder profile with conspicuous increase of contrast extravasation in portal (c) and delayed (d) phases.
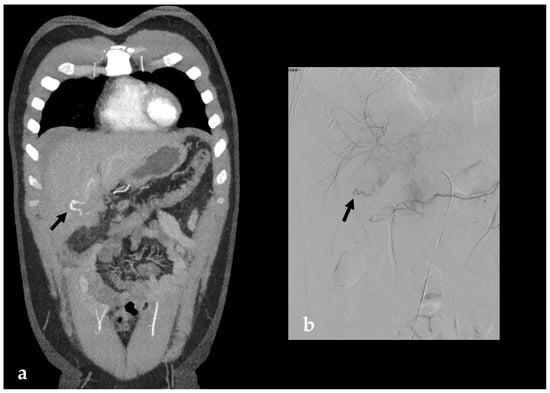
Figure 4.
Arterial phase coronal MPR CT images (a) and Digital Subtraction Angiography (DSA) (b) both showing cystic artery conspicuous bleeding. (b) DSA demonstrates the entity of the bleeding (black arrow). The patient underwent TAE successfully with super-selective embolization of the anterior branch of the cystic artery (coils and Gelfoam).
Iatrogenic injury, primarily from laparoscopic cholecystectomy, is the leading cause of CAB. Vascular damage during hepatobiliary triangle dissection, often due to diathermy, compounded by bile duct injury, delays healing and predisposes to CAP formation [14]. Histological studies reveal extensive loss of elastic and muscular fibers in CAPs, which increases susceptibility to rupture and life-threatening hemorrhagic events [15,16].
1.3. Clinical Manifestations
CAB presents with a wide range of symptoms, influenced by its etiology and the extent of the hemorrhage. Symptoms vary from mild abdominal discomfort to severe hemorrhagic shock, posing diagnostic challenges [11,17].
In traumatic cases, CAB often manifests as hemoperitoneum with abdominal pain, distension, and tenderness. Severe hemorrhage may lead to hypotension, tachycardia, and altered mental status, reflecting hemodynamic instability. Active intraperitoneal bleeding in blunt trauma frequently progresses rapidly to shock [6].
Non-traumatic and iatrogenic CAB, commonly associated with CAP, typically presents after pseudoaneurysm rupture. While Quincke’s triad—right upper quadrant pain, jaundice, and gastrointestinal bleeding—is observed in less than one-third of cases, abdominal pain (77.9%) and upper gastrointestinal bleeding (64.4%) are more frequent [10,18].
Severe CAP rupture may result in hemorrhagic shock, with 19.4% requiring emergency care [18]. Imaging often reveals biliary obstruction or hemoperitoneum, emphasizing the need for timely recognition and intervention [2,16,19].
2. Imaging
Imaging plays a pivotal role in diagnosing and managing cystic artery bleeding and pseudoaneurysms. Early and accurate identification of the bleeding source is critical for timely intervention. Modalities such as ultrasound (US), computed tomography (CT), and digital subtraction angiography (DSA) are employed based on clinical suspicion and resource availability [10,20].
FAST-US accurately detect hemoperitoneum in unstable polytraumatized patients [21,22]. In non-traumatic CAB, an ultrasound may detect hemoperitoneum [23] and cystic artery pseudoaneurysm, although few cases are reported in the literature in which the US detected the presence of macroaneurysms [24]. At B-Mode, cystic artery pseudoaneurysm appears as an anechoic cavitary lesion within the gallbladder wall. Color Doppler is mandatory to detect an arterial flow within the lesion suggestive of a pseudoaneurysm. In macroaneurysm, color Doppler US has the potential to visualize the characteristic “Yin-Yang” flow pattern and the “To and Fro” flow pattern in the spectral analysis within the pseudoaneurysm itself [24].
The pseudoaneurysm’s size and neck can be measured, but the communication between the sac and the originating artery is not easily visualized [25]. The gallbladder may appear distended and exhibit hyperechoic fluid for intraluminal blood accumulation. It is important to emphasize that although cystic artery pseudoaneurysms are rare, the low frequency with which they are incidentally detected by US, before their rupture and bleeding, may also be related to the lack of knowledge of this pathological entity [26].
Oesophagogastroduodenoscopy can detect hemorrhage in 60% of patients, and ERCP may detect hemobilia but cannot locate the source of bleeding [1].
For these reasons, multiphase CT represents the cornerstone modality for evaluating suspected cystic artery bleeding and the presence of pseudoaneurysm with high sensitivity, and provides additional insight into the underlying etiology. CT also plays a critical role in the planning of percutaneous treatment by enabling the identification of aberrant vasculature and anatomical variations [27].
In a non-enhanced CT, characteristically, one key finding is the presence of hyperdense fluid that embraces the gallbladder; this typical anatomical disposition of the hemoperitoneum should alert radiologists [28]. Following intravenous (IV) contrast administration, in the arterial phase, extravasation of contrast material may be evident within the gallbladder fossa, potentially along the course of the cystic artery’s anterior or posterior branches.
A CAP can be identified as a hyper-enhancing focus during the arterial phase along the cystic artery branches (Figure 5, Figure 6, Figure 7 and Figure 8). This finding demonstrates a change in attenuation but not morphology in the venous and delayed phases (Figure 9 and Figure 10). Because of the possible presence of pre-existing gallbladder stones or surgical clips from a previous cholecystectomy, images should be examined carefully in different CT phases to differentiate hyperdense material appearing in an angiographic phase that should not be present at the same location in non-contrast images. Delayed images can be useful in differentiating between active extravasation or CAP and relatively benign processes [1,28,29,30].
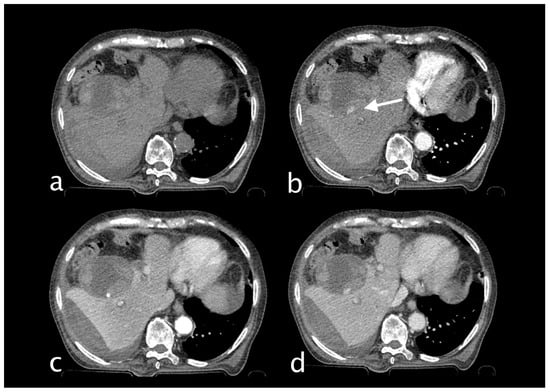
Figure 5.
82-year-old man hospitalized for pneumonia who suddenly complained of jaundice, right upper quadrant pain and sudden decline of Hb 7.1 g/dL (PRO B NP 39,473 pg/mL, n.v. < 375; PCR 9.9 mg/dL, n.v. 0.0–0.5; WBC 23.78 103/mm3 n.v. 4.2–10.5). He underwent an abdominal CT with IV contrast ((a) non-contrast, (b) arterial, (c) venous, (d) delayed phase), that showed subcapsular and pericholecystic hematoma with endoluminal active bleeding originating from a cystic artery pseudoaneurysm (CAP) ((b), white arrow). The patient underwent TAE successfully (coils).
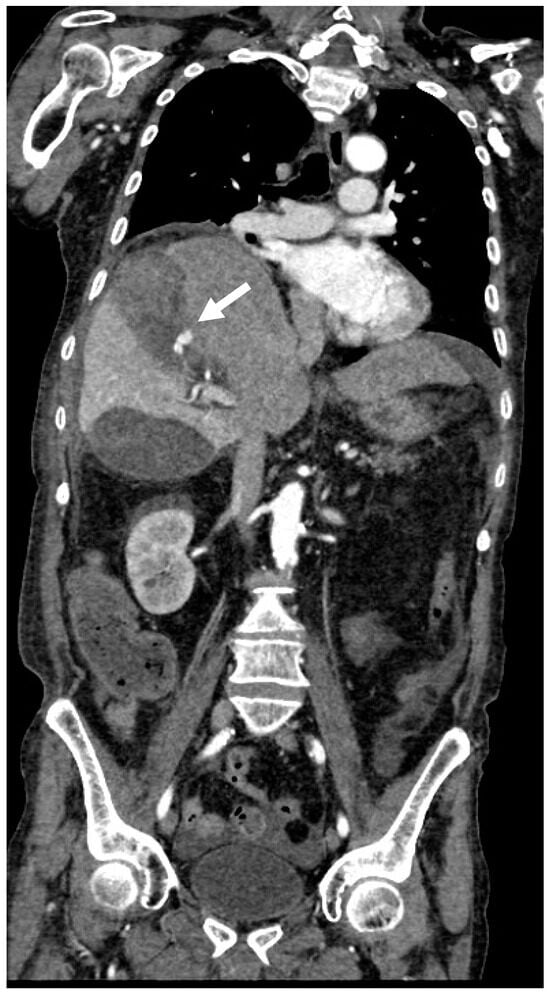
Figure 6.
Coronal CT image in arterial phase that clearly shows CAP with irregular margins (white arrow).
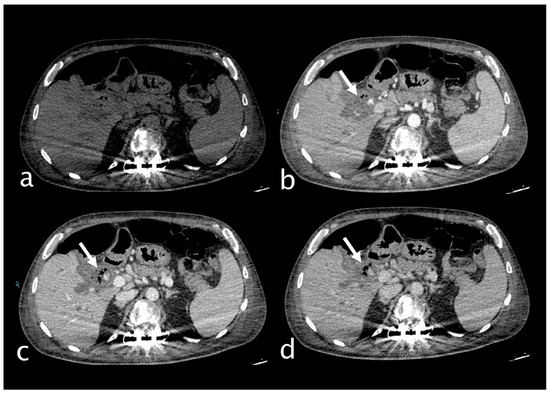
Figure 7.
58-year-old man who was hospitalized and underwent more than one ERCP for choledocholithiasis. He complained of severe right upper quadrant pain and sudden decline of Hb 7.3 g/dL (PCR 10.82 mg/dL, n.v. 0.0–0.5; WBC 18.76 103/mm3 n.v. 4.2–10.5). He underwent an abdominal CT with IV contrast (a) non-contrast, (b) arterial, (c) venous, (d) delayed phase), which showed dilated intrahepatic bile ducts and a markedly dilated common bile duct that present inhomogeneous content and air bubbles from a previous procedure and sphincterotomy. Within the common bile duct a cystic artery pseudoaneurysm was detected with active extravasation (white arrow). The patient underwent TAE successfully.
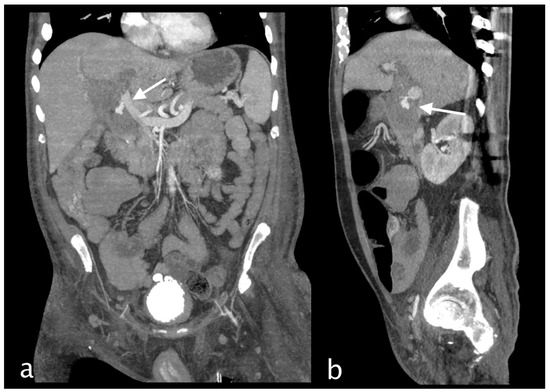
Figure 8.
MPR coronal (a) and sagittal (b) clearly demonstrate cystic branch pseudoaneurysm bleeding (arrow).
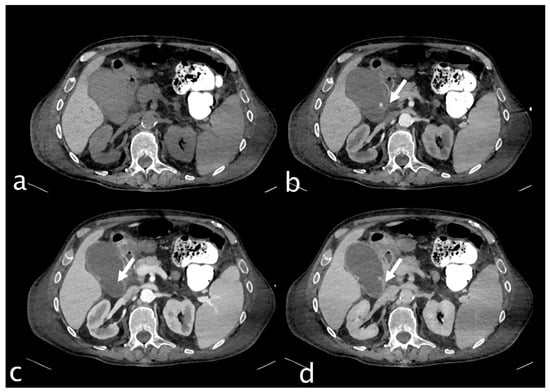
Figure 9.
74-year-old male hospitalized for hip fracture who underwent arthroplasty (Hb 8 g/dL, n.v. 13–18; PRO B NP 39,473 pg/mL, n.v. < 375; PCR 5.04 mg/dL, n.v. 0.0–0.5; WBC 7.25 103/mm3 n.v. 4.2–10.5), who suddenly complained of jaundice, right upper quadrant pain and melena. He underwent an abdominal CT with IV contrast ((a) non-contrast, (b) arterial, (c) venous, (d) delayed phase), which showed a distended gallbladder with slightly hyperdense content and a rounded hyperattenuating focus (white arrow) that did not change form and was isodense to a blood pool in the venous and delayed phases. Diagnosis: CAP with no active bleeding. The patient underwent cholecystectomy and was discharged.
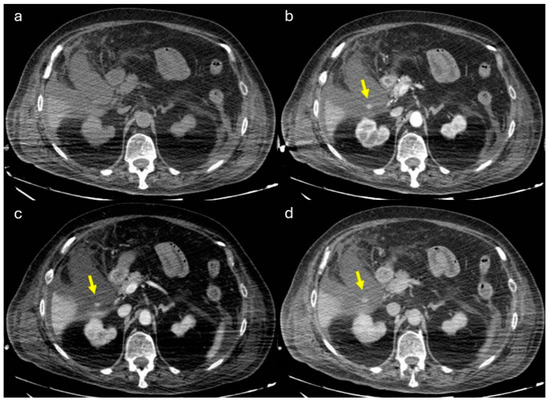
Figure 10.
80-y.o. woman with lung infection (candidiasis) with worsening of inflammatory markers. (a) Non-contrast axial CT imaging demonstration of perihepatic and perisplenic fluid collections, and the presence of fluid in the gallbladder bed. The gallbladder appears increased in size. (b) Axial CT image shows the presence of globular enhancing vascular image (yellow arrow) along the gallbladder wall in arterial phase. The globular vascular image did not change form and was isodense to a blood pool in the venous and delayed phases (c,d). Diagnosis: CAP with no active bleeding.
DSA allows real-time visualization of vascular dynamics, identifying active extravasation, pseudoaneurysms, or arteriovenous fistulas with unparalleled accuracy (Figure 4). DSA is particularly invaluable in cases where non-invasive imaging is inconclusive or when immediate therapeutic intervention is warranted [3,19].
DSA also plays a pivotal role in assessing collateral blood flow. This evaluation is essential when embolizing major hepatic vessels, as it prevents ischemic complications in the liver parenchyma. For instance, significant collateral flow from the left hepatic artery can obviate concerns about right hepatic artery occlusion during embolization [15,17].
3. Treatment
CAB, whether related or not to CAP, is a rare event, and the therapeutic approach relies on the patient’s general condition and the risk from the anaesthesiologist.
Surgery with cholecystectomy and proximal ligation or clipping of the cystic artery is considered the gold standard, but an emergency cholecystectomy can present challenges for surgeons who must manage the hemorrhage [1].
Although there are no established guidelines, transarterial embolization (TAE) nowadays represents the preferred therapeutic approach to restore hemodynamic stability [31,32].
Given the increasing role of interventional treatment as the preferred strategy in these conditions, we conducted a comprehensive systematic review of the literature on percutaneous embolization for cystic artery bleeding, including cases related to CAP and non-CAP etiologies.
3.1. Results
3.1.1. Study Selection and Characteristics
This systematic review identified 462 articles for initial screening based on predefined inclusion and exclusion criteria. After removing duplicate records, 97 articles remained. Screening of titles and abstracts excluded 365 articles for lack of relevance. Following a full-text review and application of inclusion criteria, 64 articles were included in the final analysis [2,3,10,13,14,15,16,17,19,20,25,26,29,30,31,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81]. The selection process was summarized in a PRISMA flow diagram (Figure 11).
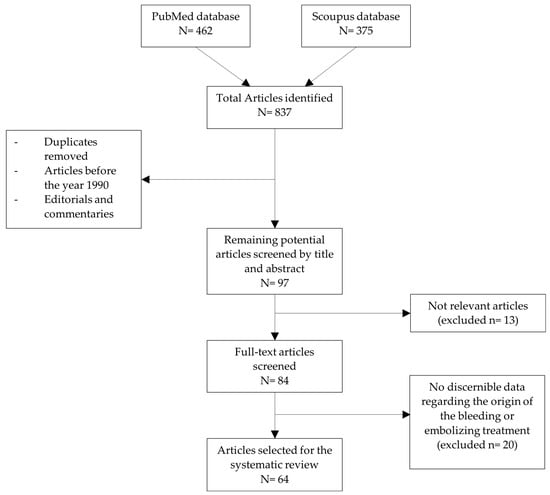
Figure 11.
PRISMA flow diagram for study selection.
3.1.2. Patient Demographics and Clinical Presentation
Data from 64 studies encompassing 90 patients were analyzed (Table A1). The median age was 64.5 years (±16.45), with a median of 67.5 years (95% CI, 63.2–71.0) and a range of 16 to 91 years. A predominance of males was observed, with 59 males (65.5%) and 31 females (34.5%).
The most common etiology was acute cholecystitis (52 cases, 58%), including two cases of cholecystocolic fistula [29,48]. Other etiologies included iatrogenic injury (15 cases, 17%), primarily post-cholecystectomy (nine cases, 10%); tumor bleeding (four cases, 4.5%); and chronic cholecystitis (four cases, 4.5%). Less frequent causes were duodenal ulcer (3%, three cases, [41]), Mirizzi syndrome (three cases, 3%, [15,43,47]), acute pancreatitis (two cases, 2%, [61,80]), and trauma (two cases, 2% [3,41]). Singular cases included arteriovenous malformation [77], Bouveret syndrome [33], spontaneous rupture of the cystic artery [16], cystic artery–gallbladder fistula [17], and xanthogranulomatous cholecystitis [76].
Presenting symptoms included right upper quadrant pain (57 cases, 63%), melena (29 cases, 32%), hematemesis (24 cases, 27%), and jaundice (18 cases, 20%). Hemodynamic instability was noted in 9% of patients (eight cases). A minority of patients (three cases, 3%) presented with nonspecific symptoms, such as nausea, anorexia, or incidental imaging findings [3,38,77].
3.1.3. Imaging and Diagnostic Findings
Contrast-enhanced computed tomography (CECT) was the diagnostic modality of choice in all cases except one, where US alone was performed before digital DSA. Imaging modality was not specified in the studies by Liu B et al. [64] and Kim HC et al. [41].
Endoscopy was performed in 43 patients, revealing active bleeding or blood oozing in 22 cases. DSA identified pseudoaneurysms in 64 cases (64/70 91%), with rupture and active bleeding in 30 cases (43%). Bleeding from the cystic artery was documented in five cases, including two with cystic artery–gallbladder fistulas [17,51]. A single case of arteriovenous malformation of the gallbladder fed by two dilated cystic arteries was reported [77]. DSA findings were not specified in the study by Kim HC et al. [41].
3.1.4. Management and Outcomes
TAE was performed in all patients except two, where percutaneous embolization was used [34,66].
Coils were the most frequently used embolic agents in TAE, applied alone in 49 cases (49/88 56%) or combined with gelfoam (three cases), thrombin (one case), or stent placement (one case).
N-butyl cyanoacrylate (NBCA) was used in 18 cases (18/88 20.5%), either alone or combined with coils (two cases), lipiodol (two cases), or a combination of lipiodol, coils, and gelfoam (two cases). Other agents included gelfoam alone (four cases, 4/88 4.5%) and PVA particles (500–700 µm) in three cases, either alone or with gelfoam or coils. Rarely used materials included blood clot [41] and Onyx. In percutaneous embolization cases, NBCA and thrombin were utilized, respectively.
Complications were reported in eight patients. Ischemic cholecystitis occurred in four patients [16,41], hepatic ischemia in two cases [2,53], and one case each of liver abscess treated with antibiotics [20] and peritonism with worsening abdominal pain post-embolization [78].
Cholecystectomy was performed in 29 patients. Additionally, one patient underwent ligation of the right hepatic artery, and another required an extended right hepatectomy.
Most patients achieved complete recovery. However, nine patients died, seven of whom died postoperatively or of multiorgan failure secondary to sepsis.
3.2. Materials and Methods
This review was conducted following the “PICO” method and “Preferred Reporting Items for Systematic Review and Meta-Analysis” (PRISMA) statement guidelines. The study did not directly involve humans, and did not require the Institutional Review Board approval of our department.
The systematic review was performed using PubMed, Scopus, and Embase databases, using the following medical subject headings (MeSH), keywords and EMBASE Subject Headings (EMTREE): “cystic artery”, “bleeding”, “hemorrhage”, “pseudoaneurysm”, “rupture” and “embolization”.
The study was carried out utilizing the specified headings in combination with the following Boolean operators: (bleeding OR hemorrhage OR haemorrhage OR pseudoaneurysm OR rupture) AND (cystic artery) AND (embolization OR embolisation). The titles, abstracts, and reference lists of the retrieved publications were screened based on predefined inclusion and exclusion criteria. Full-text articles were then reviewed in detail to assess their eligibility for inclusion.
The initial search period was arbitrarily set to begin in 1990, with screening conducted between September 2024 and October 2024 by two independent authors (F.T. and R.C.). Discrepancies were resolved through consensus or a third reviewer (S.T.).
The inclusion criteria were studies, including case reports and case series, reporting cystic artery vascular bleeding or vascular injury (pseudoaneurysm or fistula) treated with embolization (transarterial and/or percutaneous).
The exclusion criteria were recurring articles from the same authors, duplicate articles, articles not written in English, publications before the year 1990, editorials and commentaries, review articles without new case data, studies infrom which the exact origin of the bleeding (e.g., hepatic artery) could not be determined, studies that use angiography but not embolization, and studies in which embolizing treatment was not specified.
In doubtful cases, articles were included in this stage were named “uncertain” and required the evaluation of the full text to make the final decision.
Data extracted from the publications included the first author, year of publication, study design, sample size, patient demographics (age and sex), underlying etiology, presenting symptoms, initial imaging modality (ultrasound, CTA, MRI, or DSA), use of endoscopy, DSA findings, and type of interventional procedure performed. Technical details of the embolization procedure, including the embolic material used and complications, were also collected. Additionally, the need for subsequent surgery or intervention and clinical outcomes, when reported, were documented.
4. Discussion
Percutaneous selective cystic artery embolization (CAE) has emerged as an efficacious treatment strategy in the acute setting for patients with CAP or CAB, and it can be performed with high success rates and cessation of bleeding in up to 90% of patients [1,29,40,41].
CAE offers advantages such as reduced mortality and morbidity, improved identification of the bleeding vessel, and higher rates of achieving hemorrhage control.
This systematic review consolidates data from 90 cases, providing comprehensive insights into its etiology, presentation, diagnostic strategies, treatment modalities, and outcomes.
Acute cholecystitis, identified in 58% of cases, leads to local vascular damage due to prolonged inflammation and enzymatic degradation of the arterial wall. Iatrogenic causes, particularly from laparoscopic cholecystectomy, accounted for 17% of cases, emphasizing the need for meticulous surgical techniques to avoid inadvertent vascular injury. Malignancy-associated CAP, though less common (4.5%), underscores the importance of considering vascular complications in advanced gallbladder carcinoma or HCC [37,41,49].
The clinical spectrum of CAB is broad, with gastrointestinal bleeding as the most common presentation. Hematemesis (27%) and melena (32%) were predominant, accompanied by hemodynamic instability in severe cases (9%). Right upper quadrant pain, present in 63% of cases, reflects the inflammatory or ischemic component of the gallbladder pathology [1,82].
CT angiography represents the diagnostic modality of choice, utilized in 99% of cases. DSA, although invasive, confirmed pseudoaneurysm and active extravasation in all cases where performed, guiding therapeutic embolization. Endoscopic findings, while variable, reveal active bleeding or blood oozing in 50% of cases, providing critical clues in hemobilia cases.
Transarterial embolization represents the preferred approach for managing cystic artery bleeding, particularly in urgent hemodynamic stabilization cases. This minimally invasive technique achieves rapid hemostasis, demonstrating high technical success and minimal procedural risks [31].
The choice of embolic agent varied, with coils being the most frequently used (56%), followed by N-butyl cyanoacrylate (20.5%), each offering specific advantages and limitations [13,19].
Coil embolization is the preferred method due to its versatility in treating vessels of varying sizes and its ability to be introduced without the risk of increased pressure within the vascular lesion [71]. Coils provide durable occlusion, making them ideal for pseudoaneurysms with well-defined arterial feeders.
Minor complications, such as coil migration, were reported in isolated cases [25]. However, their utility is limited in high-flow bleeding or complex vascular anatomies, where alternative agents may be more appropriate [20].
Liquid embolic agents such as N-butyl cyanoacrylate (NBCA) provide an effective solution for high-flow bleeding or tortuous vasculature. NBCA polymerizes rapidly upon contact with blood, forming a permanent occlusion [83]. Its efficacy in patients with coagulopathies is particularly noteworthy, as its action is independent of coagulation status. Despite its advantages, NBCA requires technical expertise due to risks of non-target embolization and catheter adhesion [83].
Combining agents, such as NBCA with Lipiodol or gelfoam, have been described in cases requiring additional hemostatic efficacy [20,35].
Temporary agents like gelfoam offer a temporary occlusion, achieving hemostasis through mechanical obstruction and are particularly effective in low-flow conditions.
However, the temporary nature of gelfoam may result in recurrent bleeding, necessitating repeat embolization [16,84].
The selection of embolic material should be tailored to the patient’s specific clinical scenario. For high-flow bleeding or complex vascular anatomy, NBCA or a combination of coils and NBCA may be preferred. Coils remain optimal for localized lesions with well-defined anatomy, while gelfoam may be considered for temporary hemostasis in select cases. Adjunctive agents such as thrombin or lipiodol can further enhance the efficacy of embolization when used judiciously [20,35].
Thrombin direct injection into the pseudoaneurysm under Ultrasound and Doppler guidance has also been reported, but it is considered a non-selective procedure with potential collateral effects such as bowel and liver infarcts [34,66].
Although TAE is generally safe, complications can arise, typically related to ischemic injury or non-target embolization. Ischemic cholecystitis, occurring in approximately 4.5% of cases, is among the most frequently reported complications [16,41]. This condition necessitates prompt diagnosis with imaging and early intervention, such as cholecystectomy or drainage, to prevent sepsis [16,41].
Hepatic ischemia, though rare, is more likely with extensive embolization involving the hepatic artery [2,53]. Conservative management, including hydration and close monitoring, is often sufficient; however, severe cases may require surgical revascularization [20]. Non-target embolization, associated primarily with liquid agents like NBCA, underscores the importance of meticulous technique and selective catheterization. Infections and abscess formation have also been observed, particularly in patients with pre-existing biliary infections [20]. These complications are typically managed with antibiotics and abscess drainage [20].
Post-procedural monitoring is crucial for identifying these potential complications, and super selective embolization of the anterior or posterior branch of the cystic artery, when possible, reduces the risk of post-procedural complications thanks to the anastomotic network [8].
The need for cholecystectomy after embolization remains a point of debate. In this review, 32% of patients underwent cholecystectomy, primarily to address the underlying pathology, such as gallbladder gangrene, or to prevent recurrence. Early cholecystectomy, particularly in patients with gallbladder necrosis or severe inflammation, was associated with favorable outcomes. Conversely, a conservative approach with observation was effective in patients without persistent symptoms or high-risk features. The timing of cholecystectomy should be individualized based on the patient’s clinical stability and the resolution of acute inflammation [71,80].
The outcomes of TAE for cystic artery bleeding are generally favorable. Most patients achieve complete recovery [83]. Complications are infrequent, and the mortality rate remains low. These results underscore the efficacy and safety of TAE as a first-line treatment in this setting.
5. Conclusions
Isolated CAB is rare, occurring in traumatic, non-traumatic, or idiopathic cases. CT is crucial for diagnosing trauma-related CAB, and for identifying hemoperitoneum near the gallbladder with active extravasation. Non-operative trauma management often involves transarterial embolization. In non-traumatic or iatrogenic cases, prior cholecystectomy or inflammation should prompt suspicion of CAP. Even in unrelated exams, early CT detection of CAPs can enable timely intervention, preventing rupture and life-threatening complications while improving patient outcomes.
Despite advancements in diagnostic and therapeutic techniques, CAP remains a diagnostic challenge due to its rarity and nonspecific presentation. Future research should focus on developing standardized diagnostic protocols and exploring novel therapeutic agents to enhance clinical outcomes. Additionally, long-term follow-up studies are needed to assess the durability of embolization and the risk of recurrence.
Author Contributions
Conceptualization S.T., F.T., D.G.C. and R.C.; Methodology F.T. and R.C.; Formal Analysis F.T., D.G.C. and R.C.; Investigation F.T. and R.C.; Data Curation S.T., F.T., D.G.C. and R.C.; Writing—Original Draft Preparation F.T., D.G.C. and R.C.; Writing—Review & Editing F.T., R.C. and C.B.; Visualization S.P., F.D.S., F.P., G.F., G.S., P.R. and M.S.; Supervision, A.B. and S.T.; Project Administration S.T. All authors meet the ICMJE Recommendations for authorship credit. All authors have read and approved the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have contributed equally. The authors had full access to the data and take full responsibility for its integrity. All authors have read and agree to the manuscript as written. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
For this retrospective analysis, it was not necessary to request authorization from the ethics committee. The contents of this paper are consistent with the principles of the Declaration of Helsinki in the latest version.
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The author(s) received no financial support for the research, authorship and/or publication of this article.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Summary of characteristics of the selected study.
Table A1.
Summary of characteristics of the selected study.
| Authors/Year | Type of the Study | Number of Patients | Sex | Age | Etiology | Main Symptoms | Type of Lesion | Interventional Treatment | Embolic Agents | Procedure-Related Complications | Procedures After Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sarmento Costa M et al., 2024 [33] | case report | 1 | M | 88 | Bouveret syndrome (bilioenteric fistula) | Hematemesis | Pseudoaneurysm | TAE | Coils | N/R | No | Recovered |
| Giurazza F et al., 2024 [34] | technical report | 1 | M | 71 | Acute cholecystitis | N/R | Pseudoaneurysm | PE | NBCA | N/R | No | Recovered |
| Heidari A et al., 2024 [35] | case report | 1 | F | 64 | Acute cholecystitis | Abdominal pain, hematemesis | Pseudoaneurysm | TAE | PVA (500–700 µm) + gelfoam | N/R | Cholecystectomy | Recovered |
| Khawjah A et al., 2024 [2] | case report | 1 | M | 54 | Acute cholecystitis | Abdominal pain, hematemesis, jaundice | Pseudoaneurysm rupture | TAE | Coils + gelfoam | Hepatic ischemia | Cholecystectomy | Recovered |
| Anns MK et al., 2024 [36] | case report | 1 | M | 64 | Iatrogenic (laparoscopic cholecistectomy) | Hematemesis, melena | Pseudoaneurysm | TAE | Coils | N/R | No | Recovered |
| Okamoto S et al., 2024 [37] | case report | 1 | M | 80 | Neoplastic (HCC) | Abdominal pain, melena, jaundice | Pseudoaneurysm rupture | TAE | NBCA + Lipiodol + gelfoam + coils | N/R | No | Recovered |
| Rais A et al., 2024 [13] | case report | 1 | F | 71 | Acute cholecystitis | Abdominal pain | Pseudoaneurysm | TAE | NBCA + Lipiodol | N/R | Cholecystectomy | Recovered |
| Robbie R et al., 2024 [25] | case report | 1 | M | 83 | Acute cholecystitis | Bleeding per rectum | Pseudoaneurysm | TAE | Coils | N/R | No | Recovered |
| Saha B et al., 2024 [38] | case report | 1 | M | 70 | Acute cholecystitis | Abdominal pain | Pseudoaneurysm | TAE | Coils | N/R | Cholecistectomy | Recovered |
| Sibria D et al., 2024 [19] | case report | 1 | F | 38 | Iatrogenic (laparoscopic cholecistectomy) | Abdominal pain, jaundice, bleeding per rectum, melena | Pseudoaneurysm | TAE | Coils | N/R | No | Recovered |
| Mie T et al., 2024 [39] | case report | 1 | F | 78 | Acute cholecystitis | Abdominal pain, jaundice | Pseudoaneurysm rupture | TAE | NBCA + Lipiodol | N/R | No | Recovered |
| Khan H et al., 2023 [40] | case report | 1 | M | 88 | Acute cholecystitis | Abdominal pain, jaundice | Pseudoaneurysm rupture | TAE | Coils | N/R | Cholecystectomy | Recovered |
| Kim HC et al., 2023 [41] | retrospective study | 1 | M | 43 | Acute cholecystitis | Melena | N/R | TAE | blood clot | N/R | Cholecystectomy | Recovered |
| retrospective study | 1 | F | 34 | Iatrogenic (cholecystostomy) | Hemoperitoneum | N/R | TAE | Gelfoam | N/R | N/R | Recovered | |
| retrospective study | 1 | F | 54 | Neoplastic | Melena | N/R | TAE | NBCA | N/R | N/R | Recovered | |
| retrospective study | 1 | F | 49 | Acute cholecystitis | Hematemesis | N/R | TAE | NBCA | Ischemic cholecystitis | N/R | Death from MOF, sepsis | |
| retrospective study | 1 | M | 75 | Acute cholecystitis | Hematochezia | N/R | TAE | NBCA | N/R | N/R | Recovered | |
| retrospective study | 1 | F | 52 | Iatrogenic (RFA) | Hemoperitoneum | N/R | TAE | NBCA | N/R | N/R | Recovered | |
| retrospective study | 1 | F | 71 | Neoplastic | Jaundice | N/R | TAE | NBCA | N/R | N/R | Recovered | |
| retrospective study | 1 | M | 74 | Acute cholecystitis | Blood in cholecystostomy tube | N/R | TAE | NBCA | N/R | N/R | Death from MOF, sepsis | |
| retrospective study | 1 | M | 48 | Trauma | Hemoperitoneum | N/R | TAE | NBCA | Ischemic cholecystitis | N/R | Recovered | |
| retrospective study | 1 | M | 48 | Acute cholecystitis | Blood in cholecystostomy tube | N/R | TAE | NBCA | Ischemic cholecystitis | N/R | Death from MOF, sepsis | |
| retrospective study | 1 | M | 80 | Acute cholecystitis | Hematemesis | N/R | TAE | NBCA | N/R | N/R | Recovered | |
| retrospective study | 1 | F | 78 | Acute cholecystitis | Hematemesis | N/R | TAE | NBCA | N/R | N/R | Death from pneumonia | |
| retrospective study | 1 | F | 78 | Duodenal ulcer | Hematemesis | N/R | TAE | Coils | N/R | Cholecystectomy | Recovered | |
| retrospective study | 1 | M | 67 | Duodenal ulcer | Melena | N/R | TAE | NBCA | N/R | N/R | Recovered | |
| retrospective study | 1 | F | 56 | Duodenal ulcer | Melena | N/R | TAE | Gelfoam | N/R | N/R | Death from MOF, sepsis | |
| retrospective study | 1 | M | 78 | Acute cholecystitis | Abdominal pain | N/R | TAE | NBCA | N/R | Cholecystectomy | Recovered | |
| retrospective study | 1 | M | 64 | Acute cholecystitis | Blood in cholecystostomy tube | N/R | TAE | NBCA | N/R | N/R | Recovered | |
| retrospective study | 1 | M | 80 | Acute cholecystitis | Hemoperitoneum | N/R | TAE | NBCA | N/R | N/R | Death from MOF, sepsis | |
| retrospective study | 1 | M | 85 | Iatrogenic (cholecystostomy) | Blood in cholecystostomy tube | N/R | TAE | NBCA | N/R | N/R | Recovered | |
| retrospective study | 1 | M | 70 | Iatrogenic (cholecystostomy) | Abdominal pain | N/R | TAE | NBCA | N/R | N/R | Recovered | |
| Liu YL et al., 2023 [42] | case report | 1 | M | 81 | Acute cholecystitis | Abdominal pain, melena, hemobilia | Pseudoaneurysm with active bleeding | TAE | Coils | N/R | Cholecystostomy | Recovered |
| Shrivastava A et al., 2023 [26] | case report | 1 | M | 41 | Acute cholecystitis | Abdominal pain, melena | Pseudoaneurysm | TAE | Coils | N/R | Cholecystostomy + cholecystectomy | Recovered |
| Williams T et al., 2023 [43] | case report | 1 | M | 61 | Mirizzi syndrome | Abdominal pain | Pseudoaneurysm | TAE | Coils | N/R | Cholecystostomy | Recovered |
| Zainab R et al., 2023 [44] | case report | 1 | M | 55 | Acute cholecystitis | Abdominal pain, hematemesis, melena | Pseudoaneurysm | TAE | Coils | N/R | N/R | Recovered |
| Itagaki Y et al., 2023 [45] | case report | 1 | F | 70 | Acute cholecystitis | Jaundice | Pseudoaneurysm | TAE | Coils | N/R | Cholecystectomy | Recovered |
| Christodoulou P et al., 2022 [46] | case report | 1 | M | 67 | Iatrogenic (laparoscopic cholecistectomy) | Abdominal pain | Pseudoaneurysm rupture | TAE | Coils | N/R | Cholecystectomy | Recovered |
| Fukushima R et al., 2022 [47] | case report | 1 | F | 73 | Mirizzi syndrome | Abdominal pain, jaundice | Pseudoaneurysm rupture | TAE | Coils | N/R | Cholecystectomy | Recovered |
| Amakye DO et al., 2021 [48] | case report | 1 | M | 66 | Acute cholecystitis (cholecystocolic fistula) | Abdominal pain | Pseudoaneurysm rupture | TAE | Coils | N/R | No | Recovered |
| Mahalingam S et al., 2021 [49] | case report | 1 | M | 52 | Neoplastic (carcinoma gallbladder) | Abdominal pain, hematemesis, melena | Pseudoaneurysm rupture | TAE | NBCA | N/R | Cholecystostomy | Recovered |
| Nguyen D et al., 2021 [50] | case report | 1 | M | 74 | Acute cholecystitis | Abdominal pain, hematemesis, melena | Pseudoaneurysm with active bleeding | TAE | Coils | N/R | Cholecystectomy | Recovered |
| case report | 1 | M | 74 | Acute cholecystitis | N/R | Pseudoaneurysm with active bleeding | TAE | PVA (500–700) + coils | N/R | Cholecystectomy | Recovered | |
| Acharya S et al., 2020 [51] | case report | 1 | F | 62 | Chronic cholecystitis | Abdominal pain, bleeding per rectum | Cystic artery-gallbladder fistula with bleeding | TAE | Coils | N/R | No | Recovered |
| Carey F et al., 2020 [29] | case report | 1 | M | 47 | Acute cholecystitis (cholecystocolic fistula) | Abdominal pain, bleeding per rectum | Pseudoaneurysm with active bleeding | TAE | Coils | N/R | No | Recovered |
| Leshen M et al., 2020 [52] | case report | 1 | M | 16 | Acute cholecystitis | Abdominal pain, hematemesis | Pseudoaneurysm with active bleeding | TAE | Coils | N/R | Cholecystectomy | Recovered |
| Proença AL et al., 2020 [53] | case report | 1 | F | 73 | Iatrogenic (ERCP) | Abdominal pain, hematemesis, melena | Pseudoaneurysm with active bleeding | TAE | NBCA | Hepatic ischemia | Cholecystectomy | Recovered |
| Yam MKH et al., 2020 [54] | case report | 1 | F | 51 | Acute cholecystitis | Abdominal pain, hematemesis, melena | Pseudoaneurysm with active bleeding | TAE | PVA + Coils | N/R | Cholecystostomy + cholecystectomy | Recovered |
| Rossini M et al., 2019 [55] | case report | 1 | M | 66 | Iatrogenic (laparoscopic cholecistectomy) | Abdominal pain, hematemesis, melena | Pseudoaneurysm with active bleeding | TAE | Coils | N/R | No | Recovered |
| Sada DM et al., 2019 [56] | case report | 1 | M | 69 | Iatrogenic (cholecystostomy) | Abdominal pain | Pseudoaneurysm with active bleeding | TAE | Onyx | N/R | No | Postoperative death |
| Tanaka T et al., 2019 [57] | case report | 1 | F | 80 | Acute cholecystitis | Abdominal pain, melena | Pseudoaneurysm rupture | TAE | Coils | N/R | Endoscopic biliary drainage | Recovered |
| Kuzman MS et al., 2018 [58] | case report | 1 | F | 25 | Acute cholecystitis | Abdominal pain | Pseudoaneurysm | TAE | NBCA + Coils | N/R | Cholecystostomy + cholecystectomy | Recovered |
| Sunkara PRV et al., 2018 [14] | case report | 1 | M | 56 | Acute cholecystitis | Abdominal pain | Pseudoaneurysm rupture | TAE | Coils | N/R | Cholecystectomy | Recovered |
| Machado NO et al., 2017 [10] | case report | 1 | F | 70 | Iatrogenic (laparoscopic cholecistectomy) | Abdominal pain | Pseudoaneurysm with active bleeding | TAE | Coils | N/R | No | Recovered |
| Maddineni S et al., 2017 [59] | case report | 1 | F | 54 | Acute cholecystitis | Abdominal pain, melena | Pseudoaneurysm with active bleeding | TAE | Thrombin + Coils | N/R | Cholecystostomy | Death due to non-procedure-related complications |
| Tapnio RH et al., 2017 [60] | case report | 1 | F | 91 | Acute cholecystitis | Abdominal pain | Pseudoaneurysm with active bleeding | TAE | Coils | N/R | Cholecystectomy | Recovered |
| case report | 1 | M | 61 | Acute cholecystitis | Abdominal pain | Pseudoaneurysm with active bleeding | TAE | Coils + gelfoam | N/R | Cholecystectomy | Recovered | |
| case report | 1 | M | 91 | Acute cholecystitis | Abdominal pain | Pseudoaneurysm | TAE | Coils | N/R | Cholecystostomy | Recovered | |
| Thillai M et al., 2017 [61] | case report | 1 | M | 33 | Acute pancreatitis | Melena | Pseudoaneurysm with active bleeding | TAE | Gelfoam | N/R | Cholecystostomy | Recovered |
| Trombatore C et al., 2017 [62] | case report | 1 | M | 64 | Acute cholecystitis | Abdominal pain, jaundice | Pseudoaneurysm with active bleeding | TAE | Coils | N/R | Cholecystectomy | Recovered |
| Hall TC et al., 2016 [63] | case report | 1 | M | 88 | Acute cholecystitis | Abdominal pain, melena | Pseudoaneurysm with active bleeding | TAE | NBCA + Lipiodol + gelfoam + coils | N/R | No | Recovered |
| Liu B et al., 2016 [64] | case report | 1 | M | 82 | Iatrogenic (laparoscopic cholecistectomy) | Hemobilia | Pseudoaneurysm with active bleeding | TAE | Coils + stent | N/R | No | Recovered |
| Shelmerdine SC et al., 2015 [31] | case report | 1 | M | 72 | Acute cholecystitis | Abdominal pain, hematemesis, melena | Pseudoaneurysm with active bleeding | TAE | Coils | N/R | No | Recovered |
| Aljiffry MM et al., 2014 [65] | case report | 1 | M | 57 | Acute cholecystitis | Abdominal pain | Active bleeding from cystic artery | TAE | Gelfoam | N/R | Cholecystectomy | Recovered |
| Kulkarni V et al., 2014 [15] | case report | 1 | M | 55 | Mirizzi syndrome | Abdominal pain, jaundice, melena | Pseudoaneurysm | TAE | Coils | N/R | Cholecystectomy | Recovered |
| Kumar A et al., 2014 [66] | case report | 1 | F | 45 | Iatrogenic (laparoscopic cholecistectomy) | Hemobilia | Pseudoaneurysm with active bleeding | PE | Thrombin (400 units) | N/R | No | Recovered |
| Mokrane FZ et al., 2013 [67] | case report | 1 | M | 67 | Acute cholecystitis | Hematemesis | Pseudoaneurysm with active bleeding | TAE | Coils | N/R | No | Recovered |
| Nana GR et al., 2013 [68] | case report | 1 | M | 74 | Acute cholecystitis | Abdominal pain, hematemesis, jaundice | Pseudoaneurysm | TAE | Coils | N/R | Cholecystostomy | Recovered |
| case report | 1 | F | 79 | Acute cholecystitis | Abdominal pain, melena, jaundice | Pseudoaneurysm | TAE | Coils | N/R | No | Recovered | |
| Priya H et al., 2013 [17] | case report | 1 | M | 22 | Spontaneous | Abdominal pain, hematemesis, melena, jaundice | Fistula (cystic artery-gallbladder) | TAE | Coils | N/R | Cholecystectomy | Recovered |
| Chong JJR et al., 2012 [69] | case report | 1 | M | 56 | Acute cholecystitis | Abdominal pain, hematemesis | Pseudoaneurysm | TAE | Coils | N/R | Cholecystectomy | Recovered |
| Petrou A et al., 2012 [70] | case report | 1 | F | 34 | Iatrogenic (laparoscopic cholecistectomy) | Abdominal pain, hematemesis | Pseudoaneurysm | TAE | Coils | N/R | Ligation of right hepatic artery | Recovered |
| Siddiqui NA et al., 2011 [30] | case report | 1 | M | 58 | Acute cholecystitis | Abdominal pain, jaundice | Pseudoaneurysm | TAE | Coils | N/R | Cholecystectomy | Recovered |
| Desai AU et al., 2010 [71] | case report | 1 | F | 78 | Chronic cholecystitis | Abdominal pain, melena, jaundice | Pseudoaneurysm | TAE | Coils | N/R | No | Recovered |
| Hague J et al., 2010 [72] | case series | 1 | M | 83 | Acute cholecystitis | Abdominal pain, hemoperitoneum | Pseudoaneurysm | TAE | Coils | N/R | No | Recovered |
| case series | 1 | M | 79 | Acute cholecystitis | Abdominal pain, hemobilia | Pseudoaneurysm | TAE | Coils | N/R | No | Recovered | |
| case series | 1 | M | 83 | Acute cholecystitis | Abdominal pain, melena | Pseudoaneurysm | TAE | Coils | N/R | No | Death from esophageal carcinoma within 2 months | |
| Nkwam N et al., 2010 [73] | case report | 1 | M | 71 | Acute cholecystitis | Abdominal pain | Pseudoaneurysm | TAE | Coils | N/R | Cholecystostomy | Recovered |
| Osada H et al., 2010 [3] | case report | 1 | M | 62 | Trauma | Asymptomatic | Pseudoaneurysm | TAE | Coils | N/R | No | Recovered |
| Contini S et al., 2009 [74] | case report | 1 | M | 58 | Chronic cholecystitis | Abdominal pain, melena, jaundice | Active bleeding | TAE | Coils | N/R | Cholecystectomy | Recovered |
| Mullen R et al., 2009 [75] | case series | 1 | F | 75 | Chronic cholecystitis | Abdominal pain, hematemesis, melena | Pseudoaneurysm | TAE | Coils | N/R | No | Recovered |
| case series | 1 | M | 82 | Acute cholecystitis | Abdominal pain | Pseudoaneurysm | TAE | Coils | N/R | No | Recovered | |
| Ouazzani A et al., 2009 [16] | case report | 1 | M | 74 | Spontaneous | Abdominal pain | Active bleeding (rupture) | TAE | Particles (500–700 µm) | Ischemic cholecystitis | Cholecystectomy | Recovered |
| Nakase Y et al., 2008 [20] | case report | 1 | F | 63 | Iatrogenic (laparoscopic cholecistectomy) | Abdominal pain, melena, hematemesis | Pseudoaneurysm | TAE | NBCA + Coils | Liver abscess treated with antibiotics | No | Recovered |
| Shimada K et al., 2008 [76] | case report | 1 | M | 68 | Xanthogranulomatous cholecystitis | Jaundice, hemobilia | Pseudoaneurysm | TAE | Coils | N/R | Extended right hepatectomy | Recovered |
| Osada H et al., 2007 [77] | case report | 1 | F | 78 | Arteriovenous malformation | Asymptomatic | Arteriovenous malformation | TAE | Coils | N/R | No | Blood flow in AVM persisted; patient developed HCC recurrence after 4 years |
| Saluja SS et al., 2007 [78] | case report | 1 | F | 43 | Acute cholecystitis | Hematemesis, melena | Pseudoaneurysm | TAE | Coils + gelfoam | A day later the patient had increasing abdominal pain and appearance of peritoneal signs localized to the RUQ of the abdomen | Cholecystectomy | Recovered |
| Maeda A et al., 2002 [79] | case report | 1 | M | 62 | Acute cholecystitis | Abdominal pain, jaundice | Pseudoaneurysm | TAE | Coils | N/R | Cholecystectomy | Recovered |
| Delgadillo X et al., 1999 [80] | case report | 1 | M | 28 | Acute pancreatitis | Abdominal pain, hematemesis | Pseudoaneurysm | TAE | Coils | N/R | No | Recovered |
| England RE et al., 1998 [81] | case report | 1 | F | 71 | Acute cholecystitis | Abdominal pain, jaundice | Pseudoaneurysm | TAE | Coils | N/R | No | Death from MOF, sepsis |
References
- Taghavi, S.M.J.; Kumar, M.J.; Prabha, R.D.; Puhalla, H.; Sommerville, C. Cystic Artery Pseudoaneurysm: Current Review of Aetiology, Presentation, and Management. Surg. Res. Pract. 2021, 2021, 4492206. [Google Scholar] [CrossRef] [PubMed]
- Khawjah, A.; Khair, M.M.; Goubran, R. An unusual case of acute cholecystitis complicated by haemobilia and Mirizzi-like obstruction: A case report and review of literature. Ann. Med. Surg. 2024, 86, 3646–3651. [Google Scholar] [CrossRef]
- Osada, H.; Ohno, H.; Watanabe, W.; Okada, T.; Nakada, K.; Honda, N. Cystic artery bleeding due to blunt gallbladder injury: Computed tomography findings and treatment with transcatheter arterial embolization. Jpn. J. Radiol. 2010, 28, 162–165. [Google Scholar] [CrossRef]
- Hugh, T.B.; Kelly, M.D.; Li, B. Laparoscopic anatomy of the cystic artery. Am. J. Surg. 1992, 163, 593–595. [Google Scholar] [CrossRef]
- Andall, R.G.; Matusz, P.; du Plessis, M.; Ward, R.; Tubbs, R.S.; Loukas, M. The clinical anatomy of cystic artery variations: A review of over 9800 cases. Surg. Radiol. Anat. SRA 2016, 38, 529–539. [Google Scholar] [CrossRef]
- Ara, H.; Fakoya, A.O. Novel Variations in the Celiac and Hepatobiliary Arterial Anatomy: A Cadaveric Case Report. Cureus 2024, 16, e60813. [Google Scholar] [CrossRef] [PubMed]
- Asghar, A.; Priya, A.; Prasad, N.; Patra, A.; Agrawal, D. Variations in morphology of cystic artery: Systematic review and meta-analysis. Clin. Ter. 2024, 175, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Balija, M.; Huis, M.; Nikolić, V.; Štulhofer, M. Laparoscopic visualization of the cystic artery anatomy. World J. Surg. 1999, 23, 703–707, discussion 707. [Google Scholar] [CrossRef]
- Gupta, R.M.; Kumar, A.; Hariprasad, C.P.; Kumar, M. Anatomical variations of cystic artery, cystic duct, and gall bladder and their associated intraoperative and postoperative complications: An observational study. Ann. Med. Surg. 2023, 85, 3880–3886. [Google Scholar] [CrossRef]
- Machado, N.O.; Al-Zadjali, A.; Kakaria, A.K.; Younus, S.; Rahim, M.A.; Al-Sukaiti, R. Hepatic or Cystic Artery Pseudoaneurysms Following a Laparoscopic Cholecystectomy: Literature review of aetiopathogenesis, presentation, diagnosis and management. Sultan Qaboos Univ. Med. J. 2017, 17, e135–e146. [Google Scholar] [CrossRef]
- Wen, F.; Dong, Y.; Li, W.; Guo, Q.Y. Hemobilia After Laparoscopic Cholecystectomy: Imaging Features and Management of an Unusual Complication. Surg. Laparosc. Endosc. Percutaneous Tech. 2016, 26, e18–e24. [Google Scholar] [CrossRef]
- She, W.; Tsang, S.; Poon, R.; Cheung, T. Gastrointestinal bleeding of obscured origin due to cystic artery pseudoaneurysm. Asian J. Surg. 2017, 40, 320–323. [Google Scholar] [CrossRef]
- Rais, A.; Benabderrazik, B.; El Bouardi, N.; Akammar, A.; Haloua, M.; Alami, B.; Alaoui, M.Y.L.; Boubou, M.; Maaroufi, M. Cholecystitis-related cystic artery pseudoaneurysm: Case report. Radiol. Case Rep. 2024, 19, 2156–2159. [Google Scholar] [CrossRef]
- Praveen Kumar Sunkara, P.R.V.; Shah, P.K.; Rakshit, K.; Choudhary, S.R.; Bohidar, N.P.; Dubey, S.K. Rupture of Cystic Artery Pseudoaneurysm: A Rare Complication of Acute Cholecystitis. Indian J Surg. 2018, 80, 87–89. [Google Scholar] [CrossRef]
- Kulkarni, V.; Deshmukh, H.; Gupta, R. Pseudoaneurysm of anomalous cystic artery due to calculous cholecystitis. BMJ Case Rep. 2014, 2014, bcr2014207069. [Google Scholar] [CrossRef] [PubMed]
- Ouazzani, A.; Bataille, D.; Boutkhil, A.; Guérin, E.; Lefebvre, J.-C.; Vaneukem, P. Spontaneous Cystic Artery Rupture: A Rare Cause of Haemoperitoneum. Acta Chir. Belg. 2009, 109, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Priya, H.; Anshul, G.; Alok, T.; Saurabh, K.; Ranjit, N.; Romesh, L.; Deborshi, S. Emergency cholecystectomy and hepatic arterial repair in a patient presenting with haemobilia and massive gastrointestinal haemorrhage due to a spontaneous cystic artery gallbladder fistula masquerading as a pseudoaneurysm. BMC Gastroenterol. 2013, 13, 43. [Google Scholar] [CrossRef]
- Patil, N.S.; Kumar, A.H.; Pamecha, V.; Gattu, T.; Falari, S.; Sinha, P.K.; Mohapatra, N. Cystic artery pseudoaneurysm—A rare complication of acute cholecystitis: Review of literature. Surg. Endosc. 2022, 36, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Sibria, D.; Elawad, M.; Aker, L.J.; Barah, A.; Almokdad, O.; Ladumor, S.B.; Mohamed, M.A.; Al Rashid, A.A.; Alahmad, Y.M.; Kambal, A.; et al. Cystic Artery Stump Pseudoaneurysm Following Laparoscopic Cholecystectomy: A Case Report. Cureus 2024, 16, e64551. [Google Scholar] [CrossRef]
- Nakase, Y.; Takagi, T.; Fukumoto, K.; Kassai, K.; Yamagami, T.; Itani, K.; Miyagaki, T. Hemobilia and cystic artery stump pseudoaneurysm associated with liver abscess after a laparoscopic cholecystectomy: Report of a case. Surg. Today 2008, 38, 567–571. [Google Scholar] [CrossRef]
- Lin, K.-T.; Lin, Z.-Y.; Huang, C.-C.; Yu, S.-Y.; Huang, J.-L.; Lin, J.-H.; Lin, Y.-R. Prehospital ultrasound scanning for abdominal free fluid detection in trauma patients: A systematic review and meta-analysis. BMC Emerg. Med. 2024, 24, 7. [Google Scholar] [CrossRef] [PubMed]
- Netherton, S.; Milenkovic, V.; Taylor, M.; Davis, P.J. Diagnostic accuracy of eFAST in the trauma patient: A systematic review and meta-analysis. CJEM 2019, 21, 727–738. [Google Scholar] [CrossRef]
- Tamburrini, S.; Consoli, L.; Garrone, M.; Sfuncia, G.; Lugarà, M.; Coppola, M.G.; Piccirillo, M.; Toto, R.; Stella, S.M.; Sofia, S.; et al. The “Black Pattern”, a Simplified Ultrasound Approach to Non-Traumatic Abdominal Emergencies. Tomography 2022, 8, 798–814. [Google Scholar] [CrossRef]
- Taouk, J.; Lacomblez, D.; Bosschaert, P. Doppler Ultrasound Diagnosis of Cystic Artery Pseudo-Aneurysm Causing Hemobilia. J. Belg. Soc. Radiol. 2024, 108, 50. [Google Scholar] [CrossRef]
- Robbie, R.; Amrita, R.; Adrian, C.; Alexander, S.; Shastri, S.; Maharaj, P. Cystic artery pseudoaneurysm. Radiol. Case Rep. 2024, 19, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Jindal, G.; Khan, L.; Chaube, R. A Rare Case of Cystic Artery Pseudoaneurysm because of Cholecystitis Managed with Non-invasive Technique. Middle East J. Dig. Dis. 2023, 15, 285–288. [Google Scholar] [CrossRef]
- Scaglione, M.; Masala, S.; Iacobellis, F.; Tonerini, M.; Sica, G.; Liguori, C.; Saba, L.; Tamburrini, S. Imaging in Non-Traumatic Emergencies. Tomography 2023, 9, 1133–1136. [Google Scholar] [CrossRef]
- Wittenberg, A.; Minotti, A.J. CT Diagnosis of Traumatic Gallbladder Injury. AJR Am. J. Roentgenol. 2005, 185, 1573–1574. [Google Scholar] [CrossRef]
- Carey, F.; Rault, M.; Crawford, M.; Lewis, M.; Tan, K. Case report: Cystic artery pseudoaneurysm presenting as a massive per rectum bleed treated with percutaneous coil embolization. CVIR Endovasc. 2020, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.A.; Chawla, T.; Nadeem, M. Cystic artery pseudoaneurysm secondary to acute cholecystitis as cause of haemobilia. Case Rep. 2011, 2011, bcr0720114480. [Google Scholar] [CrossRef]
- Shelmerdine, S.C.; Ameli-Renani, S.; Lynch, J.O.; Gonsalves, M. Transarterial catheter embolisation for an unusual cause of upper gastrointestinal haemorrhage. BMJ Case Rep. 2015, 2015, bcr2014206837. [Google Scholar] [CrossRef]
- Ini’, C.; Distefano, G.; Sanfilippo, F.; Castiglione, D.G.; Falsaperla, D.; Giurazza, F.; Mosconi, C.; Tiralongo, F.; Foti, P.V.; Palmucci, S.; et al. Embolization for acute nonvariceal bleeding of upper and lower gastrointestinal tract: A systematic review. CVIR Endovasc. 2023, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Sarmento Costa, M.; Gravito-Soares, M.; Gravito-Soares, E.; Figueiredo, P. Unexpected cause of upper gastrointestinal bleeding: Beyond the typical complications of gallstone disease. Rev. Esp. Enfermedades Dig. 2024. [Google Scholar] [CrossRef] [PubMed]
- Giurazza, F.; Corvino, F.; Pane, F.; Di Serafino, M.; Niola, R. Combined DSA- and US-guided management of acute bleeding: Effectiveness of percutaneous glue embolization in six cases. J. Ultrasound 2024, 27, 179–184. [Google Scholar] [CrossRef]
- Heidari, A.; Ghane, Y.; Heidari, N.; Kasraianfard, A.; Kargar, M.; Moradi, A.M. Successful management of a ruptured cystic artery pseudoaneurysm with embolization and cholecystectomy: A case report. Clin. Case Rep. 2024, 12, e9427. [Google Scholar] [CrossRef]
- Anns, K.M.; Khan, F.; Aman, M.; Shahid, J.; Haq, T.U.I.; Memon, W.A.; Saeed, M.A.; Khalid, A.; Ahmed, K.A.H.M.; Akram, S. Pseudoaneurysm of cystic artery stump after laparoscopic cholecystectomy managed successfully with branch hepatic artery embolization using jail technique. J. Surg. Case Rep. 2024, 2024, rjae152. [Google Scholar] [CrossRef]
- Okamoto, S.; Matsui, Y.; Komoto, S.; Hiraki, T. Transarterial Embolization for Cystic Artery Pseudoaneurysm Caused by Hepatocellular Carcinoma Rupture in the Gallbladder: A Case Report. Cureus 2024, 16, e56400. [Google Scholar] [CrossRef]
- Saha, B.; Parasar, K.; Kodali, R.; Anwar, S. Ruptured cystic artery pseudoaneurysm: A clinical insight. BMJ Case Rep. 2024, 17, e259152. [Google Scholar] [CrossRef]
- Mie, T.; Sasaki, T.; Matsueda, K.; Okamoto, T.; Hirai, T.; Ishitsuka, T.; Yamada, M.; Nakagawa, H.; Furukawa, T.; Takeda, T.; et al. Ruptured cystic artery pseudoaneurysm after self-expandable metal stent placement for malignant biliary obstruction. DEN Open 2024, 4, e304. [Google Scholar] [CrossRef]
- Khan, H.; Lourdusamy, V.; Bansal, R. Cystic Artery Pseudoaneurysm Secondary to Cholecystitis: A Rare Cause of Hemobilia. Cureus 2023, 15, e39161. [Google Scholar]
- Kim, H.-C.; Jeong, Y.S.; Han, K.; Kim, G.M. Transcatheter arterial embolization of cystic artery bleeding. Front. Surg. 2023, 10, 1160149. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-L.; Hsieh, C.-T.; Yeh, Y.-J.; Liu, H. Cystic artery pseudoaneurysm: A case report. World J. Clin. Cases 2023, 11, 242–248. [Google Scholar] [CrossRef]
- Williams, T.; Maher, A.; Redmond, K.; Yeung, S.; Ko, B.S. Hemobilia in the setting of cystic artery pseudoaneurysm secondary to type I Mirizzi syndrome. Clin. J. Gastroenterol. 2023, 16, 605–609. [Google Scholar] [CrossRef]
- Zainab, R.; Tahir, M.M.; Khalid, D.; Ali, M.; Zaidi, M.; Abro, K. Transarterial embolisation of cystic artery pseudo aneurysm: A rare complication of acute cholecystitis. J. Pak. Med. Assoc. 2023, 73, 1106–1107. [Google Scholar]
- Itagaki, Y.; Yamamoto, K.; Kikuchi, T.; Takano, H.; Nishigami, K.; Fukunaga, A.; Ichimura, T.; Manase, H.; Hirano, S. Laparoscopic cholecystectomy after transcatheter arterial embolisation for haemobilia due to a pseudoaneurysm in the gallbladder: A case report. SAGE Open Med. Case Rep. 2023, 11, 2050313X231166777. [Google Scholar] [CrossRef]
- Christodoulou, P.; Liapis, S.-C. Early Rupture of Iatrogenic Cystic Artery Pseudoaneurysm After Unsuccessful Laparoscopic Cholecystectomy: A Case Report. Cureus 2022, 14, e22865. [Google Scholar] [CrossRef]
- Fukushima, R.; Ishii, N.; Harimoto, N.; Araki, K.; Watanabe, A.; Tsukagoshi, M.; Hagiwara, K.; Yamanaka, T.; Shirabe, K. A case of Mirizzi syndrome accompanied by a pseudoaneurysm that ruptured into the gallbladder: Successfully treated by embolization of aneurysm and sequential surgery. Surg. Case Rep. 2022, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Amakye, D.O.; Atemnkeng, N.; Hama, E.; Person, E.B. A Case of Massive Lower Gastrointestinal Bleed from a Cystic Artery Pseudoaneurysm Bleeding through a Cholecystocolic Fistula. Am. J. Case Rep. 2021, 22, e931921-1–e931921-4. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, S.; Shaikh, O.H.; Kumbhar, U.S.; Mohan, A. Cystic artery pseudoaneurysm due to carcinoma of the gallbladder. BMJ Case Rep. 2021, 14, e241714. [Google Scholar] [CrossRef]
- Nguyen, D.; Goodwin, J.S.; Bhowmik, N.; Boiteau, G.; Potts, J. Acute Hemorrhagic Cholecystitis with Large Hemoperitoneum: Treatment with Microcoil Embolization and Subsequent Cholecystectomy. J. Radiol. Case Rep. 2021, 15, 25–34. [Google Scholar] [CrossRef]
- Acharya, S.; Mukherjee, I.; Anwar, S.; Lan, G.; Polavarapu, A. Haemobilia Secondary to Spontaneous Cystic Artery-Gallbladder Fistula: A Unique Gastrointestinal Anomaly. Cureus 2020, 12, e9457. [Google Scholar] [CrossRef] [PubMed]
- Leshen, M.; Hubert, J.; Cantos, A. Pediatric cystic artery pseudoaneurysm embolization. Clin. Imaging 2020, 61, 80–83. [Google Scholar] [CrossRef]
- Proença, A.L.; Gomes, F.V.; Costa, N.; Bilhim, T.; Luz, J.H.; Coimbra, É. Transarterial Embolization of Iatrogenic Cystic Artery Pseudoaneurysm. GE-Port. J. Gastroenterol. 2020, 27, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Yam, M.K.; Sim, S.W.; Tam, K.Y.; Li, Y.L. A 51-year-old female presenting with shock due to hemorrhagic cholecystitis. Radiol. Case Rep. 2020, 15, 2547–2549. [Google Scholar] [CrossRef]
- Rossini, M.; Bonati, E.; Cozzani, F.; Marcato, C.; Del Rio, P. Hemobilia due to cystic artery pseudoaneurysm following cholecystectomy: Diagnosis and management, a case report. Acta Biomed. 2019, 90, 595–598. [Google Scholar] [CrossRef]
- Sada, D.M.; Metwalli, Z.A. Cystic Artery Hemorrhage after Cholecystostomy Catheter Exchange Treated with Transcatheter Embolization. Semin. Interv. Radiol. 2019, 36, 108–110. [Google Scholar] [CrossRef]
- Tanaka, T.; Takakura, K.; Maruyama, Y.; Hidaka, A.; Nakano, M.; Torisu, Y.; Saruta, M. Hemobilia Derived from Cystic Artery Pseudoaneurysm. Case Rep. Gastroenterol. 2019, 13, 89–94. [Google Scholar] [CrossRef]
- Kuzman, M.S.; Adiamah, A.; Higashi, Y.; Gomez, D. Rare case of cystic artery pseudoaneurysm. BMJ Case Rep. 2018, 2018, bcr-2017-223789. [Google Scholar] [CrossRef] [PubMed]
- Maddineni, S.; Lim, M.M.D.; McCabe, S.; Rozenblit, G. Transcatheter embolization of a cystic artery pseudoaneurysm in a cirrhotic patient with perforated acute cholecystitis. Indian J. Radiol. Imaging 2017, 27, 521–523. [Google Scholar] [CrossRef]
- Tapnio, R.H.; Kolber, M.K.; Shukla, P.A.; Berkowitz, E. Transcatheter Embolization of Cystic Artery Pseudoaneurysms Secondary to Acute Cholecystitis. Vasc. Endovasc. Surg. 2017, 51, 498–500. [Google Scholar] [CrossRef]
- Thillai, M.; Sethi, P.; Menon, R.N.; Kader, N.P. Cystic artery pseudoaneurysm following acute necrotising pancreatitis. BMJ Case Rep. 2017, 2017, bcr-2016-218891. [Google Scholar] [CrossRef] [PubMed]
- Trombatore, C.; Scilletta, R.; Bellavia, N.; Trombatore, P.; Lio, V.M.S.; Petrillo, G.; Di Cataldo, A. Acute hemobilia from a pseudoaneurysm of the cystic artery arising from the left hepatic artery: Case report and literature review. Int. J. Surg. Case Rep. 2017, 37, 60–64. [Google Scholar] [CrossRef]
- Hall, T.C.; De Rover, W.S.; Habib, S.; Kumaran, M. Cystic artery pseudoaneurysm secondary to acute cholecystitis: An unusual cause for haemobilia. BJR|Case Rep. 2016, 2, 20150423. [Google Scholar] [CrossRef]
- Liu, B.; Lewis, A.R.; Ward, T.J. Cystic Artery Pseudoaneurysm. J. Vasc. Interv. Radiol. 2016, 27, 694. [Google Scholar] [CrossRef] [PubMed]
- Aljiffry, M.M.; Almulhim, A.N.; Jamal, M.H.; Hassanain, M.M. Acute cholecystitis presenting with massive intra-abdominal haemorrhage. J. Surg. Case Rep. 2014, 2014, rju019. [Google Scholar] [CrossRef]
- Kumar, A.; Sheikh, A.; Partyka, L.; Contractor, S. Cystic artery pseudoaneurysm presenting as a complication of laparoscopic cholecystectomy treated with percutaneous thrombin injection. Clin. Imaging 2014, 38, 522–525. [Google Scholar] [CrossRef]
- Mokrane, F.-Z.; Alba, C.G.; Lebbadi, M.; Mejdoubi, M.; Moulabbi, M.; Lombard, F.; Lengellé, F.; Aveillan, M. Pseudoaneurism of the cystic artery treated with hyperselective embolisation alone. Diagn. Interv. Imaging 2013, 94, 641–643. [Google Scholar] [CrossRef] [PubMed]
- Nana, G.R.; Gibson, M.; Speirs, A.; Ramus, J.R. Upper gastrointestinal bleeding: A rare complication of acute cholecystitis. Int. J. Surg. Case Rep. 2013, 4, 761–764. [Google Scholar] [CrossRef]
- Chong, J.J.; O’Connell, T.; Munk, P.L.; Yang, N.; Harris, A.C. Case of the Month #176: Pseudoaneurysm of the Cystic Artery. Can. Assoc. Radiol. J. 2012, 63, 153–155. [Google Scholar] [CrossRef]
- Petrou, A.; Brennan, N.; Soonawalla, Z.; Silva, M.A. Hemobilia Due to Cystic Artery Stump Pseudoaneurysm Following Laparoscopic Cholecystectomy: Case Presentation and Literature Review. Int. Surg. 2012, 97, 140–144. [Google Scholar] [CrossRef]
- Desai, A.; Saunders, M.; Anderson, H.; Howlett, D. Successful Transcatheter Arterial Embolisation of a Cystic Artery Pseudoaneurysm Secondary to Calculus Cholecystitis: A Case Report. J. Radiol. Case Rep. 2010, 4, 18–22. [Google Scholar] [CrossRef]
- Hague, J.; Brennand, D.; Raja, J.; Amin, Z. Cystic Artery Pseudoaneurysms in Hemorrhagic Acute Cholecystitis. Cardiovasc. Interv. Radiol. 2010, 33, 1287–1290. [Google Scholar] [CrossRef] [PubMed]
- Nkwam, N.; Heppenstall, K. Unruptured Pseudoaneurysm of the cystic artery associated with acute calculus cholecystitis. J. Surg. Case Rep. 2010, 2010, 4. [Google Scholar] [CrossRef]
- Contini, S.; Uccelli, M.; Sassatelli, R.; Pinna, F.; Corradi, D. Gallbladder ulcer eroding the cystic artery: A rare cause of hemobilia. Am. J. Surg. 2009, 198, e17–e19. [Google Scholar] [CrossRef] [PubMed]
- Mullen, R.; Suttie, S.A.; Bhat, R.; Evgenikos, N.; Yalamarthi, S.; McBride, K.D. Microcoil Embolisation of Mycotic Cystic Artery Pseudoaneurysm: A Viable Option in High-Risk Patients. Cardiovasc. Interv. Radiol. 2009, 32, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Sakamoto, Y.; Esaki, M.; Kosuge, T. Pseudoaneurysm of the Cystic Artery Associated with Xanthogranulomatous Cholecystitis. Dig. Surg. 2008, 25, 8–9. [Google Scholar] [CrossRef]
- Osada, H.; Honda, N.; Takahashi, T.; Oku, S.; Watanabe, W.; Okada, T.; Ohno, H.; Hondo, M.; Nishimura, K. Arteriovenous malformation of the gallbladder: CT and angiographic findings. Radiat. Med. 2007, 25, 73–75. [Google Scholar] [CrossRef]
- Saluja, S.S.; Ray, S.; Gulati, M.S.; Pal, S.; Sahni, P.; Chattopadhyay, T.K. Acute cholecystitis with massive upper gastrointestinal bleed: A case report and review of the literature. BMC Gastroenterol. 2007, 7, 12. [Google Scholar] [CrossRef]
- Maeda, A.; Kunou, T.; Saeki, S.; Aono, K.; Murata, T.; Niinomi, N.; Yokoi, S. Pseudoaneurysm of the cystic artery with hemobilia treated by arterial embolization and elective cholecystectomy. J. Hepato-Biliary-Pancreat. Surg. 2002, 9, 755–758. [Google Scholar] [CrossRef]
- Delgadillo, X.; Berney, T.; de Perrot, M.; Didier, D.; Morel, P. Successful Treatment of a Pseudoaneurysm of the Cystic Artery with Microcoil Embolization. J. Vasc. Interv. Radiol. JVIR 1999, 10, 789–792. [Google Scholar] [CrossRef]
- England, R.; Marsh, P.; Ashleigh, R.; Martin, D. Case report: Pseudoaneurysm of the cystic artery: A rare cause of haemobilia. Clin. Radiol. 1998, 53, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Akatsu, T.; Tanabe, M.; Shimizu, T.; Handa, K.; Kawachi, S.; Aiura, K.; Ueda, M.; Shimazu, M.; Kitajima, M. Pseudoaneurysm of the Cystic Artery Secondary to Cholecystitis as a Cause of Hemobilia: Report of a Case. Surg. Today 2007, 37, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Chen, L.; Zhao, B.; Huang, H.; Lu, Z.; Su, H. Transcatheter arterial embolization for massive hemobilia with N-butyl cyanoacrylate (NBCA) Glubran 2. Acta Radiol. 2022, 63, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Saldinger, P.F.; Wang, J.Y.; Boyd, C.; Lang, E. Cystic artery stump pseudoaneurysm following laparoscopic cholecystectomy. Surgery 2002, 131, 585–586. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).