Abstract
Celiac disease (CD), also known as gluten-sensitive enteropathy, is an inflammatory autoimmune reaction triggered by ingestion of gluten in genetically predisposed subjects. Celiac disease is often associated with a wide range of disorders, caused by immune responses and by malabsorption with subsequent nutritional deficiencies. Prevalent neurologic manifestations are ataxia, epilepsy, cerebral calcification, cerebral white matter lesions, peripheral neuropathy and myopathy, but also cognitive impairment. The study aimed to identify emerging and urgent research domains in order to establish a CD-specific patient-tailored protocol that includes both psychological and neuropsychological evaluations. We performed a systematic search of MEDLINE, PubMed, SCOPUS, Web of Science and Cochrane library in November 2022. We conducted a descriptive analysis of the characteristics of the included literature. Based on the exclusion/inclusion criteria, a total of seven articles were included in the scoping review process. This review demonstrated the lack of research on CD-related cognitive impairment key features and tries to focus on both cognitive and psychological manifestations as well as their two-way interaction. We tried to establish the specific areas involved, in order to have a comprehensive view of this condition and trying to determine a correct way of assessing CD cognitive impairment and its correlations with psychological distress and personal coping skills to a chronic condition. Nevertheless, research on this topic is progressively increasing and future studies should address specific key points.
1. Introduction
Celiac disease (CD), also known as gluten-sensitive enteropathy, is an inflammatory autoimmune reaction triggered by ingestion of gluten in genetically predisposed subjects.
It is a malabsorptive disease in which gluten-containing food ingestion provokes intestinal mucosa injuries with varying degrees of villous atrophy and the only effective treatment is a strict gluten-free diet (GFD) which can improve nutrient absorption and ameliorate mucosal lesions, unless a refractory sprue occurs [1,2].
In western European countries, the prevalence is around 1%, similar to USA levels, with some unexplained differences between countries: the prevalence is 2.4% in Finland, 0.3% in Germany and 0.7% in Italy [3].
CD shows a wide range of clinical presentations, for example according to the Oslo definition, its clinical spectrum includes four main patterns [2]:
- Typical CD: The classic manifestation of CD with full expression of gluten-sensitive enteropathy associated with malabsorption, and positive duodenal biopsy and serology for Endomysial (IgA) and Tissue Transglutaminase antibodies (tTG).
- Atypical CD: Full expression of gluten-sensitive enteropathy found in association with non-gastrointestinal symptoms.
- Silent CD: Patients presenting a normal biopsy but positive serology.
- Refractory CD: Patients with severe mucosal injury but do not respond to a GFD.
The clinical manifestations of the disease in CD patients depend on the severity of mucosal damage, and are measured with MARSH scores, which are used to describe the stages of injury of duodenal or small bowel mucosa [4]. A study on the effects of a GFD on mucosal healing demonstrated that gluten withdrawal, independently of diet duration, can improve the villous height to crypt depth ratio (V/C) and reduce inflammation, but on the other hand, the epithelium still maintains some long-term damage [5].
In childhood, symptomatology usually exacerbates after ablactation and includes nutritional deficiencies and anemia, followed by other manifestations such as stunted growth, short stature, muscle wasting, hypotonia, abdominal distension and watery diarrhea [2,6]. Concerning adulthood, CD may onset at any age, and adults often complain of diarrhea, steatorrhea, weight loss, flatulence and abdominal pain, but many others may have no gastrointestinal symptoms [7]. An emerging topic in CD is that it is also associated with a wide range of extraintestinal and neurological symptomatology.
Raiteri et al. in their comparative analysis [8] defined the most frequent extraintestinal manifestations in CD patients, ranging from oral–dental abnormalities (dental enamel and recurrent oral aphthous stomatitis) as suggested by ESsCD 2019 guidelines, to reproductive disorders (delayed sexual development, amenorrhea, infertility and recurrent miscarriage) and other manifestations such as chronic fatigue, joint and muscle pain and reduced bone mineral density.
Pantic et al. [9] in their 2022 literature review collected studies focused on the occurrence of another extraintestinal manifestation in CD patients: thrombotic events (TE). The most common areas involved in thrombosis seems to be hepatic veins (30.9%), followed by deep venous thrombosis and pulmonary thromboembolism.
This study underlines the fact that CD can be considered as a risk factor for TEs, present in 58.7% of the sample.
Other relevant neurological manifestations could be ataxia, epilepsy, cerebral calcification, cerebral white matter lesions, peripheral neuropathy and myopathy and even cognitive impairments [10,11].
Regarding the unclear etiology of the neurological manifestations in CD patients, a study by Volta et al. [12] showed that modifications in immune system functioning, resulting in anti-ganglioside serum positivity, can lead to both neurological and cognitive impairment in this population.
In particular, the study found a high prevalence of anti-ganglioside antibodies, especially anti-GM1, in CD patients with neurological alterations, suggesting a strong relationship between anti-ganglioside serum positivity and neurological symptoms in celiac disease.
The authors also found that the introduction of a gluten-free diet induced serum normalization in about 50% of celiac patients presenting neurological dysfunctions. Another notable study by Cervio et al. [13] showed that serum positivity to antineuronal antibodies could also lead to neurologic impairment in celiac disease patients, triggering neuronal apoptosis.
The authors suggested the inclusion of both anti-ganglioside and antineuronal antibodies assessments in CD patients’ clinical protocols. Gastrointestinal manifestations often show a correlation with neurological symptoms and cognitive impairment, due to their temporally close onset [14].
In the last 20 years, few studies have investigated in-depth the implications of CD-related cognitive impairments; this is an emerging topic to define the main mental functions involved, as well the cognitive and emotional patterns.
The study aimed to identify the research domains in order to establish a CD-specific patient-tailored protocol that includes both psychological and neuropsychological evaluation. We tried to establish the specific areas involved to have a comprehensive view of this condition and try to determine a correct way of assessing CD cognitive impairment and its correlations with psychological distress and personal coping skills to a chronic condition.
2. Materials and Methods
We conducted a scoping review to map the literature about celiac disease-associated cognitive impairment. The framework outlined by Arksey and O’Malley (2005) in their methodological paper on scoping studies was adopted. Our aim was to summarize and disseminate the currently available evidence describing cognitive impairment features in CD patients and to propose key points for future research.
Specifically, the research objectives of this review are:
- To summarize the current evidence on the features of CD-associated cognitive impairments.
- To identify gaps and limits in the literature that may require further research.
- To identify relevant research questions, particularly, the determinants of future approaches.
2.1. Search Strategy
We performed a systematic search of MEDLINE via PubMed, including SCOPUS, Web of Science and Cochrane library in November 2022 with the advanced queries: (Celiac disease) AND (Cognitive impairment), (Celiac disease) AND (Dementia). The range of the search strategy was restricted to the 2000–2022 period.
2.2. Inclusion and Exclusion Criteria
We included all literature related to CD-associated cognitive impairment published in English from 2000 to 2022, excluding reviews, clinical trials and qualitative studies. Exclusion criteria were the absence of neuropsychological assessment, non-human studies and non-CD studies.
2.3. Article Selection and Data Extraction
The reviewers independently screened all titles, abstracts and full texts and resolved disagreements by consensus or consultation with a third reviewer. The following information were extracted: (i) title, (ii) authors, (iii) journal, (iv) publication or posted date, (v) type of article/study, (vi) topic, (viii) objectives of study and (ix) results.
2.4. Statistical Analysis
We conducted a descriptive analysis of the characteristics of the included literature. We described the source, publication date, type of article/study and topic of article/study to examine the existing gaps in research. We exclusively reviewed studies which included a neuropsychological assessment. We conducted this scoping review in accordance with the PRISMA flowchart (Page, 2021), reported in Figure 1.
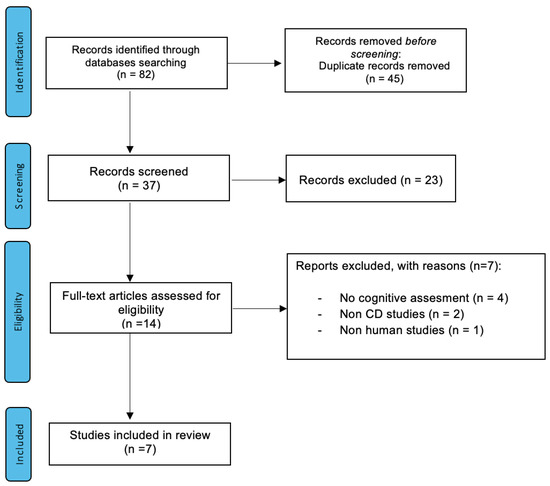
Figure 1.
PRISMA flowchart of selection process for the scoping review of celiac disease-associated cognitive impairment articles/studies until May 2021.
3. Results
3.1. Search Results
Our initial search returned n° 82 papers from our database. After duplicate and inadequate articles were removed, we identified n° 14 records that underwent title and abstract review for relevance. Seven articles were excluded after a full-text review. Figure 1 illustrates the study selection flowchart.
3.2. Characteristics of Included Articles/Studies
The articles included in the scoping review were mainly case–control studies (n° = 6) as well as one cohort study.
3.3. Source of Articles/Studies
All articles/studies have been published in peer-reviewed journals. The platform of publication was generally with a gastroenterological outlook (Nutrients, Gastroenterology, Digestive and Liver Disease and Journal of Clinical Gastroenterology) and involving mostly Italian and British authors. Moreover, looking at the posted date of publication, interest in this topic seemed to be increasing progressively over time, with five studies between 2020 and 2021.
3.4. Type of Articles/Studies
The types of published articles are reported in Table 1b.

Table 1.
(a) Publishers of articles/studies. (b) Characteristics of celiac disease-associated cognitive impairment studies.
Table 1b reports the included studies. The most relevant research topic was the investigation of cognitive impairment associated with celiac disease. Following this, the next most relevant topics were on the linked emotional features, the ameliorative effect of gluten free diet and finally on neuroimaging/TMS investigation of celiac patients’ brains.
All of the seven reviewed studies [15,16,17,18,19,20,21] were focused on CD-related cognitive impairment and four of them [15,16,17,18] also evaluated ameliorative effects of a gluten-free diet.
The seven included papers [15,16,17,18,19,20,21] also focused on the correlation between emotional features (especially depression, anxiety and quality of life) and cognitive impairment. Finally, three of the reviewed papers made use of Magnetic Resonance Imaging (MRI) data and Transcranial Magnetic Stimulation (TMS) techniques to investigate white matter changes [19], interhemispheric excitability [20] and central cholinergic functioning [21].
Table 2 contains the details of the research protocol from each of the papers.
3.5. Cognitive Modification in CD
The cognitive impairments associated with CD was the main topic of our study. The psychological measurem ents applied to evaluate global cognition in CD were as follows: the Mini Mental State Evaluation (MMSE) [15], Montreal Cognitive Assessment (MoCA) [20,21], Wechsler Adult Intelligence Scale (WAIS) [18], Addenbrooke’s Cognitive Examination (ACE-R) [17] and Subtle Cognitive Impairment Test (SCIT) [16]. All psychological tests are standardized tests and commonly used in cognitive and psychological assessing in aging.
A relevant point was that most of the reviewed studies highlighted impaired performance in CD patients.
With regard to global cognition, Casella et al. [15] evidenced significant differences (p = 0.02) in MMSE raw scores in >65-year-old patients in comparison with sex/age matched controls. In addition, Fisicaro et al. [20] and Lanza et al. [21] highlighted a significant difference in MoCA between 15 de novo patients and healthy controls. Longarini et al. [17] assessed global functioning with ACE-R and evidenced significant impairment (p = 0.02) correlated with depressive symptoms. On contrary, Lichtwark et al. [16] did not find significant modifications during the 52-week pre-post study protocol.
Cognitive functioning also underwent a domain-specific analysis. The most used test was the Trial Making Test (TMT), performed in four out of eight studies [15,16,18,19], which evaluates visual search, processing speed, mental flexibility and executive functions. Casella et al. [15] demonstrated a significant difference between CD patients and healthy controls in TMT B-A scores only while Litchtwark et al. [16] highlighted a substantial improvement in TMT A performance, between week 0 and week 52 of GFD. In contrast, in both Croall et al. studies [18,19], TMT resulted in non-significant differences between CD patients (mean age = 65) and healthy controls.
Several memory tests, Rey Auditory Verbal Learning test (RAVLT) [15,16], Story Recall [15,18], Rey–Osterrieth complex figure (ROCF) [16,18], Digit Span Test [18,19] and semantic/episodic memory evaluations [15], were performed in five out of seven studies and a memory decline was assessed in four of them.
Casella et al. [15] found significant differences in semantic memory (p = 0.03) and in Digit Span scores (p = 0.007) in elderly CD patients (≥65 years) compared to healthy controls and Licthwark et al. [16] found a substantial improvement in the ROCF test (visual memory), both in 3 min and 30 min recall between week 0 and week 52 of GFD in CD patients (mean age 30.5). Similar results were shown by Croall et al. in their pilot study on 54 CD patients (mean age 44) [18], which found an underperformance in visual (p < 0.001) and verbal memory (p = 0.046) in both newly diagnosed (NCD) and established celiac (ECD) patients compared to healthy controls. Two studies [16,19] evaluated reaction time; the first showed a significant improvement at SCIT-RTh (CD patients mean age 30.5) from baseline 580 ms to 530 ms at the week 52 follow-up and the second found a slower performance in the reaction time task in CD patients (mean score 621.2 ms) compared to controls (mean score 583.9 ms).
Regarding other functions, one study [15] also found significant differences in the Semantic Fluency Test (p = 0.03), Ideo-motor test (p < 0.001) and bucco-facial apraxia tests (p < 0.002) between elderly (≥65 years) CD patients and sex/age matched controls.
All the other tests (Table 2) performed in the seven studies did not show any significant impairment in CD patients.

Table 2.
Characteristics of the reviewed studies.
Table 2.
Characteristics of the reviewed studies.
| Authors | Topic | Sample Size | Target Sample | Tests | Outcomes |
|---|---|---|---|---|---|
| Casella et al. (2012) [15] | C.I. + GFD + EMOT. FEATURES | n° 18 | Patients aged ≥65 at the time of CD diagnosis All tested t-Tg or i-GA antibodies and villous atrophy (MARSH III in 16 patients, MARSH I-II in 2 patients) | -MMSE -TMT (A, B, B-A) -Rey list -Verbal Fluency -Verbal span -Short story test -Digit symbol -Semantic memory -Episodic memory -Ideo-motor apraxia -Bucco-facial apraxia -GDS | “Raw score” significantly lower in CD than controls for: -Mini Mental Test Examination (p = 0.02), -Trial making test (p = 0.001), -Semantic fluency (p = 0.03), -Digit Symbol Test (p = 0.007), -Ideo-motor apraxia (p < 0.001), -Bucco-facial apraxia (p < 0.002). “Equivalent score” lower in CD than controls for: -Semantic memory (p < 0.01), -Ideo-motor apraxia (p = 0.007). Barthel Index of functional performance was similar in the 2 groups. |
| Lichtwark et al. (2014) [16] | C.I. + GFD + EMOT. FEATURES | n° 11 | CD diagnosed patients (MARSH III or MARSH I and II + antibodies positivity) GFD less than 4 weeks prior to the enrollment Excellent adherence to diet Mean age = 30 (Age range = 22–39) | -SCIT -TMT A&B-ROCF COWAT -RAVLT -Grooved Pegboard Task -WTAR -STPI (anxiety and depression subscales) | At week 52: MARSH improved significantly (p = 0.001) Tissue transglutaminase decreased from mean of 58.4 at baseline to 16.8 U/mL at week 52 (p = 0.025). Significant improvement at: -verbal fluency, -attention, -motor functions. Improvement showed strong correlation with improvements in Marsh scores and tissue transglutaminase antibody levels (r = 0.377–0.735; all p< 0.05). Cognition was impaired in people with untreated CD. |
| Longarini et al. (2019) [17] | C.I. + GFD + EMOT. FEATURES | n° 53 | Adults with symptoms and signs compatible with CD Mean age = 34 (Age range = 18–50) | -ACE-R -INECO -FAQ -BDI -STAI | 33 CD patients (66%) compared to healthy controls, CD cases and disease controls had: -impaired cognitive performance (p = 0.02 and p = 0.04, respectively), -functional impairment (p < 0.01), -higher depression (p < 0.01). CD patients compared to controls: -Similar cognitive performance, -Anxiety, -Nonsignificant lower depression scores compared with disease controls. Abnormal cognitive functions detected in adult NCD not disease-specific. Cognitive dysfunction was related to presence of prolonged symptoms due to a chronic disease. |
| Croall et al. (2020) [18] | C.I. + GFD + EMOT. FEATURES | n° 54 | NCD = 19 (Newly diagnosed CD); ECD = 35 (Established CD) Mean age = 44 (Age range = 18–70) | -ToPF -WAIS (Block design, vocabulary, matrix reasoning, similarities) -TMT -COWAT -Digit span -Story recall -CVLT -ROCF -Digit-symbol coding -SOIP -BDAE (verbal agility) -SF-36 (QOL) | NCD and ECD underperformed compared to controls, by comparable degrees in: -visual memory (overall model: p < 0.001), -verbal memory (p = 0.046). The ECD group underperformed only in visuo-constructive abilities (p = 0.050). QoL: NCD = lower vitality (p = 0.030), ECD = more bodily pain (p = 0.009). Dysfunction appears established at the point of diagnosis, after which it (predominantly) stabilizes. |
| Croall et al. (2020) [19] | C.I. + EMOT. FEATURES + NEURO IMAGING | n° 104 | 65% female, Mean age = 63 | -Digit span -Biobank’s fluid intelligence -TMT B-A -Pair matching task 6 questions exploring: -anxiety -depression -happiness with own health -suicidal thoughts -thoughts of self-harm | Compared with control individuals, participants with celiac disease had significant deficits in: -reaction time (p = 0.004). Significantly higher proportions had indications of: -anxiety (p = 0.025), -depression (p = 0.015), -thoughts of self-harm (p = 0.025), -health-related unhappiness (p = 0.010). White matter changes in the brains of participants with celiac disease. |
| Fisicaro et al. (2021) [20] | C.I. + EMOT. FEATURES + TMS | n° 15 | 15 right-handed, neurologically asymptomatic, de novo CD patients Mean age 34.1 | -MOCA -HDRS -TMS | -MoCA and HDRS scored significantly worse in CD patients. -iSP and cSP significantly shorter in duration in CD, positive correlation between the MoCA and iSP -Intracortical and interhemispheric motor disinhibition observed in CD, suggesting the involvement of GABA-mediated cortical and callosal circuitries. |
| Lanza et al. (2021) [21] | C.I. + EMOT. FEATURES + TMS | n° 15 | 15 right-handed, neurologically asymptomatic, de novo CD patients Mean age 34.1 | -MOCA -HDRS -TMS | Central cholinergic functioning not affected in de novo CD patients compared to healthy controls. Statistically significant difference in MoCA, but no overt cognitive impairment in CD patients. |
3.6. Effects of Gluten-Free Diet
Four studies also evaluated the ameliorative effects of a gluten-free diet (GFD) on cognitive impairment in CD patients.
Casella et al. [15], evaluated eighteen ≥65-year-old CD patients who were on a GFD (mean duration = 5.5 years). Eleven patients tested as t-TG antibody negative and seven patients tested intermittently as t-TG antibody positive or negative (probable low adherence to GFD) during annual follow-up testing. This study did not find any difference in cognitive functioning between the two groups, underlining that cognitive performance is worse in elderly CD patients compared to healthy controls despite adherence and duration of GFD.
Lichtwark et al. [16] explored the link between cognitive improvement and mucosal injury amelioration in a study on 11 CD patients (MARSH III or MARSH I and II and antibodies positivity, mean age 30.5), who just started gluten withdrawal (no more than 4 weeks). Duodenal biopsy and serum testing showed that the MARSH scores improved significantly and tissue transglutaminase antibody concentrations decreased at week 52. Neuropsychological testing showed a substantial improvement in performance between week 0 and week 52 of GFD adherence (at 1 year follow-up), highlighting a strong correlation between cognitive impairment, mucosal healing and tissue transglutaminase antibody levels (r = 0.377–0.735; all p < 0.05). The results obtained with these studies suggest that cognition is impaired in people with untreated CD and that it can improve after starting a GFD in correlation with simultaneous healing of the small bowel mucosa.
Longarini et al. [17] suggested that in young untreated CD adults, cognitive functions including orientation, attention, concentration, memory, language fluency and visual–spatial abilities are impaired, and recommended additional studies investigating ameliorative long-term effects of GFD on cognitive functioning.
A recent pilot study [18] evaluated 54 CD patients (age range 18–70 years) at different disease and diet stages, dividing them in two groups: 19 NCD (newly diagnosed CD) and 35 ECD (established CD), compared to 21 healthy controls with the additional aim to understand the impact of a GFD on cognitive performance. The results showed that the NCD and ECD groups underperformed relative to controls by similar degrees, in visual and verbal memory. Meanwhile, the ECD group also underperformed in visuo-constructive abilities compared to controls.
3.7. Emotional Features
All the reviewed studies [15,16,17,18,19,20,21] evaluated emotional features, especially anxiety, depression and quality of life (QoL) alongside the cognitive assessment, with the aim to define possible links between these two dimensions in CD patients. Casella et al. [15] evaluated depression with the Geriatric Depression Scale (GDS) and did not find significant differences between groups. The lower performance at speed and attention was comparable to non-CD depressed elderly patients, suggesting focusing on the presence of depression when assessing cognitive impairment in elderly celiac patients. Longarini et al. [17] investigated the presence of depression and anxiety with the Beck Depression Inventory (BDI) and Stait Trait Anxiety Inventory (STAI). CD cases and disease controls (mostly Irritable Bowel Syndrome, IBS) had the worst cognitive performance and higher depression than healthy controls.
CD patients showed similar cognitive performance and anxiety compared with IBS controls. The authors suggested that cognitive impairment is not CD-specific but is related to the chronicity of the condition. Croall et al.’s pilot study [18] focused on quality of life in celiac patients (mean age 44) using the eight outcomes of the Short Form Health Survey 36 (SF-36) questionnaire: physical roles, bodily pain, vitality, general health, social functioning, emotional roles and mental health. The authors found significant differences compared to healthy controls in the bodily pain dimension especially in established CD patients and vitality dimension especially for newly diagnosed. Moreover, they found no correlation between cognitive scores and SF-36 outcomes. Later in 2020, the same author [19] alongside a cognitive assessment, evaluated emotive differences between a sample of 104 CD patients (mean age 63) and control individuals with six questions exploring anxiety, depression, happiness with own health, suicidal thoughts and thoughts of self-harm.
The authors reported worsened mental well-being in the CD sample. The significant results were: higher prevalence of anxiety, depression, thoughts of self-harm and health-related unhappiness.
Recently, Fisicaro et al. [20] and Lanza et al. [21] confronted performance on the MoCA (Montreal Cognitive Assessment) and HDSR (Hamilton Depression Rating Scale) between the same sample of 15 neurologically asymptomatic, de novo CD patients (mean age 34.1) and 15 age-matched healthy controls. The studies revealed worse MoCA and HDSR scores in CD individuals compared to controls. Finally, Licthwark et al. [16] did not find significant improvements between week 0 and 52 in the Anxiety and Depression subscales of the State Inventory Personality Inventory (STPI), as well as no significant correlation between STPI and mucosal healing (MARSH scores) or serum tTG levels in CD patients (mean age 30.5).
3.8. Neuroimaging and TMS
Three of the studies [19,20,21] performed neuroimaging investigations (MRI) and Transcranial Magnetic stimulation (TMS) to evaluate the presence of organic damage in celiac patients’ brains (mean age 63). Croall et al. in their population-based study [19] collected MRI data including T1-Weighted Images, FLAIR and DTI and found extensive white matter changes, with a progression pattern similar to the first stages of vascular dementia which, according to the authors, cannot be recovered with GDF and could be related to the slower reaction time highlighted by the cognitive evaluation. In 2021, in CD patients (mean age 34.1) on normal diet, Fisicaro et al. [20] non-invasively explored the interhemispheric functioning using TMS to find causality between brain activations and different types of sensory, motor and cognitive functions.
The authors revealed worse MoCA and HDSR scores in celiac individuals compared to controls, highlighting evidence of intracortical and interhemispheric motor disinhibition, in terms of a shorted cSP duration. Lanza et al. [21] conducted another evaluation on the same sample of 15 CD patients (mean age 34.1), aiming to analyze central cholinergic functioning. Results show no impairment in thesede novo CD patients compared to age-matched healthy controls.
4. Discussion
The aim of this scoping review was to map and determine the current literature on the presence and characteristic features of cognitive impairment in celiac disease patients. Considering exclusion/inclusion criteria, a total of seven articles [15,16,17,18,19,20,21] were included in the scoping review process. These studies focused on cognitive impairment [15,16,17,18,19,20,21], as well as on ameliorative effects of a gluten-free diet [15,16,17,18] and on correlations between psychological features and cognitive decline. Cognitive impairment in celiac patients is one of the atypical manifestations of the disease, affecting various domains of celiac individuals’ cognition before the introduction of a strict GFD [14].
Regarding the cognitive impairments, in the USA, Edwards et al. [22] conducted a nationwide survey, aiming to understand cognitive symptoms associated with gluten exposure in 1396 individuals with self-reported celiac disease (n°1143 CD) and nonceliac gluten sensitivity (n°253 NCGS). The data included the presence, timing, length and peak of cognitive symptoms after gluten intake and about which specific symptoms they have experienced. Neurocognitive manifestations after gluten ingestion were widely reported (90.1% of participants). The most common symptoms reported were difficulty concentrating, forgetfulness, detachment, grogginess and mental confusion and most participants reported a peak which occurred in the first 24 h after ingestion and a so-called GINI (gluten-induced neurocognitive impairment) that lasted 1–2 days.
Just few studies found in the current literature carried out neuropsychological testing [15,16,17,18,19,20,21] in CD patients and most of them demonstrated various impairment configurations which often entirely regressed with a gluten free diet, alongside mucosal healing and serum normalization [16].
The included studies focused on looking for cognitive impairments in celiac disease patient of different ages, not exclusively in elderly patients who are usually more prone to be affected by cognitive functioning deficits.
Four studies [16,17,20,21] addressed their focus on patients with a mean age ranging between 30 [16] and 34.1 years [17,20,21]. The study by Lichtwark et al. [16] confirmed the presence of cognitive impairment in CD patients (age range 22–39) which almost entirely regressed after gluten withdrawal, underlining the fact that cognitive functioning improves along with mucosal healing. These interesting findings, according to the authors, hint at the future possibility of replacing invasive biopsies with cognitive assessments to evaluate mucosal healing.
Longarini et al. [17] evaluated cognitive impairment in middle-aged CD adults (age range 18–50), finding an anomalous cognitive performance in newly diagnosed CD individuals.
The authors suggest that this impairment is not directly caused by CD, but results from a dysfunctional coping to this chronic condition and its prolonged symptoms.
Fisicaro et al. and Lanza et al. [20,21] also focused on a middle-aged sample (mean age 34.1), finding significant differences at the Montreal cognitive assessment and Hamilton depression scores between CD patients and healthy controls, although the authors did not find an evident impairment.
The results of these studies suggest that the impaired cognitive performance highlighted in CD patients could be secondary to a depressive condition.
The other two studies [15,19] evaluated over 60 patients (≥65 years and patients with a mean age of 63 years). The first study performed by Casella et al. [15] found impaired cognition in over 65 CD patients compared to age-matched healthy controls. Noticeably, the underperformance at attention and processing speed task did not differ from the performance shown by elderly non-CD patients with depression (no GDS scores difference between two groups), suggesting again the need to evaluate the role of depressive symptoms in exacerbation of cognitive impairment in CD individuals.
Croall et al. in their population study [19] focused on CD patients not following a GFD with a mean age of 63, and confirmed cognitive impairment and the presence of white matter changes. With regard to depressive symptoms, the authors highlighted the presence of thoughts of self-harm and health-related unhappiness which are, in all likelihood, dependent on living with a chronic disease.
The last study, the pilot study by Croall et al. [18], used a sample with a wide age range (18–70) which entirely covers the age ranges set by the previously mentioned studies.
The authors found an established cognitive impairment at the time of CD diagnosis, which regresses after introducing a gluten free diet.
All the reviewed studies found cognitive impairment in CD samples compared to healthy controls regardless of the age of patients, but the most interesting feature, confirmed by most of the included studies, seems to be the frequent association between cognitive impairment and a mild depressive condition, which, combined with the daily difficulties of living with a chronic disease, could probably be the exacerbating cause of the cognitive under performance encountered.
Future research should focus on these topics, with some methodological improvements. Firstly, the sample size should be increased to clearly determine the prevalence of cognitive impairment in CD individuals and the patients should be followed for at least one year with the aim to define cognitive and psychological changes between the moment of diagnosis and a certain period on a GFD. The samples should include patients presenting various degrees of GFD adherence to better understand effect of gluten withdrawal on cognition and emotional features besides the relationship between mucosal healing and cognitive improvement. With this aim, specific tests should be performed to precisely assess GFD adherence levels as well as to evaluate the psychological factors that could influence adherence, including psychological distress and personal ability coping to a chronic disease. Following the lead of Lichtwark et al. [16], in future studies, a comparison between neuropsychological findings and MARSH [4] scores or antibody serum concentrations will be needed in order to define links between cognitive impairment and mucosal injury and confirm whether neuropsychological testing could ever replace biopsies in diagnosis and follow-up phases [16]. Moreover, when selecting celiac patients, those presenting gastrointestinal symptoms should be included because G.I. manifestations are often correlated with a higher risk of cognitive impairment and psychological distress [14]. Most of the reviewed studies used cognitive screening tools such as MMSE which possibly lacked sensitivity to subtle impairments; future studies should select sufficiently sensitive tests, such as the SCIT [23] performed by Licthwark et al. [16], with the aim to identify and monitor minor cognitive declines, which may not reach cut-off scores, as well as finding small differences between celiac patients and healthy controls as well as monitoring minor changes in the same patient. Based on Croall et al.’s [19] findings about extensive white matter damage in CD patients and its implications on cognitive functioning especially for those who did not follow a GFD, future studies should also include MRI data to assess organic brain injury.
An emerging topic in clinical management of CD focuses on the relation between cognitive impairment and vitamin deficiencies. In clinical practice, untreated celiac disease can lead to malabsorption and nutritional deficiencies. CD patients often show low levels of calcium, iron, vitamin B (especially B12), A, D and folate [24] which improve after starting a gluten-free diet [15]. Notably, nutritional deficiencies can occur even following a strict GFD [25]. Most consequences for celiac patients come from vitamin B and vitamin D: low levels of B vitamins can lead to a high plasma concentration of homocysteine, a modifiable risk factor for vascular diseases and cognitive impairment [26]. Vitamin D deficiency is a common sign found in CD patients which could lead to neurological diseases and osteoporosis [27]. Interesting clinical perspective might be consistent with further research conducted to assess whether vitamin supplementation could prevent or treat cognitive decline in CD patients, as well as defining the possible role of vitamin deficiencies in the onset of CD.
This review was performed taking into account psychosomatic medicine [28], which provides a holistic approach to patients, based on the Biopsychosocial model by Engel. This framework is focused on comprehending how psychosocial factors can influence individual vulnerability to diseases and how they can affect the course and outcomes of health issues [29]. For this reason, we strongly believe that a full comprehensive study should try correlate cognitive impairment in celiac patients, to psychological dimensions.
First of all, priority should be given to an accurate psychological distress evaluation, investigating depression, stress and anxiety dimensions and their possible effects on both cognitive impairment and adherence to GFD.
It is an established fact that anxiety is often associated with gastrointestinal diseases such as irritable bowel syndrome (IBS) [30]. It could negatively influence the frequency and intensity of gastrointestinal symptomatology, affecting the individual’s well-being and quality of life.
In our case, anxiety was often associated with CD and may worsen both G.I. and cognitive symptoms and may persist after GFD introduction [31].
Determining the presence of stressful events in the patients’ life is crucial when facing gastrointestinal diseases [32,33], celiac disease included, due to the possibility that they could promote the development and maintenance of cognitive, emotional and gastrointestinal manifestations of CD even after gluten withdrawal.
In addition, following the route drawn by psychosomatic medicine [28], personality factors should be assessed [34], in order to define the influence of personality traits on CD atypical manifestations and to try to determine their predictive value for higher risks of cognitive impairment and psychological distress related to the chronic effect of celiac disease.
5. Conclusions
In conclusion, this review suggests the need to implement into clinical practice and related scientific perspective an extension of the range of evaluations performed during the diagnosis process and at follow-ups, looking at the possibility of integrating both neuropsychological and psychological assessments as well as neuroimaging tools to establish a CD-specific patient-tailored protocol. This protocol includes a comprehensive and integrated patient approach which includes all the dimensions (biological, cognitive and psychological), that directly influence the celiac disease pathway. Biopsychosocial approached to the patient could be used as the personalized medicine perspective to perform efficient clinical therapy over time.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are thankful for the collaboration with Dott J. Ranieri in the reviewing process.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; De Giorgio, R.; Catassi, C.; Fasano, A. Celiac disease: A comprehensive current review. BMC Med. 2019, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.C.; Biagi, F.; Fasano, A.; Green, P.H.R.; Hadjivassiliou, M.; Kaukinen, K.; Kelly, C.P.; Leonard, J.N.; et al. The Oslo definitions for coeliac disease and related terms. Gut 2013, 62, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Mustalahti, K.; Catassi, C.; Reunanen, A.; Fabiani, E.; Heier, M.; McMillan, S.; Murray, L.; Metzger, M.-H.; Gasparin, M.; Bravi, E.; et al. The prevalence of celiac disease in Europe: Results of a centralized, international mass screening project. Ann. Med. 2010, 42, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.E.; Lee, H.E.; Wu, T.-T. Histologic evaluation in the diagnosis and management of celiac disease: Practical challenges, current best practice recommendations and beyond. Hum. Pathol. 2022, 132, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Mandile, R.; Maglio, M.; Mosca, C.; Marano, A.; Discepolo, V.; Troncone, R.; Auricchio, R. Mucosal Healing in Celiac Disease: Villous Architecture and Immunohistochemical Features in Children on a Long-Term Gluten Free Diet. Nutrients 2022, 14, 3696. [Google Scholar] [CrossRef] [PubMed]
- Ascher, H.; Holm, K.; Kristiansson, B.; Maki, M. Different features of coeliac disease in two neighbouring countries. Arch. Dis. Child. 1993, 69, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tapia, A.; Hill, I.D.; Kelly, C.P.; Calderwood, A.H.; Murray, J.A. American College of Gastroenterology ACG clinical guidelines: Diagnosis and management of celiac disease. Am. J. Gastroenterol. 2013, 108, 656–676. [Google Scholar] [CrossRef]
- Raiteri, A.; Granito, A.; Giamperoli, A.; Catenaro, T.; Negrini, G.; Tovoli, F. Current guidelines for the management of celiac disease: A systematic review with comparative analysis. WJG 2022, 28, 154–176. [Google Scholar] [CrossRef] [PubMed]
- Pantic, N.; Pantic, I.; Jevtic, D.; Mogulla, V.; Oluic, S.; Durdevic, M.; Nordin, T.; Jecmenica, M.; Milovanovic, T.; Gavrancic, T.; et al. Celiac Disease and Thrombotic Events: Systematic Review of Published Cases. Nutrients 2022, 14, 2162. [Google Scholar] [CrossRef] [PubMed]
- Cooke, W.T.; Smith, W.T. Neurological disorders associated with adult coeliac disease. Brain 1966, 89, 683–722. [Google Scholar] [CrossRef] [PubMed]
- Hadjivassiliou, M.; Grünewald, R.A.; Davies-Jones, G.A.B. Gluten sensitivity as a neurological illness. J. Neurol. Neurosurg. Psychiatry 2002, 72, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Volta, U.; De Giorgio, R.; Granito, A.; Stanghellini, V.; Barbara, G.; Avoni, P.; Liguori, R.; Petrolini, N.; Fiorini, E.; Montagna, P.; et al. Anti-ganglioside antibodies in coeliac disease with neurological disorders. Dig. Liver Dis. 2006, 38, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Cervio, E.; Volta, U.; Verri, M.; Boschi, F.; Pastoris, O.; Granito, A.; Barbara, G.; Parisi, C.; Felicani, C.; Tonini, M.; et al. Sera of Patients with Celiac Disease and Neurologic Disorders Evoke a Mitochondrial-Dependent Apoptosis In Vitro. Gastroenterology 2007, 133, 195–206. [Google Scholar] [CrossRef]
- Hu, W.T.; Murray, J.A.; Greenaway, M.C.; Parisi, J.E.; Josephs, K.A. Cognitive Impairment and Celiac Disease. Arch. Neurol. 2006, 63, 1440. [Google Scholar] [CrossRef]
- Casella, S.; Zanini, B.; Lanzarotto, F.; Ricci, C.; Marengoni, A.; Romanelli, G.; Lanzini, A. Cognitive performance is impaired in coeliac patients on gluten free diet: A case–control study in patients older than 65 years of age. Dig. Liver Dis. 2012, 44, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Lichtwark, I.T.; Newnham, E.D.; Robinson, S.R.; Shepherd, S.J.; Hosking, P.; Gibson, P.R.; Yelland, G.W. Cognitive impairment in coeliac disease improves on a gluten-free diet and correlates with histological and serological indices of disease severity. Aliment. Pharmacol. Ther. 2014, 40, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Longarini, G.; Richly, P.; Temprano, M.; Costa, A.F.; Vázquez, H.; Moreno, M.L.; Niveloni, S.; López, P.; Smecuol, E.; Mazure, R.; et al. A Prospective Study on Cognitive Impairment in Middle-aged Adults with Newly Diagnosed Celiac Disease. J. Clin. Gastroenterol. 2019, 53, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Croall, I.D.; Tooth, C.; Venneri, A.; Poyser, C.; Sanders, D.S.; Hoggard, N.; Hadjivassiliou, M. Cognitive Impairment in Coeliac Disease with Respect to Disease Duration and Gluten-Free Diet Adherence: A Pilot Study. Nutrients 2020, 12, 2028. [Google Scholar] [CrossRef]
- Croall, I.D.; Sanders, D.S.; Hadjivassiliou, M.; Hoggard, N. Cognitive Deficit and White Matter Changes in Persons with Celiac Disease: A Population-Based Study. Gastroenterology 2020, 158, 2112–2122. [Google Scholar] [CrossRef]
- Fisicaro, F.; Lanza, G.; D’Agate, C.C.; Ferri, R.; Cantone, M.; Falzone, L.; Pennisi, G.; Bella, R.; Pennisi, M. Intracortical and Intercortical Motor Disinhibition to Transcranial Magnetic Stimulation in Newly Diagnosed Celiac Disease Patients. Nutrients 2021, 13, 1530. [Google Scholar] [CrossRef]
- Lanza, G.; Fisicaro, F.; D’Agate, C.C.; Ferri, R.; Cantone, M.; Falzone, L.; Pennisi, G.; Bella, R.; Hadjivassiliou, M.; Pennisi, M. Preserved central cholinergic functioning to transcranial magnetic stimulation in de novo patients with celiac disease. PLoS ONE 2021, 16, e0261373. [Google Scholar] [CrossRef] [PubMed]
- Edwards George, J.B.; Aideyan, B.; Yates, K.; Voorhees, K.N.; O’Flynn, J.; Sweet, K.; Avery, K.; Ehrlich, A.; Bast, A.; Leffler, D.A. Gluten-induced Neurocognitive Impairment: Results of a Nationwide Study. J. Clin. Gastroenterol. 2022, 56, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Yelland, G.; Bruce, K.; Robinson, S.; Smith, J. Validity of a screening tool for detecting subtle cognitive impairment in the middle-aged and elderly. CIA 2014, 2014, 2165–2176. [Google Scholar] [CrossRef]
- Cardo, A.; Churruca, I.; Lasa, A.; Navarro, V.; Vázquez-Polo, M.; Perez-Junkera, G.; Larretxi, I. Nutritional Imbalances in Adult Celiac Patients Following a Gluten-Free Diet. Nutrients 2021, 13, 2877. [Google Scholar] [CrossRef] [PubMed]
- Hallert, C.; Grant, C.; Grehn, S.; Grännö, C.; Hultén, S.; Midhagen, G.; Ström, M.; Svensson, H.; Valdimarsson, T. Evidence of poor vitamin status in coeliac patients on a gluten-free diet for 10 years: Vitamin status in treated coeliac patients. Aliment. Pharmacol. Ther. 2002, 16, 1333–1339. [Google Scholar] [CrossRef]
- Smith, A.D.; Refsum, H.; Bottiglieri, T.; Fenech, M.; Hooshmand, B.; McCaddon, A.; Miller, J.W.; Rosenberg, I.H.; Obeid, R. Homocysteine and Dementia: An International Consensus Statement1. JAD 2018, 62, 561–570. [Google Scholar] [CrossRef]
- Zmijewski, M.A. Vitamin D and Human Health. IJMS 2019, 20, 145. [Google Scholar] [CrossRef]
- Fava, G.A.; Sonino, N. Psychosomatic Medicine: Emerging Trends and Perspectives. Psychother. Psychosom. 2000, 69, 184–197. [Google Scholar] [CrossRef]
- Porcelli, P.; Sonino, N. Fattori Psicologici Che Influenzano le Malattie: Una Nuova Classificazione per il DSM-5; Giovanni Fioriti: Roma, Italy, 2008; ISBN 978-88-87319-98-9. [Google Scholar]
- Fond, G.; Loundou, A.; Hamdani, N.; Boukouaci, W.; Dargel, A.; Oliveira, J.; Roger, M.; Tamouza, R.; Leboyer, M.; Boyer, L. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): A systematic review and meta-analysis. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, 651–660. [Google Scholar] [CrossRef]
- Rostami-Nejad, M.; Taraghikhah, N.; Ciacci, C.; Pourhoseingholi, M.A.; Barzegar, F.; Rezaei-Tavirani, M.; Aldulaimi, D.; Zali, M.R. Anxiety Symptoms in Adult Celiac Patients and the Effect of a Gluten-Free Diet: An Iranian Nationwide Study. Inflamm. Intest. Dis. 2020, 5, 42–47. [Google Scholar] [CrossRef]
- Mawdsley, J.E. Psychological stress in IBD: New insights into pathogenic and therapeutic implications. Gut 2005, 54, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.M.; Gramling, S.E.; Mancini, T. The influence of life stress, personality, and learning history on illness behavior. J. Behav. Ther. Exp. Psychiatry 1994, 25, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, P.; De Carne, M.; Todarello, O. Prediction of Treatment Outcome of Patients with Functional Gastrointestinal Disorders by the Diagnostic Criteria for Psychosomatic Research. Psychother. Psychosom. 2004, 73, 166–173. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).