The Role of Neuroglia in Administrating Nerve Blockers and Anesthesia to Patients
Abstract
1. Introduction
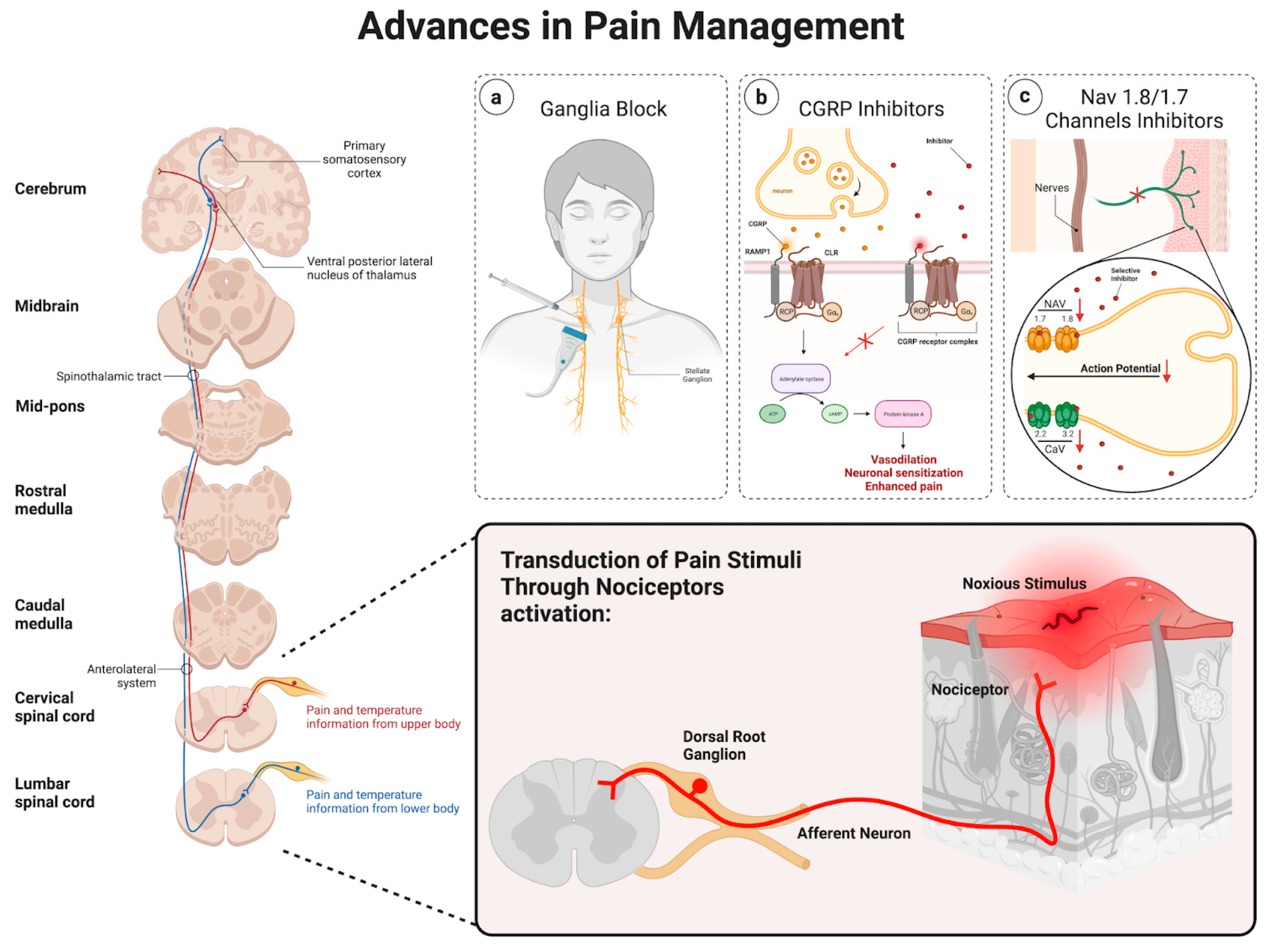
2. Pathophysiology of Pain
2.1. Ascending Pathway
2.2. Descending Pathway
2.3. Advances in Pain Management
2.4. Ganglia Injections
2.5. Calcitonin Gene-Related Peptide
2.6. NaV 1.8/1.7 Channels
3. Introduction to Various Nerve Blockers Used in Anesthesia
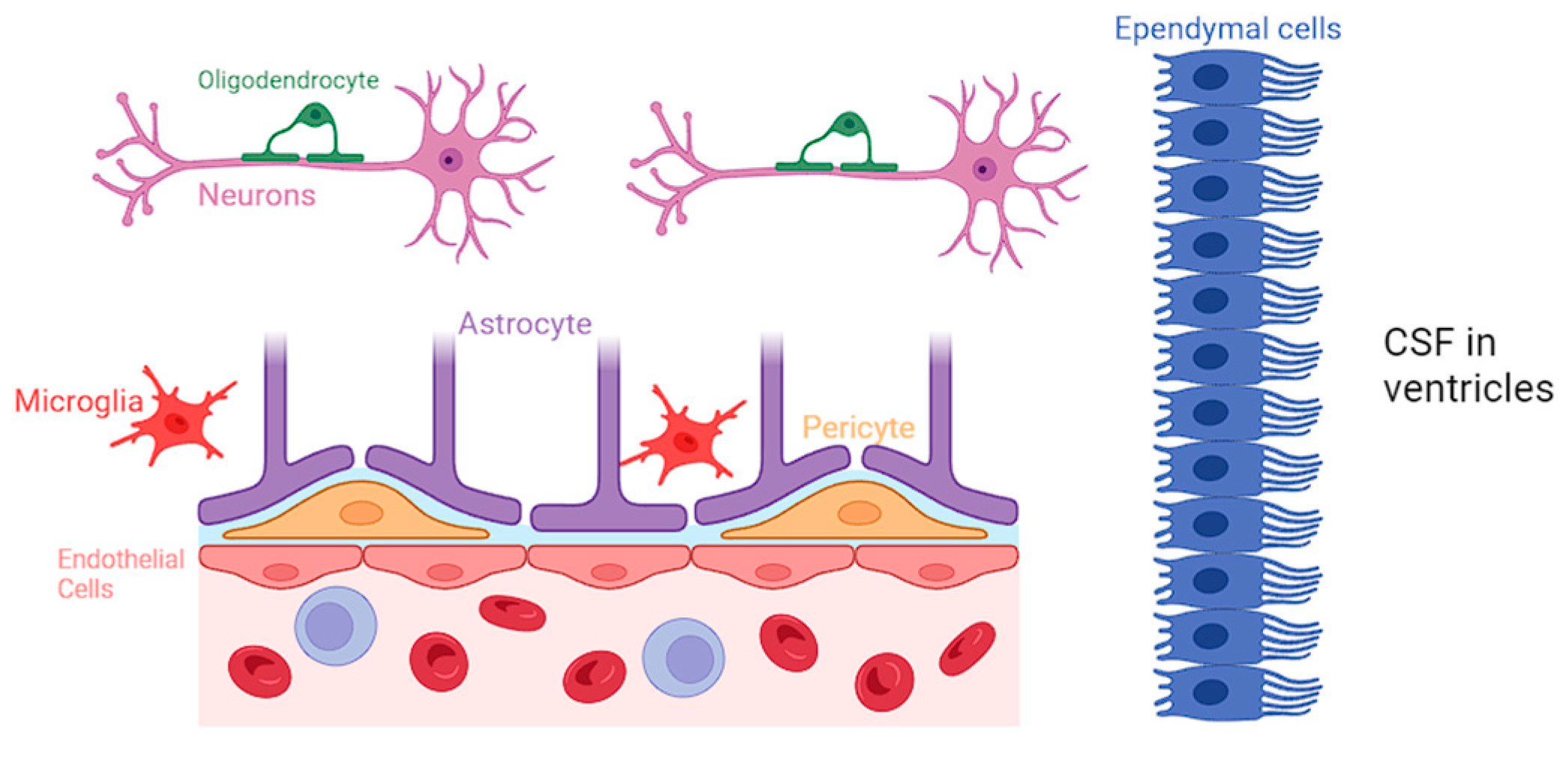
Impact of Lack of Neuroglia in Neurologic Disorders
4. Future Developments in Alternative Nerve-Blockage Therapies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gawdi, R.; Shumway, K.R.; Emmady, P.D. Physiology, Blood Brain Barrier. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Obermeier, B.; Verma, A.; Ransohoff, R.M. The blood-brain barrier. Handb. Clin. Neurol. 2016, 133, 39–59. [Google Scholar] [CrossRef]
- Cichorek, M.; Kowiański, P.; Lietzau, G.; Lasek, J.; Moryś, J. Neuroglia-development and role in physiological and pathophysiological processes. Folia Morphol. 2021, 80, 766–775. [Google Scholar] [CrossRef]
- Cekanaviciute, E.; Buckwalter, M.S. Astrocytes: Integrative Regulators of Neuroinflammation in Stroke and Other Neurological Diseases. Neurother. J. Am. Soc. Exp. NeuroTherapeutics 2016, 13, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Bordey, A. The astrocyte odyssey. Prog. Neurobiol. 2008, 86, 342–367. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, R.; Avila, M.; Gonzalez, J.; El-Bachá, R.S.; Báez, E.; García-Segura, L.M.; Jurado Coronel, J.C.; Capani, F.; Cardona-Gomez, G.P.; Barreto, G.E. Astrocytic modulation of blood brain barrier: Perspectives on Parkinson’s disease. Front. Cell. Neurosci. 2014, 8, 211. [Google Scholar] [CrossRef]
- Rochfort, K.D.; Cummins, P.M. The blood-brain barrier endothelium: A target for pro-inflammatory cytokines. Biochem. Soc. Trans. 2015, 43, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson, P.T.; Davis, T.P. Regulation of blood-brain barrier integrity by microglia in health and disease: A therapeutic opportunity. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2020, 40 (Suppl. S1), S6–S24. [Google Scholar] [CrossRef]
- Gullotta, G.S.; Costantino, G.; Sortino, M.A.; Spampinato, S.F. Microglia and the Blood-Brain Barrier: An External Player in Acute and Chronic Neuroinflammatory Conditions. Int. J. Mol. Sci. 2023, 24, 9144. [Google Scholar] [CrossRef]
- da Fonseca, A.C.; Matias, D.; Garcia, C.; Amaral, R.; Geraldo, L.H.; Freitas, C.; Lima, F.R. The impact of microglial activation on blood-brain barrier in brain diseases. Front. Cell. Neurosci. 2014, 8, 362. [Google Scholar] [CrossRef]
- Simons, M.; Nave, K.A. Oligodendrocytes: Myelination and Axonal Support. Cold Spring Harb. Perspect. Biol. 2015, 8, a020479. [Google Scholar] [CrossRef]
- Seo, J.H.; Maki, T.; Maeda, M.; Miyamoto, N.; Liang, A.C.; Hayakawa, K.; Pham, L.D.; Suwa, F.; Taguchi, A.; Matsuyama, T.; et al. Oligodendrocyte precursor cells support blood-brain barrier integrity via TGF-β signaling. PLoS ONE 2014, 9, e103174. [Google Scholar] [CrossRef]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Attwell, D.; Mishra, A.; Hall, C.N.; O’Farrell, F.M.; Dalkara, T. What is a pericyte? J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2016, 36, 451–455. [Google Scholar] [CrossRef]
- Jäkel, S.; Dimou, L. Glial Cells and Their Function in the Adult Brain: A Journey through the History of Their Ablation. Front. Cell. Neurosci. 2017, 11, 24. [Google Scholar] [CrossRef]
- Stapor, P.C.; Sweat, R.S.; Dashti, D.C.; Betancourt, A.M.; Murfee, W.L. Pericyte dynamics during angiogenesis: New insights from new identities. J. Vasc. Res. 2014, 51, 163–174. [Google Scholar] [CrossRef]
- Gaceb, A.; Özen, I.; Padel, T.; Barbariga, M.; Paul, G. Pericytes secrete pro-regenerative molecules in response to platelet-derived growth factor-BB. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2018, 38, 45–57. [Google Scholar] [CrossRef]
- Brown, L.S.; Foster, C.G.; Courtney, J.M.; King, N.E.; Howells, D.W.; Sutherland, B.A. Pericytes and Neurovascular Function in the Healthy and Diseased Brain. Front. Cell. Neurosci. 2019, 13, 282. [Google Scholar] [CrossRef]
- Sonar, S.A.; Lal, G. Blood-brain barrier and its function during inflammation and autoimmunity. J. Leukoc. Biol. 2018, 103, 839–853. [Google Scholar] [CrossRef]
- De Ridder, D.; Adhia, D.; Vanneste, S. The anatomy of pain and suffering in the brain and its clinical implications. Neurosci. Biobehav. Rev. 2021, 130, 125–146. [Google Scholar] [CrossRef]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef] [PubMed]
- Thanh, N.X.; Tanguay, R.L.; Manhas, K.J.P.; Kania-Richmond, A.; Kashuba, S.; Geyer, T.; Pereira, J.X.; Wasylak, T. Economic burden of chronic pain in Alberta, Canada. PLoS ONE 2022, 17, e0272638. [Google Scholar] [CrossRef] [PubMed]
- Yong, R.J.; Mullins, P.M.; Bhattacharyya, N. Prevalence of chronic pain among adults in the United States. Pain 2022, 163, e328–e332. [Google Scholar] [CrossRef]
- Maniadakis, N.; Gray, A. The economic burden of back pain in the UK. Pain 2000, 84, 95–103. [Google Scholar] [CrossRef]
- Dubin, A.E.; Patapoutian, A. Nociceptors: The sensors of the pain pathway. J. Clin. Investig. 2010, 120, 3760–3772. [Google Scholar] [CrossRef] [PubMed]
- Lacroix-Fralish, M.L.; Mogil, J.S. Progress in genetic studies of pain and analgesia. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 97–121. [Google Scholar] [CrossRef]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef]
- Bourne, S.; Machado, A.G.; Nagel, S.J. Basic anatomy and physiology of pain pathways. Neurosurg. Clin. N. Am. 2014, 25, 629–638. [Google Scholar] [CrossRef]
- Millan, M.J. Descending control of pain. Prog. Neurobiol. 2002, 66, 355–474. [Google Scholar] [CrossRef]
- Bagley, E.E.; Ingram, S.L. Endogenous opioid peptides in the descending pain modulatory circuit. Neuropharmacology 2020, 173, 108131. [Google Scholar] [CrossRef]
- Gunduz, O.H.; Kenis-Coskun, O. Ganglion blocks as a treatment of pain: Current perspectives. J. Pain Res. 2017, 10, 2815–2826. [Google Scholar] [CrossRef]
- Ramer, M.S.; Bisby, M.A. Adrenergic innervation of rat sensory ganglia following proximal or distal painful sciatic neuropathy: Distinct mechanisms revealed by anti-NGF treatment. Eur. J. Neurosci. 1999, 11, 837–846. [Google Scholar] [CrossRef]
- Makharita, M.Y.; Amr, Y.M.; El-Bayoumy, Y. Effect of early stellate ganglion blockade for facial pain from acute herpes zoster and incidence of postherpetic neuralgia. Pain Physician 2012, 15, 467–474. [Google Scholar]
- Lipov, E.; Ritchie, E.C. A review of the use of stellate ganglion block in the treatment of PTSD. Curr. Psychiatry Rep. 2015, 17, 599. [Google Scholar] [CrossRef]
- O’Connell, N.E.; Wand, B.M.; Gibson, W.; Carr, D.B.; Birklein, F.; Stanton, T.R. Local anaesthetic sympathetic blockade for complex regional pain syndrome. Cochrane Database Syst. Rev. 2016, 7, CD004598. [Google Scholar] [CrossRef]
- Abdi, S.; Zhou, Y.; Patel, N.; Saini, B.; Nelson, J. A new and easy technique to block the stellate ganglion. Pain Physician 2004, 7, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, W.E.; Zhang, J.M. Efficacy of stellate ganglion blockade for the management of type 1 complex regional pain syndrome. South. Med. J. 2006, 99, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.D.; Jung, S.S.; Kim, H.S.; Yun, D.H.; Kim, D.H.; Chon, J.; Hong, D.W. Efficacy of ultrasonography guided stellate ganglion blockade in the stroke patients with complex regional pain syndrome. Ann. Rehabil. Med. 2012, 36, 633–639. [Google Scholar] [CrossRef]
- Meier, P.M.; Zurakowski, D.; Berde, C.B.; Sethna, N.F. Lumbar sympathetic blockade in children with complex regional pain syndromes: A double blind placebo-controlled crossover trial. Anesthesiology 2009, 111, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Abramov, R. Lumbar sympathetic treatment in the management of lower limb pain. Curr. Pain Headache Rep. 2014, 18, 403. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Dash, H.H. Sympathetic blockade for the relief of chronic pain. J. Indian Med. Assoc. 2001, 99, 698–703. [Google Scholar] [PubMed]
- Erdine, S. Celiac ganglion block. Agri Agri (Algoloji) Dernegi’nin Yayin. Organidir = J. Turk. Soc. Algol. 2005, 17, 14–22. [Google Scholar]
- Nitschke, A.M.; Ray, C.E., Jr. Percutaneous neurolytic celiac plexus block. Semin. Interv. Radiol. 2013, 30, 318–321. [Google Scholar] [CrossRef]
- Wang, P.J.; Shang, M.Y.; Qian, Z.; Shao, C.W.; Wang, J.H.; Zhao, X.H. CT-guided percutaneous neurolytic celiac plexus block technique. Abdom. Imaging 2006, 31, 710–718. [Google Scholar] [CrossRef]
- Yasuda, I.; Wang, H.P. Endoscopic ultrasound-guided celiac plexus block and neurolysis. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2017, 29, 455–462. [Google Scholar] [CrossRef]
- Eisenberg, E.; Carr, D.B.; Chalmers, T.C. Neurolytic celiac plexus block for treatment of cancer pain: A meta-analysis. Anesth. Analg. 1995, 80, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.V.; Candido, K.D.; Raja, O.; Knezevic, N.N. Celiac plexus block in the management of chronic abdominal pain. Curr. Pain Headache Rep. 2014, 18, 394. [Google Scholar] [CrossRef]
- Bosscher, H. Blockade of the superior hypogastric plexus block for visceral pelvic pain. Pain Pract. Off. J. World Inst. Pain 2001, 1, 162–170. [Google Scholar] [CrossRef]
- Waldman, S.D.; Wilson, W.L.; Kreps, R.D. Superior hypogastric plexus block using a single needle and computed tomography guidance: Description of a modified technique. Reg. Anesth. 1991, 16, 286–287. [Google Scholar]
- Plancarte, R.; Amescua, C.; Patt, R.B.; Aldrete, J.A. Superior hypogastric plexus block for pelvic cancer pain. Anesthesiology 1990, 73, 236–239. [Google Scholar] [CrossRef]
- de Leon-Casasola, O.A.; Kent, E.; Lema, M.J. Neurolytic superior hypogastric plexus block for chronic pelvic pain associated with cancer. Pain 1993, 54, 145–151. [Google Scholar] [CrossRef]
- Plancarte, R.; de Leon-Casasola, O.A.; El-Helaly, M.; Allende, S.; Lema, M.J. Neurolytic superior hypogastric plexus block for chronic pelvic pain associated with cancer. Reg. Anesth. 1997, 22, 562–568. [Google Scholar]
- Scott-Warren, J.T.; Hill, V.; Rajasekaran, A. Ganglion impar blockade: A review. Curr. Pain Headache Rep. 2013, 17, 306. [Google Scholar] [CrossRef]
- Wemm, K., Jr.; Saberski, L. Modified approach to block the ganglion impar (ganglion of Walther). Reg. Anesth. 1995, 20, 544–545. [Google Scholar] [PubMed]
- Foye, P.M.; Buttaci, C.J.; Stitik, T.P.; Yonclas, P.P. Successful injection for coccyx pain. Am. J. Phys. Med. Rehabil. 2006, 85, 783–784. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, O.H.; Sencan, S.; Kenis-Coskun, O. Pain Relief due to Transsacrococcygeal Ganglion Impar Block in Chronic Coccygodynia: A Pilot Study. Pain Med. 2015, 16, 1278–1281. [Google Scholar] [CrossRef] [PubMed]
- Lawson, S.N.; Crepps, B.; Perl, E.R. Calcitonin gene-related peptide immunoreactivity and afferent receptive properties of dorsal root ganglion neurones in guinea-pigs. J. Physiol. 2002, 540 Pt 3, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Ruscheweyh, R.; Forsthuber, L.; Schoffnegger, D.; Sandkühler, J. Modification of classical neurochemical markers in identified primary afferent neurons with Abeta-, Adelta-, and C-fibers after chronic constriction injury in mice. J. Comp. Neurol. 2007, 502, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, S.; Ossipov, M.H.; Johnson, K.W. The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. Pain 2017, 158, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Brain, S.D.; Williams, T.J.; Tippins, J.R.; Morris, H.R.; MacIntyre, I. Calcitonin gene-related peptide is a potent vasodilator. Nature 1985, 313, 54–56. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Calcitonin gene-related peptide and its receptors: Molecular genetics, physiology, pathophysiology, and therapeutic potentials. Endocr. Rev. 1996, 17, 533–585. [Google Scholar] [CrossRef]
- Benemei, S.; Nicoletti, P.; Capone, J.G.; Geppetti, P. CGRP receptors in the control of pain and inflammation. Curr. Opin. Pharmacol. 2009, 9, 9–14. [Google Scholar] [CrossRef]
- Russell, F.A.; King, R.; Smillie, S.J.; Kodji, X.; Brain, S.D. Calcitonin gene-related peptide: Physiology and pathophysiology. Physiol. Rev. 2014, 94, 1099–1142. [Google Scholar] [CrossRef]
- Edvinsson, L. CGRP and migraine: From bench to bedside. Rev. Neurol. 2021, 177, 785–790. [Google Scholar] [CrossRef]
- Olesen, J.; Diener, H.C.; Husstedt, I.W.; Goadsby, P.J.; Hall, D.; Meier, U.; Pollentier, S.; Lesko, L.M.; BIBN 4096 BS Clinical Proof of Concept Study Group. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N. Engl. J. Med. 2004, 350, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Bigal, M.E.; Dodick, D.W.; Krymchantowski, A.V.; VanderPluym, J.H.; Tepper, S.J.; Aycardi, E.; Loupe, P.S.; Ma, Y.; Goadsby, P.J. TEV-48125 for the preventive treatment of chronic migraine: Efficacy at early time points. Neurology 2016, 87, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Bigal, M.E.; Edvinsson, L.; Rapoport, A.M.; Lipton, R.B.; Spierings, E.L.; Diener, H.C.; Burstein, R.; Loupe, P.S.; Ma, Y.; Yang, R.; et al. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of chronic migraine: A multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol. 2015, 14, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Bigal, M.E.; Escandon, R.; Bronson, M.; Walter, S.; Sudworth, M.; Huggins, J.P.; Garzone, P. Safety and tolerability of LBR-101, a humanized monoclonal antibody that blocks the binding of CGRP to its receptor: Results of the Phase 1 program. Cephalalgia Int. J. Headache 2014, 34, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L.; Haanes, K.A.; Warfvinge, K.; Krause, D.N. CGRP as the target of new migraine therapies-successful translation from bench to clinic. Nat. Rev. Neurol. 2018, 14, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, G.; McMahon, S.B. The physiological function of different voltage-gated sodium channels in pain. Nat. Rev. Neurosci. 2021, 22, 263–274. [Google Scholar] [CrossRef]
- Alles, S.R.A.; Smith, P.A. Peripheral Voltage-Gated Cation Channels in Neuropathic Pain and Their Potential as Therapeutic Targets. Front. Pain Res. 2021, 2, 750583. [Google Scholar] [CrossRef]
- Hameed, S. Nav1.7 and Nav1.8: Role in the pathophysiology of pain. Mol. Pain 2019, 15, 1744806919858801. [Google Scholar] [CrossRef] [PubMed]
- Emery, E.C.; Luiz, A.P.; Wood, J.N. Nav1.7 and other voltage-gated sodium channels as drug targets for pain relief. Expert Opin. Ther. Targets 2016, 20, 975–983. [Google Scholar] [CrossRef]
- Stewart, J.W.; Dickson, D.; Van Hal, M.; Aryeetey, L.; Sunna, M.; Schulz, C.; Alexander, J.C.; Gasanova, I.; Joshi, G.P. Ultrasound-guided erector spinae plane blocks for pain management after open lumbar laminectomy. Eur. Spine J. 2023. Correction in Eur. Spine J. 2023. [Google Scholar] [CrossRef]
- Mehlmann, F.M.G.; Ferraro, L.H.C.; Sousa, P.C.C.B.D.; Cunha, G.P.; Bergamaschi, E.C.Q.A.; Takeda, A. Ultrasound-guided selective nerve blocks for trigger finger surgeries to maintain flexion/extension of fingers-Case series. Braz. J. Anesthesiol. 2019, 69, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Curatolo, M.; Bogduk, N. Diagnostic blocks for chronic pain. Scand. J. Pain. 2010, 1, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Piraccini, E.; Munakomi, S.; Chang, K.V. Stellate Ganglion Blocks. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK507798/ (accessed on 24 December 2023).
- Bogduk, N. On diagnostic blocks for lumbar zygapophysial joint pain. F1000 Med. Rep. 2010, 2, 57. [Google Scholar] [CrossRef]
- Gupta, R.; Madanat, L.; Jindal, V.; Gaikazian, S. Celiac Plexus Block Complications: A Case Report and Review of the Literature. J. Palliat. Med. 2021, 24, 1409–1412. [Google Scholar] [CrossRef]
- Erdine, S. Complications of Splanchnic and Celiac Plexus Block. In Complications of Pain-Relieving Procedures; Erdine, S., Staats, P.S., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar] [CrossRef]
- John, R.S.; Dixon, B.; Shienbaum, R. Celiac Plexus Block. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK531469/ (accessed on 24 December 2023).
- Avila Hernandez, A.N.; Singh, P. Epidural Anesthesia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK542219/ (accessed on 24 December 2023).
- Kompoliti, K.; Horn, S.S. Chapter 55—Drug-Induced and Iatrogenic Neurological Disorders. In Textbook of Clinical Neurology, 3rd ed.; Goetz, C.G., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2007; pp. 1285–1318. [Google Scholar] [CrossRef]
- Bhattaram, S.; Shinde, V.S. Novel use of motor-sparing genicular nerve blocks for knee injuries in the emergency department. Am. J. Emerg. Med. 2024, 75, 196.e1–196.e4. [Google Scholar] [CrossRef] [PubMed]
- Dass, R.M.; Kim, E.; Kim, H.K.; Lee, J.Y.; Lee, H.J.; Rhee, S.J. Alcohol neurolysis of genicular nerve for chronic knee pain. Korean J. Pain. 2019, 32, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Choi, S.S.; Yoon, S.H.; So-Hee, L.; Seo, D.K.; Lee, I.G.; Woo-Jong, C.; Jin-Woo, S. Ultrasound-Guided Genicular Nerve Block for Knee Osteoarthritis: A Double-Blind, Randomized Controlled Trial of Local Anesthetic Alone or in Combination with Corticosteroid. Pain Physician 2018, 21, 41–52. [Google Scholar] [PubMed]
- Baxter, C.S.; Singh, A.; Ajib, F.A.; Fitzgerald, B.M. Intercostal Nerve Block. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK482273/ (accessed on 24 December 2023).
- Guerra-Londono, C.E.; Privorotskiy, A.; Cozowicz, C.; Hicklen, R.S.; Memtsoudis, S.G.; Mariano, E.R.; Cata, J.P. Assessment of Intercostal Nerve Block Analgesia for Thoracic Surgery: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e2133394. [Google Scholar] [CrossRef] [PubMed]
- Alexander, C.E.; De Jesus, O.; Varacallo, M. Lumbar Sympathetic Block. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK431107/ (accessed on 24 December 2023).
- Carroll, I.; Clark, J.D.; Mackey, S. Sympathetic block with botulinum toxin to treat complex regional pain syndrome. Ann. Neurol. 2009, 65, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Barreto Junior, E.P.S.; Nascimento, J.D.S.; Castro, A.P.C.R. Neurolytic block of the lumbar sympathetic chain improves chronic pain in a patient with critical lower limb ischemia. Braz. J. Anesthesiol. 2018, 68, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.; Hinson, M.R. Occipital Nerve Block. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK580523/ (accessed on 24 December 2023).
- Castillo-Álvarez, F.; Hernando de la Bárcena, I.; Marzo-Sola, M.E. Greater occipital nerve block in the treatment of headaches. Review of evidence. Med. Clín. (Engl. Ed.) 2023, 161, 113–118. [Google Scholar] [CrossRef]
- Ghanavatian, S.; Leslie, S.W.; Derian, A. Pudendal Nerve Block. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK551518/ (accessed on 24 December 2023).
- Cok, O.Y.; Eker, H.E.; Cok, T.; Akin, S.; Aribogan, A.; Arslan, G. Transsacral S2-S4 Nerve Block For Vaginal Pain Due To Pudendal Neuralgia. J. Minim. Invasive Gynecol. 2011, 18, 401–404. [Google Scholar] [CrossRef]
- Goel, V.; Patwardhan, A.M.; Ibrahim, M.; Howe, C.L.; Schultz, D.M.; Shankar, H. Complications associated with stellate ganglion nerve block: A systematic review. Reg. Anesth. Pain Med. 2019. [Google Scholar] [CrossRef]
- Jeon, Y. Therapeutic potential of stellate ganglion block in orofacial pain: A mini-review. J. Dent. Anesth. Pain Med. 2016, 16, 159–163. [Google Scholar] [CrossRef]
- Jacques, N.; Karoutsos, S.; Marais, L.; Nathan-Denizot, N. Quality of life after trigeminal nerve block in refractory trigeminal neuralgia: A retrospective cohort study and literature review. J. Int. Med. Res. 2022, 50, 3000605221132027. [Google Scholar] [CrossRef]
- Kumar, A.; Sinha, C.; Kumar, A.; Kumari, P.; Mukul, S.K. Ultrasound-guided trigeminal nerve block and its comparison with conventional analgesics in patients undergoing faciomaxillary surgery: Randomised control trial. Indian J. Anaesth. 2018, 62, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Vila-Pueyo, M.; Gliga, O.; Gallardo, V.J.; Pozo-Rosich, P. The Role of Glial Cells in Different Phases of Migraine: Lessons from Preclinical Studies. Int. J. Mol. Sci. 2023, 24, 12553. [Google Scholar] [CrossRef]
- Mungoven, T.J.; Henderson, L.A.; Meylakh, N. Chronic Migraine Pathophysiology and Treatment: A Review of Current Perspectives. Front. Pain Res. 2021, 2, 705276. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; An, Q.; Li, R.; Chen, S.; Gu, X.; An, S.; Wang, Z. Calcitonin gene-related peptide induces the histone H3 lysine 9 acetylation in astrocytes associated with neuroinflammation in rats with neuropathic pain. CNS Neurosci. Ther. 2021, 27, 1409–1424. [Google Scholar] [CrossRef]
- E Hirbec, H.; Noristani, H.N.; Perrin, F.E. Microglia Responses in Acute and Chronic Neurological Diseases: What Microglia-Specific Transcriptomic Studies Taught (and did Not Teach) Us. Front. Aging Neurosci. 2017, 9, 227. [Google Scholar] [CrossRef]
- Gerber, Y.N.; Sabourin, J.C.; Rabano, M.; Vivanco, M.; Perrin, F.E. Early functional deficit and microglial disturbances in a mouse model of amyotrophic lateral sclerosis. PLoS ONE 2012, 7, e36000. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.R. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef] [PubMed]
- Tafti, D.; Ehsan, M.; Xixis, K.L. Multiple Sclerosis. [Updated 7 September 2022]. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499849/ (accessed on 2 January 2024).
- Murphy, K.L.; Bethea, J.R.; Fischer, R. Neuropathic Pain in Multiple Sclerosis—Current Therapeutic Intervention and Future Treatment Perspectives. In Multiple Sclerosis: Perspectives in Treatment and Pathogenesis [Internet]; Zagon, I.S., McLaughlin, P.J., Eds.; Codon Publications: Brisbane, AU, USA, 27 November 2017; Chapter 4. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470151/ (accessed on 2 January 2024). [CrossRef]
- Gritsch, S.; Lu, J.; Thilemann, S.; Wörtge, S.; Möbius, W.; Bruttger, J.; Karram, K.; Ruhwedel, T.; Blanfeld, M.; Vardeh, D.; et al. Oligodendrocyte ablation triggers central pain independently of innate or adaptive immune responses in mice. Nat. Commun. 2014, 5, 5472. [Google Scholar] [CrossRef]
- Pisciotta, C.; Saveri, P.; Pareyson, D. Challenges in Treating Charcot-Marie-Tooth Disease and Related Neuropathies: Current Management and Future Perspectives. Brain Sci. 2021, 11, 1447. [Google Scholar] [CrossRef]
- Kaye, A.D.; Ridgell, S.; Alpaugh, E.S.; Mouhaffel, A.; Kaye, A.J.; Cornett, E.M.; Chami, A.A.; Shah, R.; Dixon, B.M.; Viswanath, O.; et al. Peripheral Nerve Stimulation: A Review of Techniques and Clinical Efficacy. Pain Ther. 2021, 10, 961–972. [Google Scholar] [CrossRef]
- Schmitt, H.J.; Huberth, S.; Huber, H.; Münster, T. Catheter-based distal sciatic nerve block in patients with Charcot-Marie-Tooth disease. BMC Anesthesiol. 2014, 14, 8. [Google Scholar] [CrossRef]
- Haight, E.S.; Forman, T.E.; Cordonnier, S.A.; James, M.L.; Tawfik, V.L. Microglial Modulation as a Target for Chronic Pain: From the Bench to the Bedside and Back. Anesth. Analg. 2019, 128, 737–746. [Google Scholar] [CrossRef]
- Park, J.; Park, H.J. Botulinum Toxin for the Treatment of Neuropathic Pain. Toxins 2017, 9, 260. [Google Scholar] [CrossRef] [PubMed]
- Luvisetto, S. Botulinum Neurotoxins beyond Neurons: Interplay with Glial Cells. Toxins 2022, 14, 704. [Google Scholar] [CrossRef] [PubMed]
- Crosby, N.D.; Janik, J.J.; Grill, W.M. Modulation of activity and conduction in single dorsal column axons by kilohertz-frequency spinal cord stimulation. J. Neurophysiol. 2017, 117, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Hebl, J.R.; Horlocker, T.T.; Schroeder, D.R. Neuraxial anesthesia and analgesia in patients with preexisting central nervous system disorders. Anesth. Analg. 2006, 103, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Jeanmonod, D.; Werner, B.; Morel, A.; Michels, L.; Zadicario, E.; Schiff, G.; Martin, E. Transcranial magnetic resonance imaging-guided focused ultrasound: Noninvasive central lateral thalamotomy for chronic neuropathic pain. Neurosurg. Focus 2012, 32, E1. [Google Scholar] [CrossRef] [PubMed]
- Ailani, J.; Rabany, L.; Tamir, S.; Ironi, A.; Starling, A. Real-World Analysis of Remote Electrical Neuromodulation (REN) for the Acute Treatment of Migraine. Front. Pain Res. 2022, 2, 753736. [Google Scholar] [CrossRef] [PubMed]
- Mouraux, D.; Brassinne, E.; Sobczak, S.; Nonclercq, A.; Warzée, N.; Sizer, P.S.; Tuna, T.; Penelle, B. 3D augmented reality mirror visual feedback therapy applied to the treatment of persistent, unilateral upper extremity neuropathic pain: A preliminary study. J. Man. Manip. Ther. 2017, 25, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, K.; Chen, Y.; He, C.; Christo, P.J. Stem Cell Therapy for Chronic Pain Management: Review of Uses, Advances, and Adverse Effects. Pain Physician 2017, 20, 293–305. [Google Scholar] [CrossRef]
| Procedure | Mechanism | Indication | Side Effects | Reference |
|---|---|---|---|---|
| Celiac plexus block (CPB) | Targets visceral afferent pain fibers from the liver, gallbladder, omentum, pancreas, mesentery, and stomach to the mid-transverse colon | Pain secondary to pancreatic cancer, chronic pancreatitis, and intractable abdominal pain | Transient or persistent diarrhea, paraplegia (anterior spinal artery syndrome), postural hypotension, pneumothorax | [81,82,83] |
| Epidural nerve block | Injected anesthetic in the epidural space temporarily numbs spinal nerves, blocking pain signals from spinal cord levels | Surgical procedures: pelvic fractures, cesarean delivery, labor analgesia, hepatic, gastric, and colonic surgeries Nonsurgical: myasthenia gravis, malignant hyperthermia, hyperreflexia | Hypotension, nausea, vomiting, post-puncture headache after dural perforation. [9] Incidence of transient paralysis is 0.1%; that of permanent paralysis is 0.02% [10]. Paresthesia with or without motor weakness, epidural hematoma, abscess, hypoalgesia of lower extremities | [84,85] |
| Genicular nerve block (GNB) | Anesthetizes sensory nerve terminal branches of genicular arteries or at the junction of the epiphysis and diaphysis of the femur and tibia, sparing motor function | Chronic knee osteoarthritis, post-operative knee pain, total knee arthroplasty, alternative to femoral, fascia iliaca, and adductor canal nerve blocks in knee injuries [11] | Leg muscle weakness, dizziness, and discomfort at injection site | [86,87,88] |
| Intercostal nerve block (ICNB) | Anesthetic injection to intercoastal nerves below each rib | Rib fracture neuralgia, thoracostomy analgesia, herpes zoster neuralgia, upper abdominal surgery, palliative cancer pain for rib and chest wall tumors | Self-limited bruising and soreness at the injection site. Serious: bleeding, infection, pneumothorax, nerve damage | [89,90] |
| Lumbar sympathetic nerve block | Disrupts the nerve supply from the preganglionic neurons exiting the spinal cord via the white rami of the ventral root of spinal nerves L1 to L4 and synapse at the lumbar sympathetic ganglion to the postganglionic neurons innervating the lower extremities | Sciatica, Complex Regional Pain Syndrome (CPRS), phantom limb pain, and lower limb painful ischemia | Flushing of skin, bleeding, bruising, soreness at the injection site, headache, and leg weakness on ipsilateral injection. Serious: infection, visceral injury, Horner’s syndrome | [91,92,93] |
| Occipital nerve block | C2 sensory neurons of the greater occipital nerve create a nociceptive pathway with the trigeminal nucleus caudalis, relieving compression and nerve irritation when targeted with an anesthetic | Occipital neuralgia, chronic intractable migraine, and cervicogenic and cluster headache treatment alternative in elderly and pregnant populations | Dizziness, vertigo, numbness, lightheadedness, vasovagal syncope, facial edema, and alopecia at injection if administered with steroid | [94,95] |
| Pudendal nerve block | Transcutaneous (perineal) or transvaginal approach targets the pudendal nerve trunk and its sensorimotor innervation | Pudendal neuralgia, obstetric (e.g., second stage of vaginal birth, vaginal repairs, hemorrhoidectomy), and urologic procedures (e.g.,transrectal ultrasound-guided prostate biopsy, transurethral prostatectomy) | Discomfort at the injection site, serious side effect of bladder and rectum structural injury, and pudendal artery puncture infection | [96,97] |
| Stellate ganglion block | Interrupts signals to the cervical sympathetic chain and postganglionic fibers for sympathetic innervation of upper limbs | CRPS of head and upper limbs, peripheral vascular disease, chronic post-surgical pain, postherpetic neuralgia, orofacial pain, scleroderma | Temporary pain, eyelid droopiness, fever, local blood aspiration, hematoma formation, spondylitis, and rare convulsions | [98,99] |
| Trigeminal nerve block | The ophthalmic (V1), maxillary (V2), and mandibular (V3) divisions and their corresponding nerves are blocked | Trigeminal neuralgia, pre-emptive analgesia in maxillofacial surgery | Difficulty chewing and swallowing and transient facial weakness and numbness | [100,101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, A.; Al-Bahou, R.; Thakkar, R.; Patel, D.; Foster, D.; Benjamin, J.; Pedreira, M.; Lucke-Wold, B. The Role of Neuroglia in Administrating Nerve Blockers and Anesthesia to Patients. Neuroglia 2024, 5, 13-26. https://doi.org/10.3390/neuroglia5010002
Patel A, Al-Bahou R, Thakkar R, Patel D, Foster D, Benjamin J, Pedreira M, Lucke-Wold B. The Role of Neuroglia in Administrating Nerve Blockers and Anesthesia to Patients. Neuroglia. 2024; 5(1):13-26. https://doi.org/10.3390/neuroglia5010002
Chicago/Turabian StylePatel, Anjali, Raja Al-Bahou, Rajvi Thakkar, Drashti Patel, Devon Foster, Jonathan Benjamin, Marian Pedreira, and Brandon Lucke-Wold. 2024. "The Role of Neuroglia in Administrating Nerve Blockers and Anesthesia to Patients" Neuroglia 5, no. 1: 13-26. https://doi.org/10.3390/neuroglia5010002
APA StylePatel, A., Al-Bahou, R., Thakkar, R., Patel, D., Foster, D., Benjamin, J., Pedreira, M., & Lucke-Wold, B. (2024). The Role of Neuroglia in Administrating Nerve Blockers and Anesthesia to Patients. Neuroglia, 5(1), 13-26. https://doi.org/10.3390/neuroglia5010002






