Abstract
Forest fires represent a natural element in the dynamics of forest ecosystems. This study investigated the impact of a large-scale forest fire in 2022 (ca. 1300 ha) on epigeic ground beetles (Coleoptera: Carabidae). The research was conducted in coniferous forests at six pairwise study sites: burnt and unburnt dead spruce from bark beetles, burnt and unburnt clear cut, and burnt and unburnt healthy sites. Each site was replicated in four plots, with two pitfall traps deployed within each plot. In total, 48 pitfall traps (6 × 4 × 2) were installed in April 2023. It was tested how individual sites affected the similarity of ground beetle communities, whether they contained similar life guilds, and how significantly large-scale fire affects the abundance of pyrophilous ground beetles. A total of 5952 individuals and 63 species were recorded. We observed a significant decline in abundance at clear-cut and dead spruce burnt sites (73% and 77.5%, respectively) compared to the unburnt sites. Conversely, abundance increased by 88% at the burnt healthy site compared to the unburnt healthy site. Additionally, significant differences in the number of species per trap and species richness diversity (q = 0, q = 1, q = 2) were found only between burnt and unburnt healthy sites. In general, the highest species richness in the comparison of all study sites was at unburnt clear-cut and burnt healthy sites. Communities of ground beetles responded considerably to the fire, differing significantly from unburnt sites, and demonstrating a high degree of similarity. The original healthy spruce stands had highly homogeneous communities. On the contrary, any disturbance (bark beetle calamity, clear-cut) resulted in an increase in the alpha, beta, and gamma diversities of the ground beetle communities. Burnt sites attracted pyrophilous species (Sericoda quadripunctata, Pterostichus quadrifoveolatus) at very low abundances, with the highest activity in the second half of the season. In conclusion, ground beetles demonstrated a strong short-term response to large-scale fire, forming specific communities. However, pyrophilous ground beetles were unable to occupy a large-scale fire area due to the initial low abundance. Understanding post-fire processes can provide important guidance for management in areas designated for biodiversity enhancement.
1. Introduction
Wildfires are a common part of the natural dynamics of forest ecosystems [1]. The main forest biomes regulated by wildfires are Siberian and North American boreal forests [2]. Drier and warmer periods contribute to wildfires [3] due to the weakened health of forest stands [2,4], making them more susceptible to wind disturbances and bark beetle gradations. Although permanently present dead wood is essential for forest biodiversity [5,6,7] and provides numerous ecosystem services [8,9,10,11], it also creates fuel for the spread of fires [12,13]. In Europe, forest fires are relatively infrequent, yet historically, they constituted a significant aspect of landscape settlement [14]. However, presently, human carelessness is the primary factor in initiating forest fires, with the season, time of day, and working or non-working days further influencing their occurrence [15]. The impact of forest fires extends to various components of both living and non-living ecosystems, including soil chemistry [16], vertebrates [17], invertebrates [18], and plants [19,20]. Pyroentomological research, examining relationships between fire and invertebrates, in Europe has predominantly focused on Fennoscandia and Russia [21,22,23,24,25,26], as well as the Mediterranean region [27,28]. Similar pyroentomological studies are rare in Central European regions [29,30,31,32,33], which is also related to the importance given to the fire protection of forests in Central Europe [13].
Ground beetles (Carabidae) are a frequently studied bioindicator group among epigeic beetles in various research domains. Their examination includes, e.g., the impact of tree composition in forest stands [34], the effect of forestry and agricultural management [35,36,37], and the effect of different disturbances, such as fire and wind, on their species assemblages [18,38,39]. Ground beetles, along with butterflies, represent the most extensively studied group of insects in relation to forest fires [18]. Burnt forests play a crucial role as an essential habitat for the pyrophilous species of ground beetles and other beetle species [33,40], as well as hymenopterans [30]. The reproduction of “fire-loving” (pyrophilous) beetles depends on forest fires [41]. Pyrophilous insects represent a highly specialized and endangered group of insects [40]. Pyrophilous beetles have evolved specific adaptations to thrive in extreme fire-related conditions, including exceptional olfactory abilities to detect smoke over long distances [42], infrared sensors for identifying excessively hot surfaces [43], and high dispersal and reproductive capabilities [40,44,45]. Pyrophilous ground beetles primarily use the sterility of their post-fire environment to reproduce without facing predatory competition [45]. According to the Catalogue of Palaearctic Coleoptera in Central Europe [46] there are three species of pyrophilous carabids: Sericoda quadripunctata (De Geer, 1774) and Pterostichus quadrifoveolatus (Letzner, 1852) and a species not yet recorded in the Czech Republic, Sericoda bogemannii (Gyllenhal, 1813). In contrast, pyrosaproxylic beetles, such as Buprestidae and Cerambycidae, are closely associated with burnt wood [42]. Smaller patches of wildfires and their impact on ground beetles in the Central European region have been studied, as exemplified by Blažej [33]. However, large-scale fires in forest cultural landscapes and the responses of ground beetles are not well understood and have been only partially addressed, for instance, in [31]. While fires can cause substantial damage and are generally undesirable, prescribed burning and leaving unburnt patches can be a viable management tool in open landscapes, because it supports plant diversity and does not threaten arthropods [20].
The aim of this study was to present the results of the research aimed at assessing the impact of large-scale fires on ground beetle communities in the first year after a fire in the Bohemian and Saxon Switzerland National Park. The aim was also to evaluate the potential impact of previous management in the area with respect to the approach applied to bark beetle gradation. In fact, two management approaches to bark beetle degradation have been applied in the national park: active management against bark beetle, which was based on felling and debarking of infested trees, and passive management, where trees dead after the bark beetle attack were left standing in stands. This resulted in characteristically distinct sites that were subsequently burnt. Therefore, the study area encompassed sites affected by extensive bark beetle outbreaks without logging, as well as logged clearings resulting from bark beetle infestations. Unburnt sites with a similar character served as controls for comparison. In addition, the fire also affected stands not damaged by bark beetles; therefore, burnt healthy stands were investigated and compared to the healthy spruce stands beyond the forest fire boundary, serving as controls. The following hypotheses were tested: (i) post-fire sites exhibit similar communities of ground beetles to unburnt sites; (ii) the individual site type does not affect the communities of ground beetles; (iii) individual site types contain similar life guilds; and (iv) a large-scale fire significantly impacts the abundance of pyrophilous ground beetles. The findings from this investigation may have implications for recommending potential actively controlled burning, particularly in national park areas.
2. Materials and Methods
2.1. Study Area and Design of Study
The study was primarily conducted in the Bohemian Switzerland National Park (Czech Republic), covering an area of 80 km2. Additionally, two study plots were situated in the neighboring Saxon Switzerland National Park (Germany) with an area of 93 km2, (Figure 1; centroid coordinates: 50.88 N, 14.28 E). These protected areas were designated relatively recently, in 2000 and 1990, respectively. Characterized by a relief of cobble sandstone, the study area exhibited specific ecological conditions that play a key role in determining biodiversity. The long-term mean annual temperature ranges from 6 to 8 °C, and the area is characterized by an oceanic climate with a mean annual precipitation of approximately 800 mm [47]. The majority of the forest area (60%) in these parks consists of non-native Norway spruce (Picea Abies L.) monocultures, replacing the native acidophilous European beech (Fagus sylvatica L.) forests of the Luzulo-Fagion association [48]. In the years 2018–2021, a massive bark beetle (Ips typhographus Linnaeus, 1758) gradation affected the area, causing extensive damage to spruce stands. Until 2017, forest protection measures against bark beetle gradations were implemented in the park [47]. In response, numerous stands were logged, while many others remained unlogged. On 23 July 2022, a fire was started by human activity in the territory of the NP Bohemian Switzerland, impacting a total of 1060 ha and around 250 ha of forest area in the territory of the Saxon Switzerland NP. The fire was successfully extinguished on 12 August 2022 and 19 August 2022, respectively. This incident marked the largest fire ever recorded in all of the Czech Republic and Saxony. In 2021, a total of 1517 forest fires were documented (sum 411 ha), with an average size of 0.3 ha. The vast majority of these fires were caused by human carelessness [49]. The scale of the studied fire area is shown in Figure 1. The burning was extremely spatially variable in the forest stands. The fire passed through all vegetation and vegetation types present. The highest burn severity was achieved in mature spruce forests that had died as a result of the recent bark beetle gradation, while the lowest burn severity was achieved in mature beech forests. Clear-cuts were also burnt intensively. Burning was highly variable at all spatial scales. Significant sources of variability were individual logs at the local level, and the vegetation types and topography at the landscape level. Lying logs and standing dead trees on plateaus and valley bottoms with intense burning also acted as burn centers, and therefore, the complete burning of overburden horizons to mineral soils occurred in their close proximity [50].
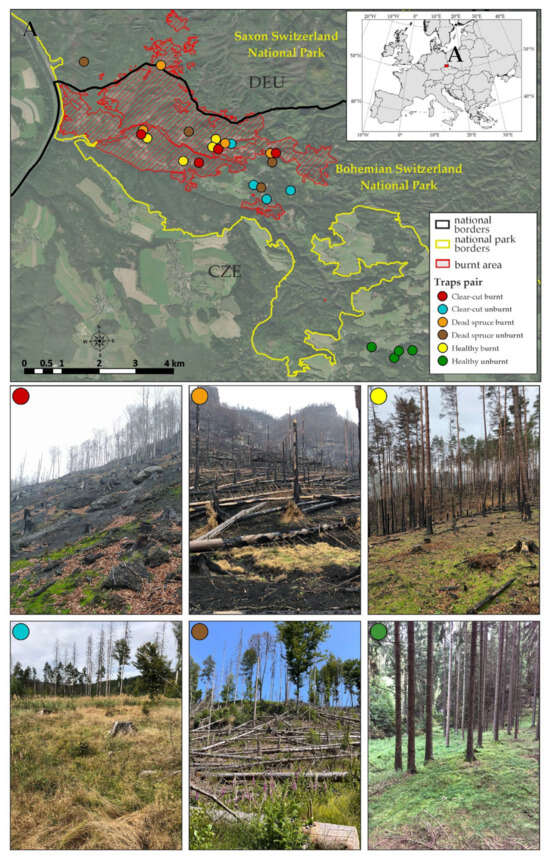
Figure 1.
The locations of the study area and research plots with fire borders indicated. Each study site is shown in a different color, and a photo of its typical appearance is attached.
Three types of coniferous forest sites affected by fire were selected (burnt sites), each with a corresponding control site of the same character (unburnt). Altogether, six sites were sampled in this study: (i) a burnt and (ii) unburnt dead spruce site after a bark beetle calamity (unlogged); (iii) a burnt and (iv) unburnt clear-cut spruce site; and (v) a burnt and (vi) unburnt healthy spruce site. As there were no fire-affected spruce stands in the area, we selected pine stand (Pinus sylvestris L.) as (v) a burnt healthy site. Healthy unburnt spruce stands (=vi) that did not show signs of decline or other severe defoliation and were not affected by fire were located outside the national park near the border within common even-aged managed forests. They had a similar structure to that found in the national park. Each site was replicated in four plots (Figure 1), with two traps deployed within each plot and placed 20 m apart. In total, 48 pitfall traps (6 × 4 × 2) were installed in April 2023.
2.2. Beetle Sampling and Identification
Epigeic ground beetles were collected using pitfall traps, which is a common and successful method for capturing these insect [51]. Each trap was mounted with a roof to prevent the evaporation of conservation liquid and washout by rain (Figure 2). Due to high temperatures on the bare dark surfaces of the burnt sites, the roofs were non-transparent to serve as a good measure against evaporation. However, this measure does not bias the species assemblages of the ground beetles [52]. The trap consisted of a larger container (1020 mL) housing a smaller container (450 mL) within it, with a funnel placed inside (Figure 2). The composition scheme of the pitfall trap used is recommended for the collection of ground beetles and for making correct conclusions [53,54]. The edge of the trap was carefully aligned with the ground to ensure the capture of even small beetles. The conservation liquid used was an 8% acetic acid solution, using commonly available vinegar. Vinegar has slightly attractive properties, mainly for the family Nitidulidae. However, the species data obtained were nearly unbiased using vinegar [55]. Vinegar was used mainly to eliminate the risk of trap destruction by wild game. Traps were installed between 19 April and 14 September 2023, and collected every three weeks. At each collection, the traps were replenished with a new 250 mL of conservation liquid. Each trap remained in the field for 147 days, resulting in a cumulative total of 7056 trap days (147 × 48). The dimensions of the trap are illustrated in Figure 2. Individuals of the family Carabidae were identified by a specialist and co-author of this paper, Oto Nakládal, as demonstrated in several faunistic studies [56,57,58,59]. Species databases were used to categorize the ground beetles into life guilds, as presented in Table 1. The taxonomy and nomenclature of the species corresponded to the concept outlined by Zicha [60] (http://www.biolib.cz, accessed on 15 December 2023). Furthermore, the species were classified based on their conservation statuses according to the IUCN Red List of Endangered Species of the Czech Republic, Invertebrates [61].

Figure 2.
Pitfall trap in the field (A), components forming the whole trap system (B), and dimensions of the trap components in cm (C).

Table 1.
Classification of the studied life-trait guilds of ground beetles.
2.3. Data Analysis
In this study, the following software were used for the analyses: R 4.3.1 [65], Canoco 5 [66].
To evaluate differences in the numbers of individuals and species per trap between sites, a generalized linear effect model was employed, with study plots as random effect. We used a Poisson distribution for the number of species, and a negative binomial distribution was applied for abundance data. For this analysis, the glmmTMB package was used [67]. The carabid communities among study sites were evaluated using non-metric multidimensional scaling (NMDS) based on the abundance data. For this analytic approach, we used the “vegan” package, employing the metaMDS function with “bray, two dimensions” [68]. The difference calculation between carabid communities was analyzed using the “adonis2” function included in the “vegan” package with 9999 randomizations. Preferences of individual carabid species to the study site were analyzed with the use of the Indicator Species Analysis (IndVal) approach [69]. For this analysis, the “indicspecies” package was employed with the “multipatt” function [70]. Sample-based rarefaction, following the methodology outlined in [71] and focusing on Hill numbers q = 0 (species richness) and q = 1 (the exponential of Shannon’s entropy index) and q = 2 (the inverse of Simpson’s concentration index), using package "iNEXT" [72], was used to evaluate gamma diversity through species richness curves. The incidence data approach, commonly employed in entomology studies, e.g., [7,73], was applied, utilizing 200 bootstrap replicates. Multivariate analysis was employed to evaluate the preference of the individual life guilds of carabid among the studied sites. We used constrained linear redundancy analysis RDA (log transformed) to calculate species preferences assigned to the surveyed life guilds. An analysis was performed with all canonical axis tests, with a research plot as a covariate. Ordination analyses were computed with 4999 unrestricted Monte Carlo permutations.
3. Results
3.1. Communities of Ground Beetles
A total of 5952 ground beetles were recorded, representing 63 species. The most abundant species was Carabus violaceus, with 852 individuals. At the same time, the ten most abundant species accounted for 82% of the entire captured assemblage of ground beetles. None of the species recorded were red-listed. The specific numbers of beetles captured at each site are detailed in Table 2. Additionally, the numbers of individuals and species per trap are shown in Figure 3. Significant differences in abundance were observed between the studied sites, particularly in the comparison of clear-cut burnt vs. unburnt sites and dead spruce burnt vs. unburnt sites. On the contrary, the difference in the number of species per trap was recorded only between healthy burnt vs. unburnt sites (Figure 3). The mean abundance of ground beetles decreased by 73% at clear-cut burnt sites and by 77.5% at dead spruce sites in comparison to their unburnt controls. On the other hand, abundance increased by 88% at burnt healthy sites compared to unburnt healthy sites. Ground beetle communities were significantly different between sites (Adonis2: R2 0.42; p < 0.001). Notably, the communities on fire-affected sites (dead spruce burnt, clear-cut burnt, healthy burnt) demonstrated a high degree of similarity among each other. In contrast, the healthy spruce sites hosted the most distinct communities (Figure 4). The gamma diversity was very similar across all sites, except for the healthy sites, which showed the lowest values (Figure 5). The highest levels of species richness (q = 0) and Shannon diversity (q = 1) were observed at the burnt healthy sites and unburnt clear-cut sites (Figure 5). Indicator species analyses revealed site preferences for 28 species, with most species (16 species) having significant preference for unburnt dead spruce sites (8 species) and burnt healthy sites (8 species) (Table 2).

Table 2.
List of recorded ground beetle species at the individual study sites. Indicator species values (InVal) are included by Dufrêne & Legendre, [69]. The results of the InVal approach are indicated by bold symbols (*** p < 0.001; ** p < 0.01; * p < 0.05; . p < 0.20).
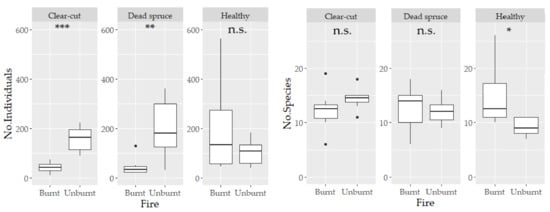
Figure 3.
Numbers of individuals and numbers of species of ground beetles per trap. Boxes indicate the interquartile 1–3Q, the solid line in the box represents the median, and the error lines are min–max values. The stars above the bars show differences analyzed using the generalized mixed effect model (*** p < 0.001; ** p < 0.01; * p < 0.05, n.s., non-significant).
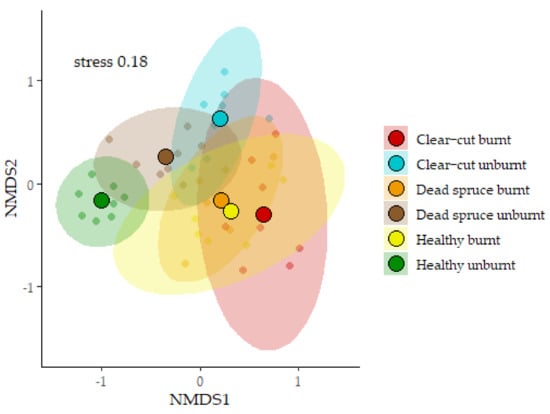
Figure 4.
Non-metric multidimensional scaling (NMDS) showing differences in the ground beetle communities of individual study sites. Each site is enclosed in an ellipse of the 95% confidence interval. Solid points are centroids.
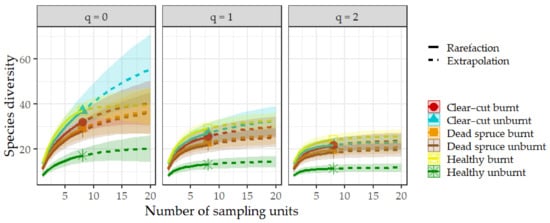
Figure 5.
Sample size-based rarefaction and extrapolation sampling of the gamma diversity, showing the Hill numbers q = 0 (species richness) and q = 1 (the exponential of Shannon’s entropy index) and q = 2 (the inverse of Simpson’s concentration index). The colored shaded areas represent 95% confidence intervals. Solid symbols depict the total number of species, and extrapolation is represented by dashed lines, extended up to double the reference sample size.
3.2. Ground Beetle Guilds
Different preferences of life guilds were recorded between study sites (Figure 6; RDA: 50.2%, pF 10.8, p < 0.0001). The numbers of ground beetle individuals and species categorized by guilds are shown in Table 3. The unburnt sites attracted the group of large ground beetles, flightless (brachypteran) species, forest (F) and cold forest (FC) species. Small-body size species and macropterans showed a preference for burnt healthy sites, while the groups with affinity to open landscapes (MM, MO) showed a preference for the unburnt clear-cut site. Pyrophilous species and two guilds of ground beetles (O = open landscape, FL = light forest) were attracted to burnt sites. The activity of all the ground beetles varied throughout the season and across study sites (Figure 7). The lowest activity was recorded at burnt sites (dead spruce, clear-cut), showing a slight increase in abundance in the second half of the season. Conversely, the highest activity was found at burnt healthy sites. A consistent decreasing trend was observed at unburnt clear-cut sites (Figure 7). In pyrophilous ground beetles (S. quadripunctata, P. quadrifoveolatus), the overwintering generation exhibited low activity based on the abundance in the trap exposition intervals, resulting in the low numbers of these species recorded in the first third of the season. However, higher activity was observed in the second half of the season (Figure 7).
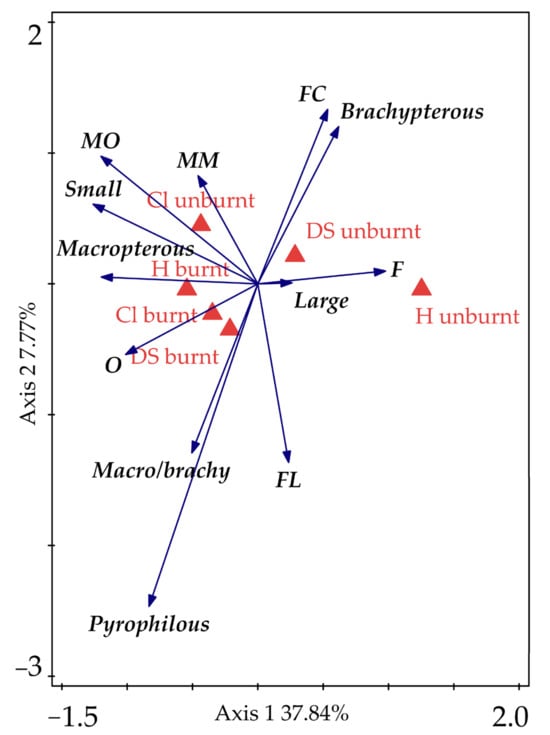
Figure 6.
Preferences of ground beetle guilds to the studied sites, redundancy analysis (RDA). Cl = clear-cut, DS = dead spruce, H = healthy.

Table 3.
Numbers of ground beetle individuals and species categorized by life trait guilds. In the category of flight ability, “both” represents the species exhibiting variations within their population, such as combinations of macropterous and brachypterous individuals.
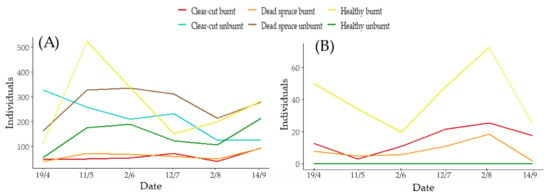
Figure 7.
Ground beetle activity over time at individual study sites (represented by colored lines): (A) all ground beetles (B) pyrophilous ground beetles. Date indicates the intervals of traps exposition in 2023.
4. Discussion
This study evaluated the effect of a forest fire on ground beetles at different study sites with different managements in the past. The evaluation of individual management methods (clear-cut, unlogged dead spruce) after a large-scale fire was insufficiently researched. Two studies in northern Europe assessed sites similarly, however, on a low-scale prescribed-fire forest plot [23,74]. Overall, interest in the study of ground beetles and the fire relationship is decreasing in recent years compared to those in butterflies and bees [18]. Below, we discuss the ground beetle response by grouping units, abundance, richness, communities, guilds, and pyrophilous ground beetles.
4.1. Ground Beetle Abundance
The impact was significant, particularly in the abundance of ground beetles on fire-affected sites, showing a substantial decrease in the mean abundance per trap of 73–77.5%. Such a decline in the abundance of ground beetles following fire is consistent with other studies [18,75]. However, an increase in abundance was observed for small-scale prescribe fires [23,74]. In contrast, on burnt healthy sites, the mean abundance of the ground beetles increased by 88% compared to unburnt healthy sites. This may be due to the severity of the fire, as healthy stands do not have as suitable conditions for burning as clearings and dead spruce where there is sufficient fuel for burning (dead wood or dry grass growth). Thus, in healthy stands, there could be fire with lower severity. Although we did not directly assess fire severity in our study, a lower fire intensity in healthy stands in the area was confirmed by Hruška et al. [50]. Such an environment emerges as highly diverse, providing niches for both pyrophilous species and those adapted to open habitats, or to sandy soils, which occupy niches formed after the litter burn caused by fire, e.g., Calathus erratus [33]. Remarkably, the P. quadrifoveolatus, N. brevicolis, and C. erratus species were significantly associated with the burnt healthy site where these species were captured in high numbers compared to the other sites. Moreover, P. quadrifoveolatus and C. erratus were captured exclusively on burnt sites. The observed increases in carabid activity and abundance in the second part of the season suggests that abundance may increase in the second year after a fire, as shown in [27]. Furthermore, Gongalsky, Persson [21] noted a significant increase in phytophagous invertebrate abundances two years after a fire. At the same time, increasing trap captures in the second half of the season may indicate that former or surviving individuals have reproduced and established the next generation of ground beetles or the recolonization of burnt sites.
4.2. Ground Beetle Richness and Communities
The ground beetle communities of the unburnt healthy site showed the lowest alpha, beta and gamma diversities and were significantly different from all the other five sites (the burnt and unburnt clear-cut sites, burnt and unburnt dead spruce sites, and burnt healthy site), which, in contrast, represented localities after a disturbation. The communities on the unburnt healthy site were also characterized by high homogeneity and minimal variations. This finding generally corresponds with the idea that homogeneous spruce stands tend to homogenize beetle communities [76,77]. Consequently, any disturbance in the forest or a mosaic of different landscape management practices can alter and enhance regional beetle diversity [78,79]. The ground beetle communities responded similarly to the fire, significantly differing from the corresponding unburnt sites. This suggests that ground beetles demonstrate a similar response to fire-affected areas regardless of the original stand conditions. Mason et al. [80] stated that fire severity can also affect ground beetle communities. In our study, ground beetles did not show significantly higher species richness (gamma) or Shannon diversity on fire-affected sites than at unburnt sites. This result contradicts the findings of [23] and the response observed in saproxylic beetles [32,79]. On the other hand, the stands studied in [23,32] were similar in character to our studied burnt healthy site. We evaluated these sites as high in species richness. Additionally, the study site in [23] had a minimal extent of the fire-affected area. The unburnt clear-cut site exhibited comparable species richness to the burnt healthy site. The high level of all assessed parameters of beetle biodiversity on clear-cuts corresponds to the high conservation value of this type of forest habitat [39,81]. This may correspond to the earlier landscape character of open forest-steppe formations regulated by, e.g., large herbivores [82]. The next potential suitability of both the burnt healthy and unburnt clear-cut sites may be attributed to the missing suction of water through the vegetation at these sites. Consequently, these sites could maintain higher humidity levels, facilitated by the shading effect of the parent stand or the growth of vegetation in the clear-cut area, in contrast to the burnt open areas exposed to full sun. Elevated humidity is known to be crucial for the richness of carabid beetles [25,83].
4.3. Pyrophilous Ground Beetle
As pyrophilous species, Sericoda quadripunctata (5 individuals) and Pterostichus quadrifoveolatus (392 individuals) were identified, the first one being the primary pyrophilous species colonizing freshly burnt areas and the second one representing a secondary pyrophilous species [33,45]. The Catalogue of Palaearctic Coleoptera in Central Europe [46] mentions another pyrophilous species in Europe, S. bogemannii, but this species has not been recorded in our study. The pyrophilous species strictly preferred post-fire sites, consistent with findings in other studies, such as Wikars’ [84]. The low abundance of pyrophilous species may be because these sites may already show characteristics of a more advanced stage of epigeic succession in the first year after the fire, or it may correspond to a low-intensity fire. Koivula et al. [85] found that S. quadripunctata seeks out and responds positively in abundance to higher fire severity. It may also be related to fire type, e.g., ref. [44] reported that S. quadripunctata is more common in sites after a crown fire. A similar structure of pyrophilous ground beetles with our study was observed two- and three years post fire [33,75]. S. quadripunctata is sensitive to predation pressure on its eggs and therefore prefers fresh burnt areas only [45]. The sensitivity to environmental sterility for S. quadripuntata is evident in the relatively slow recovery of soil arthropod groups after a fire [21,22]. Based on the ground beetle activity results, it is possible to argue that pyrophilous ground beetle species may not have reproduced in the large study area. Since the first moments after the fire, the area has been colonized by individuals from distant locations [40], allowing them to spread effectively over the large area with limited trap captures. The activity of pyrophilous ground beetles concentrated in the second half of the sampling season suggests that these are likely the next generations of the initial pyrophilous colonizers that arrived from more distant sites. The studies by Koivula et al. [85] and Blažej [33] noted that S. quadripunctata was most abundant only in the first year after a fire, suggesting that their abundance might decrease in our study sites. This may illustrate that even areas after extensive fire do not host large numbers of pyrophilous species, supporting the idea proposed in [86] that pyrophilous communities are highly fluctuating and unstable.
4.4. Ground Beetle Guilds
Large ground beetles (>10 mm) constituted the largest part of the ground beetle abundance. However, these beetles showed a notably strong negative response to the fire, resulting in a significant decrease in their abundance compared to those at unburnt sites. The genus Carabus accounted for the majority of ground beetle individuals. As a brachypteran genus without the ability to fly, it was probably negatively affected and burnt by the fire, hence the very low abundances recorded at the post-fire sites. In general, our observations indicate a clear association of large ground beetles, brachypteran species, and forest specialists with unburnt sites. The recolonization of the large-scale fire area could be hindered particularly for species such as Carabus, which has very low dispersal abilities [87]. Moreover, after a large-area fire, forest edges are less important for invertebrate movement [21]. Large ground beetles mostly preferred unburnt dead spruce sites (after a bark beetle calamity), likely due to the resemblance of these sites to an undisturbed forest environment. Additionally, the availability of better feeding opportunities in the herbaceous understory and presence of dead wood could contribute to this preference [88]. On the other hand, burnt sites contained mainly small macropteran species that are associated with open landscape biotopes. These traits play in favor of these ground beetles, and they are better able to recolonize burnt areas. This finding is consistent with those of other studies, e.g., [74].
5. Conclusions
The results of the study provide insights into the behaviors of the ground beetles after a large-scale fire in Central Europe, an event of rare occurrence at this scale in the region. The fire primarily affected allochthonous non-native spruce stands and their logged and unlogged sites. This illustrates the importance of bark beetle gradation and the accumulation of large amounts of dead wood in unmanaged stands as potential fuel for the spread of fires [13], despite the vital role dead wood plays as an irreplaceable component of biodiversity [89]. While fire is a natural phenomenon inherent in forest development [1], its occurrence is often influenced by human activities. Indeed, fires are mostly started due to human carelessness [15]. The findings of our study emphasize the crucial role of fire in enhancing the regional diversity of ground beetles, highlighting its strong impact on their abundance. Specifically, (i) ground beetle communities significantly differed between burnt and unburnt sites. (ii) Similarly, all disturbed sites (both the burnt/unburnt dead spruce/clear-cut sites and burnt healthy site) were significantly different from the unburnt healthy site (green spruce stands). Thus, these disturbances (natural as well as artificial) significantly increase regional biodiversity. (iii) Individual study sites influenced beetle life trait guilds, particularly affecting the large and brachypteran ground beetles that were burnt by the fire and their abundances were very low at all the burnt sites. Also, species associated with open habitats were more prevalent at burnt sites. (iv) Contrary to expectations, the large-scale fire did not confirm the anticipated high abundances of pyrophilous ground beetles, such as the primary pyrophilous species S. quadripunctata. Further research is needed in these large fire-affected areas to find recommendations for nature conservation and the promotion of biodiversity.
Author Contributions
Conceptualization, V.Z., O.N. and J.R.; data curation, V.Z. and O.N.; formal analysis, V.Z.; funding acquisition, J.R.; investigation, V.Z. and O.N.; methodology, V.Z. and O.N.; project administration, J.R.; resources, O.N.; visualization, V.Z.; writing—original draft, V.Z.; writing—review and editing, O.N. and J.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grant No. QK23020008, funded by the Ministry of Agriculture of the Czech Republic.
Data Availability Statement
Data available on request.
Acknowledgments
We are grateful to Markéta Macháčová, who proofread the language of this manuscript. We would also like to thank Dana Vébrová, Lukáš Blažej, and Annika Busse, officers and conservationists of the National Parks, for the permission to conduct this research. We also thank Jiří Trombik for helping with the Arcgis map generating. We are very grateful to three anonymous reviewers for high-quality comments that helped improve this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Schelhaas, M.-J.; Nabuurs, G.-J.; Schuck, A. Natural disturbances in the European forests in the 19th and 20th centuries. Glob. Chang. Biol. 2003, 9, 1620–1633. [Google Scholar] [CrossRef]
- Soja, A.J.; Tchebakova, N.M.; French, N.H.F.; Flannigan, M.D.; Shugart, H.H.; Stocks, B.J.; Sukhinin, A.I.; Parfenova, E.I.; Chapin, F.S.; Stackhouse, P.W. Climate-Induced Boreal Forest Change: Predictions Versus Current Observations. Glob. Planet. Chang. 2007, 56, 274–296. [Google Scholar] [CrossRef]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest disturbances under climate change. Nat. Clim. Chang. 2017, 7, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Millar, C.I.; Stephenson, N.L. Temperate forest health in an era of emerging megadisturbance. Science 2015, 349, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Noss, R.F.; Bussler, H.; Brandl, R. Learning from a “benign neglect strategy” in a national park: Response of saproxylic beetles to dead wood accumulation. Biol. Conserv. 2010, 143, 2559–2569. [Google Scholar] [CrossRef]
- Zumr, V.; Remeš, J.; Pulkrab, K. How to Increase Biodiversity of Saproxylic Beetles in Commercial Stands Through Integrated Forest Management in Central Europe. Forests 2021, 12, 814. [Google Scholar] [CrossRef]
- Zumr, V.; Nakládal, O.; Bílek, L.; Remeš, J. The Diameter of Beech Snags is an Important Factor for Saproxylic Beetle Richness: Implications for Forest Management and Conservation. For. Ecosyst. 2023, 10, 100143. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef]
- Wambsganss, J.; Stutz, K.P.; Lang, F. European beech deadwood can increase soil organic carbon sequestration in forest topsoils. For. Ecol. Manag. 2017, 405, 200–209. [Google Scholar] [CrossRef]
- Klamerus-Iwan, A.; Lasota, J.; Błońska, E. Interspecific Variability of Water Storage Capacity and Absorbability of Deadwood. Forests 2020, 11, 575. [Google Scholar] [CrossRef]
- Błońska, E.; Prażuch, W.; Lasota, J. Deadwood affects the soil organic matter fractions and enzyme activity of soils in altitude gradient of temperate forests. For. Ecosyst. 2023, 10, 100115. [Google Scholar] [CrossRef]
- Moriarty, K.; Cheng, A.S.; Hoffman, C.M.; Cottrell, S.P.; Alexander, M.E. Firefighter Observations of “Surprising” Fire Behavior in Mountain Pine Beetle-Attacked Lodgepole Pine Forests. Fire 2019, 2, 34. [Google Scholar] [CrossRef]
- Berčák, R.; Holuša, J.; Kaczmarowski, J.; Tyburski, Ł.; Szczygieł, R.; Held, A.; Vacik, H.; Slivinský, J.; Chromek, I. Fire Protection Principles and Recommendations in Disturbed Forest Areas in Central Europe: A Review. Fire 2023, 6, 310. [Google Scholar] [CrossRef]
- Tinner, W.; Conedera, M.; Ammann, B.; Lotter, A.F. Fire ecology north and south of the Alps since the last ice age. Holocene 2005, 15, 1214–1226. [Google Scholar] [CrossRef]
- Berčák, R.; Holuša, J.; Lukášová, K.; Hanuška, Z.; Agh, P.; Vaněk, J.; Kula, E.; Chromek, I. Forest fires in the Czech Republic—Characteristic, prevention and firefighting: Review. Zprávy Lesn. Výzkumu 2018, 63, 184–194. [Google Scholar]
- Pérez-Valera, E.; Verdú, M.; Navarro-Cano, J.A.; Goberna, M. Soil microbiome drives the recovery of ecosystem functions after fire. Soil Biol. Biochem. 2020, 149, 107948. [Google Scholar] [CrossRef]
- Lebedinskii, A.A.; Noskova, O.S.; Dmitriev, A.I. Post-fire recovery of terrestrial vertebrates in the Kerzhensky State Nature Biosphere Reserve (Central Volga Region, Russia). Nat. Conserv. Res. 2019, 4 (Suppl. 1), 45–56. [Google Scholar] [CrossRef]
- Mason, S.C.; Shirey, V.; Ponisio, L.C.; Gelhaus, J.K. Responses from bees, butterflies, and ground beetles to different fire and site characteristics: A global meta-analysis. Biol. Conserv. 2021, 261, 109265. [Google Scholar] [CrossRef]
- Dzwonko, Z.; Loster, S.; Gawroński, S. Impact of fire severity on soil properties and the development of tree and shrub species in a Scots pine moist forest site in southern Poland. For. Ecol. Manag. 2015, 342, 56–63. [Google Scholar] [CrossRef]
- Valkó, O.; Deák, B.; Magura, T.; Török, P.; Kelemen, A.; Tóth, K.; Horváth, R.; Nagy, D.D.; Debnár, Z.; Zsigrai, G.; et al. Supporting biodiversity by prescribed burning in grasslands—A multi-taxa approach. Sci. Total Environ. 2016, 572, 1377–1384. [Google Scholar] [CrossRef]
- Gongalsky, K.B.; Persson, T. Recovery of Soil Macrofauna After Wildfires in Boreal Forests. Soil Biol. Biochem. 2013, 57, 182–191. [Google Scholar] [CrossRef]
- Malmström, A. Life-History Traits Predict Recovery Patterns in Collembola Species After Fire: A 10 Year Study. Appl. Soil Ecol. 2012, 56, 35–42. [Google Scholar] [CrossRef]
- Gongalsky, K.; Midtgaard, F.; Overgaard, H. Effects of Prescribed Forest Burning on Carabid Beetles (Coleoptera: Carabidae). Entomol. Fenn. 2006, 17, 325–333. [Google Scholar] [CrossRef]
- Muona, J.; Rutanen, I. The short-term impact of fire on the beetle fauna in boreal coniferous forest. Ann. Entomol. Fenn. 1994, 31, 109–121. [Google Scholar]
- Toivanen, T.; Heikkilä, T.; Koivula, M.J. Emulating natural disturbances in boreal Norway spruce forests: Effects on ground beetles (Coleoptera, Carabidae). For. Ecol. Manag. 2014, 314, 64–74. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V.; MacGowan, I.; Makarkin, V.N.; Antropov, A.V.; Gornostaev, N.G.; Khapugin, A.A.; Dvořák, L.; Esin, M.N. Post-fire insect fauna explored by crown fermental traps in forests of the European Russia. Sci. Rep. 2021, 11, 21334. [Google Scholar] [CrossRef] [PubMed]
- Elia, M.; Lafortezza, R.; Tarasco, E.; Colangelo, G.; Sanesi, G. The spatial and temporal effects of fire on insect abundance in Mediterranean forest ecosystems. For. Ecol. Manag. 2012, 263, 262–267. [Google Scholar] [CrossRef]
- Lazarina, M.; Sgardelis, S.P.; Tscheulin, T.; Devalez, J.; Mizerakis, V.; Kallimanis, A.S.; Papakonstantinou, S.; Kyriazis, T.; Petanidou, T. The effect of fire history in shaping diversity patterns of flower-visiting insects in post-fire Mediterranean pine forests. Biodivers. Conserv. 2017, 26, 115–131. [Google Scholar] [CrossRef]
- Hochkirch, A.; Adorf, F. Effects of prescribed burning and wildfires on Orthoptera in Central European peat bogs. Environ. Conserv. 2007, 34, 225–235. [Google Scholar] [CrossRef]
- Bogusch, P.; Blažej, L.; Trýzna, M.; Heneberg, P. Forgotten role of fires in Central European forests: Critical importance of early post-fire successional stages for bees and wasps Hymenoptera. Eur. J. For. Res. 2015, 134, 153–166. [Google Scholar] [CrossRef]
- Błońska, E.; Bednarz, B.; Kacprzyk, M.; Piaszczyk, W.; Lasota, J. Effect of scots pine forest management on soil properties and carabid beetle occurrence under post-fire environmental conditions—A case study from Central Europe. For. Ecosyst. 2020, 7, 28. [Google Scholar] [CrossRef]
- Gutowski, J.M.; Sućko, K.; Borowski, J.; Kubisz, D.; Mazur, M.A.; Melke, A.; Mokrzycki, T.; Plewa, R. Post-fire beetle succession in a biodiversity hotspot: Białowieża Primeval Forest. For. Ecol. Manag. 2020, 461, 117893. [Google Scholar] [CrossRef]
- Blažej, L. Groung beetles (Coleoptera: Carabidae) of the forest burnt in Jetřichovice (Northern Bohemia). In Vlastivědný Sborník Českolipska 32/2023; BEZDĚZ: Česká Lípa, Czech Republic, 2023. [Google Scholar]
- Podrázský, V.; Remeš, J.; Farkač, J. Composition of communities of ground beetles (Coleoptera: Carabidae) in forest stands with different species structure and management system. Rep. For. Res. Rep. 2010, 55, 10–15. [Google Scholar]
- Pfiffner, L.; Luka, H. Effects of low-input farming systems on carabids and epigeal spiders—A paired farm approach. Basic Appl. Ecol. 2003, 4, 117–127. [Google Scholar] [CrossRef]
- Niemelä, J.; Koivula, M.; Kotze, D.J. The effects of forestry on carabid beetles (Coleoptera: Carabidae) in boreal forests. J. Insect Conserv. 2007, 11, 5–18. [Google Scholar] [CrossRef]
- Skłodowski, J. Consequence of the transformation of a primeval forest into a managed forest for carabid beetles (Coleoptera: Carabidae)—A case study from Białowieża (Poland). Eur. J. Entomol. 2014, 111, 639–648. [Google Scholar] [CrossRef]
- Pearce, J.L.; Venier, L.A. The use of ground beetles (Coleoptera: Carabidae) and spiders (Araneae) as bioindicators of sustainable forest management. Ecol. Indic. 2006, 6, 780–793. [Google Scholar] [CrossRef]
- Plath, E.; Trauth, C.; Gerhards, J.; Griebel, L.; Fischer, K. Dieback of Managed Spruce Stands in Western Germany Promotes Beetle Diversity. J. For. Res. 2024, 35, 48. [Google Scholar] [CrossRef]
- Bell, A.J. Like moths to a flame: A review of what we know about pyrophilic insects. For. Ecol. Manag. 2023, 528, 120629. [Google Scholar] [CrossRef]
- Schmitz, H.; Schmitz, A.; Kreiss, E.; Gebhardt, M.; Gronenberg, W. Navigation to Forest Fires by Smoke and Infrared Reception: The Specialized Sensory Systems of “Fire-Loving” Beetles. Navigation 2008, 55, 137–145. [Google Scholar] [CrossRef]
- Suckling, D.M.; Gibb, A.R.; Daly, J.M.; Chen, X.; Brockerhoff, E.G. Behavioral and Electrophysiological Responses of Arhopalus tristis to Burnt Pine and Other Stimuli. J. Chem. Ecol. 2001, 27, 1091–1104. [Google Scholar] [CrossRef] [PubMed]
- Hinz, M.; Klein, A.; Schmitz, A.; Schmitz, H.; Engelmann, J. The Impact of Infrared Radiation in Flight Control in the Australian “Firebeetle” Merimna Atrata. PLoS ONE 2018, 13, e0192865. [Google Scholar] [CrossRef] [PubMed]
- Gongalsky, K.B.; Wikars, L.-O.; Persson, T. Dynamics of pyrophilous carabids in a burned pine forest in Central Sweden. Balt. J. Coleopterol. 2003, 3, 107–111. [Google Scholar]
- Bell, A.J.; Calladine, K.S.; Wardle, D.A.; Phillips, I.D. Rapid colonization of the post-burn environment improves egg survival in pyrophilic ground beetles. Ecosphere 2022, 13, e4213. [Google Scholar] [CrossRef]
- Schmid, J. Tribe Platynini Bonelli. Catalogue of Palaearctic Coleoptera. In Archostemata—Myxophaga—Adephaga; Löbl, I., Löbl, D., Eds.; Revised and Updated Edition; Brill: Leiden, The Netherlands; Boston, MA, USA, 2017; Volume 1, pp. 642–675. [Google Scholar]
- Vébrová, D.; Härtel, H. (Eds.) Care Principles for the Czech Switzerland National Park 2022–2041; Czech Switzerland National Park Administration: Krásná Lípa, Czech Republic, 2022. [Google Scholar]
- Drozd, J.; Härtel, H.; Klitsch, M. Péče o Lesní Ekosystémy v Národním Parku České Švýcarsko. Ochrana přírody 1/2010—Péče o Přírodu a Krajinu. Available online: https://www.casopis.ochranaprirody.cz/pece-o-prirodu-a-krajinu/pece-o-lesni-ekosystemy-v-narodnim-parku-ceske-svycarsko/ (accessed on 7 January 2024).
- Czech Forest Reports. Report on the State of Forests and Forestry in the Czech Republic in 2021; Ministerstvo Zemědělství: Prague, Czech Republic, 2022; ISBN 978-80-7434-669-9. Available online: https://eagri.cz/public/portal/-q266433---jF_7lFFI/zprava-o-stavu-lesa-a-lesniho?_linka=a235209 (accessed on 4 January 2024).
- Hruška, J. Jaké Faktory Ovlivnily Vznik a Šíření Požáru v NP České Švýcarsko? Factors Involved in the Origin and Spread of the Fire in Bohemian Switzerland in 2022: In Ministry of the Environment. 2022. Available online: https://www.mzp.cz/C1257458002F0DC7/cz/news_20220106-Vedci-zmapovali-pozar-v-Ceskem-Svycarsku-Majitele-lesu-se-z-nej-musi-ponaucit-Pro-prirodu-ale-znamena-probihajici-obnova-velkou-sanci/$FILE/Studie_faktoru_pozaru_Narodni_park_Ceske_Svycarsko.pdf (accessed on 10 February 2024).
- Montgomery, G.A.; Belitz, M.W.; Guralnick, R.P.; Tingley, M.W. Standards and Best Practices for Monitoring and Benchmarking Insects. Front. Ecol. Evol. 2021, 8, 513. [Google Scholar] [CrossRef]
- Phillips, I.D.; Cobb, T.P. Effects of Habitat Structure and Lid Transparency on Pitfall Catches. Environ. Entomol. 2005, 34, 875–882. [Google Scholar] [CrossRef]
- Brown, G.R.; Matthews, I.M. A review of extensive variation in the design of pitfall traps and a proposal for a standard pitfall trap design for monitoring ground-active arthropod biodiversity. Ecol. Evol. 2016, 6, 3953–3964. [Google Scholar] [CrossRef]
- Császár, P.; Torma, A.; Gallé-Szpisjak, N.; Tölgyesi, C.; Gallé, R. Efficiency of pitfall traps with funnels and/or roofs in capturing ground-dwelling arthropods. Eur. J. Entomol. 2018, 115, 15–24. [Google Scholar] [CrossRef]
- Nakládal, O.; Havránková, E.; Zumr, V. Trapping liquids may bias the results of beetle diversity assessment. PeerJ 2023, 11, e16531. [Google Scholar] [CrossRef]
- Nakládal, O. Results of a faunistic survey of beetles (Coleoptera) in floodplain forests of the Litovelské Pomoraví Protected Landscape Area (Czech Republic, Northern Moravia) in 2006. Klapalekiana 2008, 44, 237–269. [Google Scholar]
- Nakládal, O. Results of a faunistic survey of beetles (Coleoptera) in Vrapač National Nature Reserve (Czech Republic, Northern Moravia, Litovelské Pomoraví Protected Landscape Area) in 2009. Klapalekiana 2011, 47, 213–236. [Google Scholar]
- Nakládal, O. Results of a faunistics survey of beetles (Coleoptera) in Hejtmanka Nature Reserve (Czech Republic, Northern Moravia, Litovelské Pomoraví Protected Landscape Area) in 2009. Acta Musei Beskidensis 2011, 3, 103–129. [Google Scholar]
- Nakládal, O. Results of beetles (Coleoptera) survey of Zástudánčí National Nature Reserve (Central Moravia) 2008—Part 1. Časopis Slez. Zemského Muz. 2011, 60, 63–78. [Google Scholar] [CrossRef]
- Zicha, O. BioLib: Biological Library. Available online: https://www.biolib.cz (accessed on 9 December 2023).
- Hejda, R.; Farkač, J.; Chobot, K. Red List of Threatened Species of the Czech Republic; Agentura Ochrany Přírody a Krajiny České Republiky, Příroda: Prague, Czech Republic, 2017; ISBN 978-80-88076-53-7. [Google Scholar]
- Schneider, A.; Blick, T.; Pauls, S.U.; Dorow, W.H.O. The List of Forest Affinities for Animals in Central Europe—A Valuable Resource for Ecological Analysis and Monitoring in Forest Animal Communities? For. Ecol. Manag. 2021, 479, 118542. [Google Scholar] [CrossRef]
- Lompe, A. Die Käfer Europas: Ein Bestimmungswerk im Internet, ‘Beetles of Europe: An Online Identification Resource 2002’. Available online: http://www.coleo-net.de/coleo/index.htm (accessed on 15 December 2023).
- Hůrka, K. Carabidae of the Czech and Slovak Republics; Kabourek: Zlin, Czech Republic, 1996; p. 565. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing_; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 20 December 2023).
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using Canoco 5; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. GlmmTMB balances speed and flexibility among packages for Zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package 2022. R package version 2.6-2. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 2 February 2024).
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Cáceres, M.D.; Legendre, P. Associations Between Species and Groups of Sites: Indices and Statistical Inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: iNterpolation and EXTrapolation for Species Diversity. R Package Version 3.0.0. 2022. Available online: http://chao.stat.nthu.edu.tw/wordpress/software-download/ (accessed on 25 February 2024).
- Graf, M.; Seibold, S.; Gossner, M.M.; Hagge, J.; Weiß, I.; Bässler, C.; Müller, J. Coverage based diversity estimates of facultative saproxylic species highlight the importance of deadwood for biodiversity. For. Ecol. Manag. 2022, 517, 120275. [Google Scholar] [CrossRef]
- Martikainen, P.; Kouki, J.; Heikkala, O. The Effects of Green Tree Retention and Subsequent Prescribed Burning on Ground Beetles (Coleoptera: Carabidae) in Boreal Pine-Dominated Forests. Ecography 2006, 29, 659–670. [Google Scholar] [CrossRef]
- Saint-Germain, M.; Larrivée, M.; Drapeau, P.; Fahrig, L.; Buddle, C.M. Short-Term Response of Ground Beetles (Coleoptera: Carabidae) to Fire and Logging in A Spruce-Dominated Boreal Landscape. For. Ecol. Manag. 2005, 212, 118–126. [Google Scholar] [CrossRef]
- Du Bus de Warnaffe, G.; Lebrun, P. Effects of Forest Management on Carabid Beetles in Belgium: Implications for Biodiversity Conservation. Biol. Conserv. 2004, 118, 219–234. [Google Scholar] [CrossRef]
- Zumr, V.; Nakládal, O.; Remeš, J.; Brestovanská, T.; Zumr, V. Diversity of Click Beetles in Managed Nonnative Coniferous and Native Beech Stands: Consequences of Changes in the Structural and Species Composition of Tree Stands in Central Europe. For. Ecosyst. 2022, 9, 100057. [Google Scholar] [CrossRef]
- Hilmers, T.; Friess, N.; Bässler, C.; Heurich, M.; Brandl, R.; Pretzsch, H.; Seidl, R.; Müller, J.; Butt, N. Biodiversity along Temperate Forest Succession. J. Appl. Ecol. 2018, 55, 2756–2766. [Google Scholar] [CrossRef]
- Schall, P.; Gossner, M.M.; Heinrichs, S.; Fischer, M.; Boch, S.; Prati, D.; Jung, K.; Baumgartner, V.; Blaser, S.; Böhm, S.; et al. The Impact of Even-Aged and Uneven-Aged Forest Management on Regional Biodiversity of Multiple Taxa in European Beech Forests. J. Appl. Ecol. 2018, 55, 267–278. [Google Scholar] [CrossRef]
- Mason, S.C., Jr.; Shirey, V.; Waite, E.S.; Gallagher, M.R.; Skowronski, N.S. Exploring Prescribed Fire Severity Effects on Ground Beetle (Coleoptera: Carabidae) Taxonomic and Functional Community Composition. Fire 2023, 6, 366. [Google Scholar] [CrossRef]
- Perlík, M.; Kraus, D.; Bußler, H.; Neudam, L.; Pietsch, S.; Mergner, U.; Seidel, D.; Sebek, P.; Thorn, S. Canopy Openness As the Main Driver of Aculeate Hymenoptera and Saproxylic Beetle Diversity Following Natural Disturbances and Salvage Logging. For. Ecol. Manag. 2023, 540, 121033. [Google Scholar] [CrossRef]
- Vera, F.W.M. (Ed.) Grazing Ecology and Forest History; CABI: Wallingford, UK, 2000. [Google Scholar] [CrossRef]
- Sroka, K.; Finch, O.-D. Ground Beetle Diversity in Ancient Woodland Remnants in North-Western Germany (Coleoptera, Carabidae). J. Insect Conserv. 2006, 10, 335–350. [Google Scholar] [CrossRef]
- Wikars, L.O. Dependence on Fire in Wood-living Insects: An Experiment with Burned and Unburned Spruce and Birch Logs. J. Insect Conserv. 2002, 6, 1–12. [Google Scholar] [CrossRef]
- Koivula, M.; Cobb, T.; Déchêne, A.D.; Jacobs, J.; Spence, J.R. Re-sponses of two Sericoda Kirby, 1837 (Coleoptera: Carabidae) species to forestharvesting, wildfire, and burn severity. Entomol. Fennica 2006, 17, 315–324. [Google Scholar] [CrossRef]
- Zúñiga, A.H.; Rau, J.R.; Fierro, A.; Vergara, P.M.; Encina-Montoya, F.; Fuentes-Ramírez, A.; Jaksic, F.M. Fire Severity Causes Temporal Changes in Ground-Dwelling Arthropod Assemblages of Patagonian Araucaria–Nothofagus Forests. Fire 2022, 5, 168. [Google Scholar] [CrossRef]
- Negro, M.; Casale, A.; Migliore, L.; Palestrini, C.; Rolando, A. Habitat use and movement patterns in the endangered ground beetle species, Carabus olympiae (Coleoptera: Carabidae). Eur. J. Entomol. 2008, 105, 105–112. [Google Scholar] [CrossRef]
- Cobb, T.P.; Langor, D.W.; Spence, J.R. Biodiversity and Multiple Disturbances: Boreal Forest Ground Beetle (Coleoptera: Carabidae) Responses to Wildfire, Harvesting, and Herbicide. Can. J. For. Res. 2007, 37, 1310–1323. [Google Scholar] [CrossRef]
- Parajuli, R.; Markwith, S.H. Quantity is Foremost But Quality Matters: A Global Meta-Analysis of Correlations of Dead Wood Volume and Biodiversity in Forest Ecosystems. Biol. Conserv. 2023, 283, 110100. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).