An Integrated Strategy for Preventing and Rehabilitating Dust-Induced Occupational Bronchopulmonary Diseases: A Scoping Review
Abstract
Highlights
- Dust-induced occupational bronchopulmonary diseases—often overlooked contributors to the global COPD burden—represent a significant cause of preventable morbidity and mortality among exposed workers.
- Dust-induced occupational bronchopulmonary disease is a common condition in patients with COPD, often requiring increased energy, protein, vitamin, and mineral requirements.
- Preventing COPD and OBPDs requires accurate screening using both clinical tools (e.g., HRCT, FeNO) and biological markers (e.g., IL-6, TNF-α).
- Integrating personalized nutritional support and antioxidant-based rehabilitation—such as polyphenol-rich phytotherapy—can reduce progression and improve work reintegration outcomes.
- Occupational health systems should prioritize early detection programs and structured rehabilitation tailored to dust-exposed populations.
Abstract
1. Introduction
2. Materials and Methods
2.1. Review Framework and Methodological Approach
2.2. Reporting Standards
2.3. Study Selection and Data Charting
Quality Appraisal of Included Studies
2.4. Synthesis of Results
3. Results
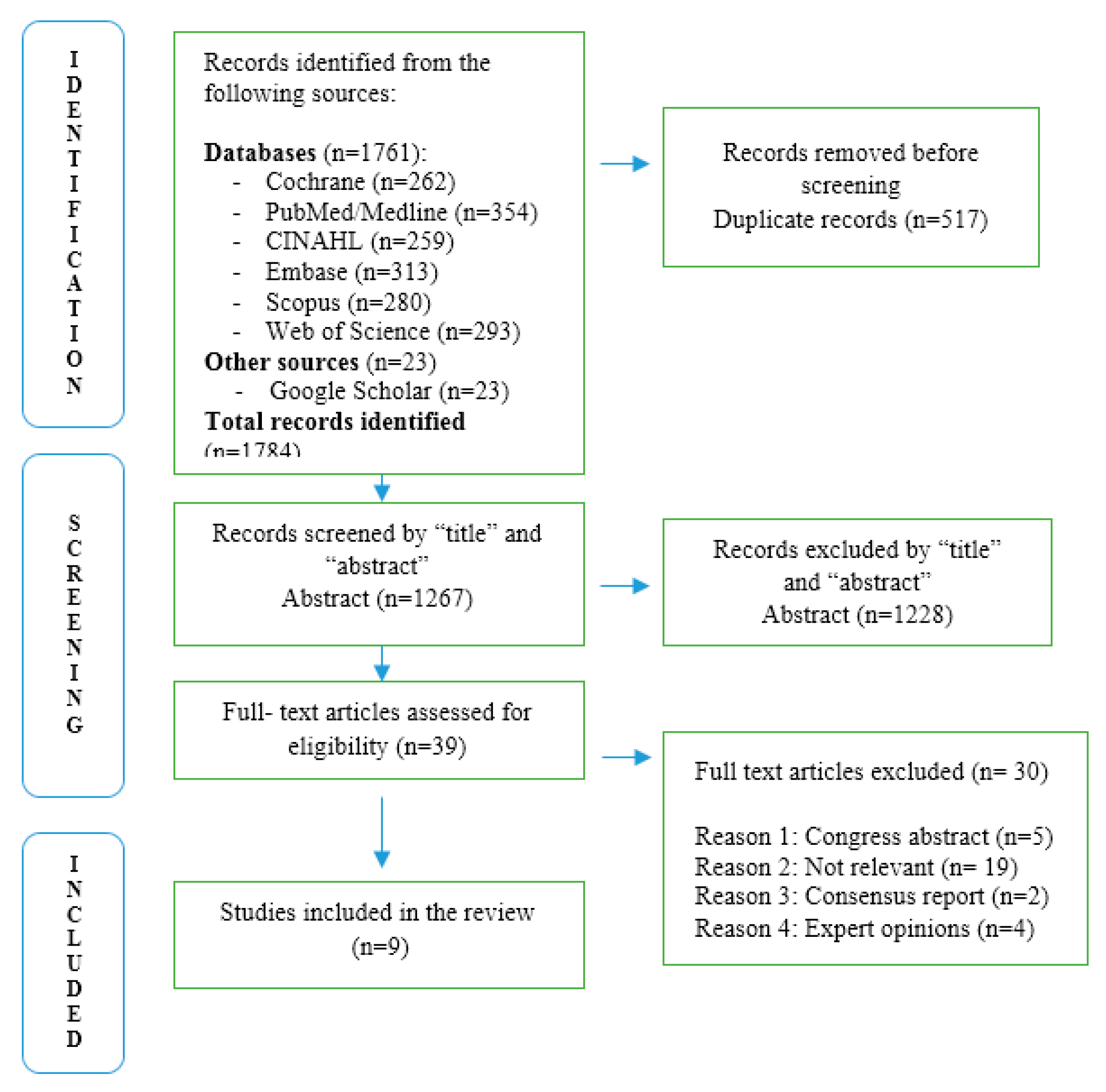
3.1. Study Selection
3.2. Characteristics of Included Studies
3.2.1. Eligibility Criteria
| Criterion Type | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Workers exposed to occupational dusts (e.g., silica, coal) with diagnosis or risk of OBPDs | Studies focused on general population, non-occupational exposures, comorbidities unrelated to OBPD |
| Study design | Systematic reviews, narrative literature reviews, observational studies, descriptive studies | Randomized controlled trials unrelated to occupational or public health interventions |
| Publication type | Peer-reviewed articles, government reports, mechanistic analyses | Non-scientific articles, case reports, conference abstracts, editorials without primary data |
| Time frame | Publications from January 2014 to January 2024 | Studies published outside this period |
| Language | English language publications | Non-English publications without accessible translation |
3.2.2. Excluded Studies
- Irrelevance to occupational exposure contexts (e.g., general pulmonary disease studies);
- Lack of empirical data or focus on unrelated health outcomes;
- Publications outside the 2014–2024 timeframe;
- Publications not written in English.
3.3. Thematic Synthesis
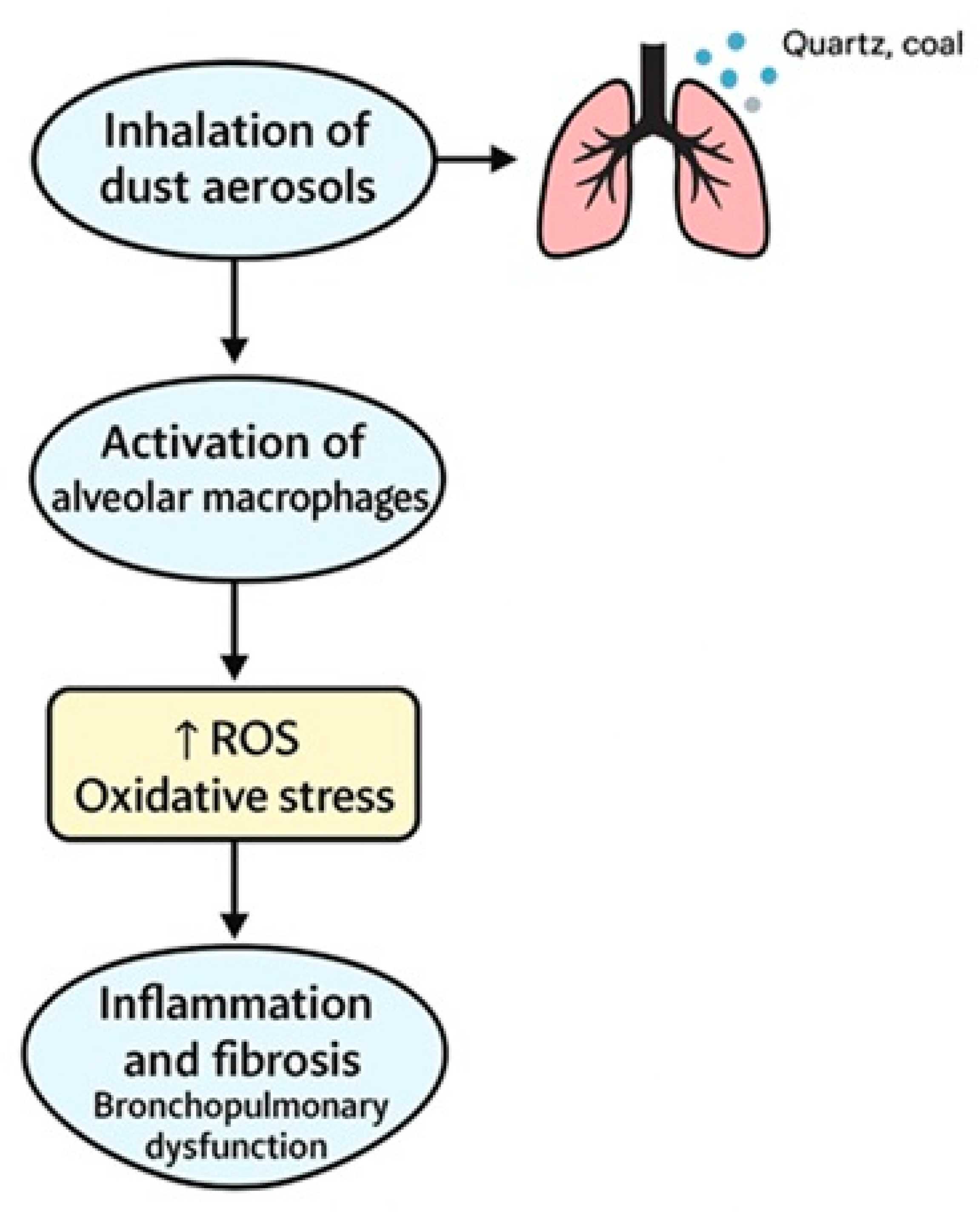
3.3.1. Risk Factors and Pathogenesis
3.3.2. Diagnostics and Clinical Criteria
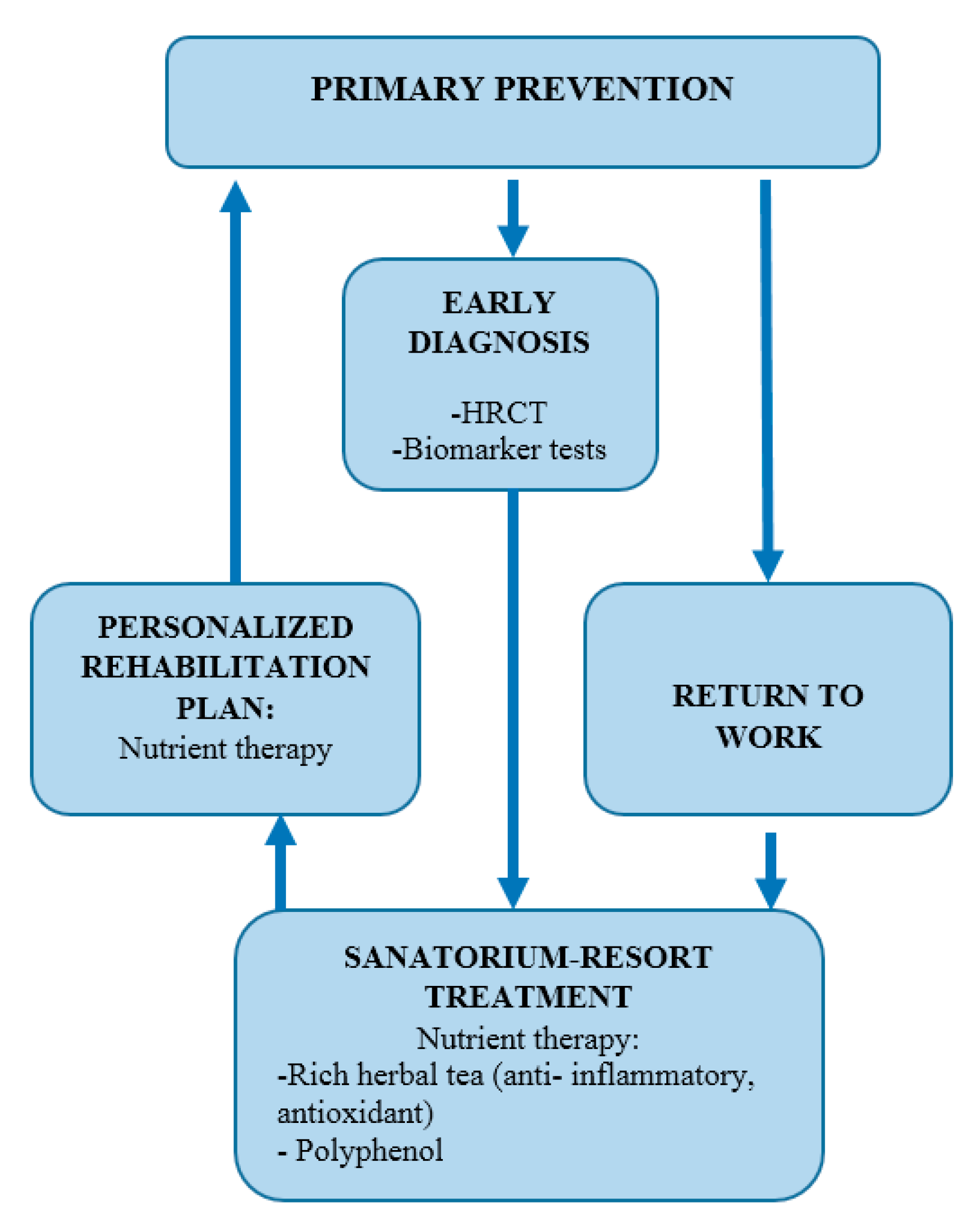
3.3.3. Prevention and Surveillance
3.3.4. Rehabilitation and Return to Work
3.4. Overview Table
| Author (Year), Country | Research Design | Sample | Research Purpose | Intervention | Results |
|---|---|---|---|---|---|
| Cullinan et al. (2017), UK and EU [1] | Review with recommendations (belongs to observational studies) | Occupational exposure cases | Identify prevention strategies for both classic and emerging exposures | Epidemiological policy, recommendations | Suggested stronger regulations and surveillance in occupational medicine |
| Sim et al. (2019), Australia [7] | Government report (belongs to observational studies) | 263 workers with silicosis | Explore rehabilitation and return-to-work models for silicosis patients | Vocational rehab program review | Identified need for customized return to work plans |
| Su et al. (2023), China [16] | Systematic review and meta-analysis | Multiple occupational cohorts | Assess incidence and risk factors of pneumoconiosis | Meta-analysis of global data | Identified key predictors including exposure duration, smoking, and silica concentration |
| Vanka et al. (2022), USA [17] | Mechanistic review (belongs to observational studies) | Preclinical and clinical | Explore dust-related immune and oxidative mechanisms | Mechanistic analysis (ROS/macrophages) | Identified fibrotic and inflammatory pathways in coal and silica exposure |
| Weissman (2022), USA [18] | Narrative review | Silicosis/CWP patients | Characterize progressive massive fibrosis (PMF) | Imaging and marker evaluation | Provided diagnostic criteria for early-stage PMF |
| Krefft et al. (2020), USA [19] | Clinical update (belongs to observational studies) | - | Update on clinical recognition and prevention of silicosis | Diagnostic algorithm and HRCT | Advocated regular screening and detailed exposure history |
| Perlman & Maier (2019), USA [20] | Narrative review | Summarize occupational lung disease management | Overview of therapies and policies | Stressed importance of early diagnosis and clinical monitoring | |
| Minov et al. (2022), North Macedonia [21] | Review (belongs to observational studies) | COPD patient cohorts | Review COPD prevalence and prevention in occupational settings | Literature and guideline review | Highlighted need for dust control and cessation of smoking |
| Hou X. et al. (2025), China [22] | Systematic review | Coal-exposed populations | Assess health effects of coal dust and evaluate control interventions | Synthesis of environmental, clinical data | Proposed evidence-based dust suppression strategies for industry |
4. Discussion
4.1. Diagnostics and Imaging Tools
4.2. Biomarkers and Genetic Factors
4.3. Preventive and Workplace Interventions
4.4. Rehabilitation and Multidisciplinary Care
4.5. Methodological and Geographic Limitations
4.6. Justification for Scoping Review Approach
4.7. Strengths
4.8. Limitations
5. Conclusions
- Early diagnostic interventions using HRCT, FeNO, and cytokine assays;
- Systematic workplace exposure surveillance and enforcement of dust control measures;
- Genetic and immunological risk profiling among vulnerable worker groups;
- Comprehensive worker education, including smoking cessation programs and occupational health literacy.
- Validation of biomarker-based screening for early disease detection;
- Cost-effectiveness analysis of screening and rehabilitation models;
- Development and deployment of digital platforms for exposure tracking and clinical decision support.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OBPD | Occupational bronchopulmonary diseases |
| COPD | Chronic obstructive pulmonary disease |
| PPE | Personal protective equipment |
| ScR | Scoping reviews |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor necrosis factor alpha |
| TGF-β | Transforming growth factor beta |
| HRCT | High-resolution computed tomography |
| FeNO | Fractional exhaled nitric oxide |
| GST | Glutathione S-transferase |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| ROS | Reactive oxygen species |
References
- Cullinan, P.; Muñoz, X.; Suojalehto, H.; Agius, R. Occupational lung diseases: From old and novel exposures to effective preventive strategies. Lancet Respir. Med. 2017, 5, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Petsonk, E.L.; Rose, C.; Cohen, R. Coal mine dust lung disease: New lessons from an old exposure. Am. J. Respir. Crit. Care Med. 2013, 187, 1178–1185. [Google Scholar] [CrossRef]
- National Research Council. Respiratory Diseases Research at NIOSH; National Academies Press: Cambridge, MA, USA, 2008. Available online: https://www.ncbi.nlm.nih.gov/books/NBK214523/ (accessed on 1 June 2025).
- Schwab, A.D.; Poole, J.A. Mechanistic and therapeutic approaches to occupational exposure-associated asthmatic disease. Curr. Allergy Asthma Rep. 2023, 23, 450–464. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Occupational Risk Factors Collaborators. Global and regional burden of disease and injury in 2016 arising from occupational exposures: A systematic analysis for the Global Burden of Disease Study 2016. Occup. Environ. Med. 2020, 77, 133–141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- International Labour Organization. The Right to Occupational Safety and Health: Still Unrealized. Available online: https://ilostat.ilo.org/blog/the-right-to-occupational-safety-and-health-still-unrealized (accessed on 5 June 2025).
- Sim, M.; Glass, D.C.; Dimitriadis, C. Return to Work and Vocational Rehabilitation for Workers with Silicosis: A Systematic Review; Monash University, Centre for Occupational and Environmental Health: Melbourne, Australia, 2019; 47p, Available online: https://research.monash.edu/en/publications/return-to-work-review-return-to-work-and-vocational-rehabilitatio (accessed on 5 June 2025).
- Esmaeil, N.; Gharagozloo, M.; Rezaei, A.; Grunig, G. Dust events, pulmonary diseases and immune system. Am. J. Clin. Exp. Immunol. 2014, 3, 20–29. [Google Scholar]
- Isaak, M.; Ulu, A.; Osunde, A.; Nordgren, T.M.; Hanson, C. Nutritional Factors in Occupational Lung Disease. Curr. Allergy Asthma Rep. 2021, 21, 24. [Google Scholar] [CrossRef]
- Liu, K.; Sun, X.; Hu, W.J.; Mei, L.Y.; Zhang, H.D.; Su, S.B.; Ning, K.; Nie, Y.F.; Qiu, L.P.; Xia, Y.; et al. Silicosis Risk in Chinese Noncoal Mines: Risk Assessment. JMIR Public Health Surveill. 2024, 10, e56283. [Google Scholar] [CrossRef] [PubMed]
- Schroedl, C.J.; HT Go, L.; Cohen, R.A. Coal mine dust lung disease: The silent coal mining disaster. Curr. Respir. Med. Rev. 2016, 12, 101–110. [Google Scholar] [CrossRef]
- Feary, J.; Lindstrom, I.; Huntley, C.C.; Suojalehto, H.; de la Hoz, R.E. Occupational lung disease: When should I think of it and why is it important? Breathe 2023, 19, 230002. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Peters, M.D.J.; Godfrey, C.; McInerney, P.; Munn, Z.; Tricco, A.C.; Khalil, H. Chapter 11: Scoping Reviews. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: Adelaide, Australia, 2020; Available online: https://synthesismanual.jbi.global (accessed on 5 June 2025).
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Su, J.; Kong, X.; Yu, X.; Zhang, X. Incidence and influencing factors of occupational pneumoconiosis: A systematic review and meta-analysis. BMJ Open 2023, 13, e065114. [Google Scholar] [CrossRef] [PubMed]
- Vanka, A.; Kleinhenz, M.E.; Maier, L.A. Pathogenesis of coal and silica dust–associated lung disease. Eur. Respir. Rev. 2022, 31, 210250. [Google Scholar] [CrossRef] [PubMed]
- Weissman, D.N. Progressive massive fibrosis: A literature review. Pharmacol. Ther. 2022, 239, 108231. [Google Scholar] [CrossRef]
- Krefft, S.D.; Hinkamp, C.A.; Weissman, D.N. Silicosis: An update for clinicians. Clin. Chest Med. 2020, 41, 709–722. [Google Scholar] [CrossRef]
- Perlman, D.M.; Maier, L.A. Occupational lung disease. Med. Clin. North Am. 2019, 103, 535–548. [Google Scholar] [CrossRef]
- Minov, J.; Karadzinska-Bislimovska, J.; Vasilevska, K. Occupational COPD: Prevalence and prevention. Expert Rev. Respir. Med. 2022, 16, 145–152. [Google Scholar]
- Hou, X.; Wei, Z.; Jiang, X.; Wei, C.; Dong, L.; Li, Y.; Liang, R.; Nie, J.; Shi, Y.; Qin, X.; et al. A comprehensive retrospect on the current perspectives and future prospects of pneumoconiosis. Front. Public Health 2025, 12, 1435840. [Google Scholar] [CrossRef]
- Zhai, R.; Liu, G.; Ge, X.; Bao, W.; Wu, C.; Yang, C.; Liang, D. Serum levels of tumor necrosis factor-α (TNF-α), interleukin 6 (IL-6), and their soluble receptors in coal workers’ pneumoconiosis. Respir. Med. 2002, 96, 829–834. [Google Scholar] [CrossRef]
- Nadif, R.; Jedlicka, A.; Mintz, M.; Bertrand, J.-P.; Kleeberger, S.; Kauffmann, F. Effect of TNF and LTA polymorphisms on biological markers of response to oxidative stimuli in coal miners: A model of gene–environment interaction. J. Med. Genet. 2003, 40, 96–103. [Google Scholar] [CrossRef] [PubMed]
- David, G.L.; Zeldin, D.C. Coal workers’ pneumoconiosis. In Encyclopedia of Molecular Mechanisms of Disease; Springer: Berlin, Germany, 2009; pp. 373–374. [Google Scholar]
- Bradford, E.; Jacobson, S.; Varasteh, J.; Comellas, A.P.; Woodruff, P.; O’Neal, W.; DeMeo, D.L.; Li, X.; Kim, V.; Cho, M.; et al. The value of blood cytokines and chemokines in assessing COPD. Respir. Res. 2017, 18, 180. [Google Scholar] [CrossRef] [PubMed]
- Selvarajah, S.; Todd, I.; Tighe, P.J.; John, M.; Bolton, C.E.; Harrison, T.; Fairclough, L.C. Multiple circulating cytokines are coelevated in chronic obstructive pulmonary disease. Mediat. Inflamm. 2016, 2016, 3604842. [Google Scholar] [CrossRef] [PubMed]
- Vanka, K.S.; Shukla, S.; Gomez, H.M.; James, C.; Palanisami, T.; Williams, K.; Chambers, D.C.; Britton, W.J.; Ilic, D.; Hansbro, P.M.; et al. Understanding the pathogenesis of occupational coal and silica dust-associated lung disease. Eur. Respir. Rev. 2022, 31, 210250. [Google Scholar] [CrossRef] [PubMed]
- LeVan, T.D.; Romberger, D.J.; Siahpush, M.; Grimm, B.L. Relationship of systemic IL-10 levels with proinflammatory cytokine responsiveness and lung function in agriculture workers. Respir. Res. 2018, 19, 166. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Verma, S.K.; Kumar, S.; Ahmad, M.K.; Nischal, A.; Singh, S.K.; Dixit, R.K. Correlation of severity of chronic obstructive pulmonary disease with potentialbiomarkers. Immunol. Lett. 2018, 196, 1–10. [Google Scholar] [CrossRef]
- Baurzhan, M.; Berkinbayev, S.; Abzaliyev, K.; Andassova, Z.; Anvarbekova, Y.; Abzaliyeva, S.; Absatarova, K.; Tanabayeva, S.; Rakhimbekova, G.; Fakhradiyev, I. Prognostic value of serum soluble ST2 in professional athletes. Retos 2022, 43, 428–437. [Google Scholar] [CrossRef]
- Baurzhan, M.; Abzaliyev, K.; Anvarbekova, Y.; Andassova, Z.; Berkinbaev, S.; Absatarova, K.; Murariu, C. Modern approaches for diagnosing transformations of the heart in qualified athletes. J. Phys. Educ. Sport 2021, 21, 813–818. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Polish Respiratory Society. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulyayev, A.E.; Absattarova, K.S.; Kairgeldina, S.A.; Dosmagambetova, R.S.; Tekebayev, K.K.; Baurzhan, M.B.; Sagandykova, N.; Dauletova, G.S. An Integrated Strategy for Preventing and Rehabilitating Dust-Induced Occupational Bronchopulmonary Diseases: A Scoping Review. Adv. Respir. Med. 2025, 93, 30. https://doi.org/10.3390/arm93040030
Gulyayev AE, Absattarova KS, Kairgeldina SA, Dosmagambetova RS, Tekebayev KK, Baurzhan MB, Sagandykova N, Dauletova GS. An Integrated Strategy for Preventing and Rehabilitating Dust-Induced Occupational Bronchopulmonary Diseases: A Scoping Review. Advances in Respiratory Medicine. 2025; 93(4):30. https://doi.org/10.3390/arm93040030
Chicago/Turabian StyleGulyayev, Alexandr E., Karlygash S. Absattarova, Sayagul A. Kairgeldina, Raushan S. Dosmagambetova, Kanat K. Tekebayev, Madina B. Baurzhan, Nazym Sagandykova, and Gaukhar Sh. Dauletova. 2025. "An Integrated Strategy for Preventing and Rehabilitating Dust-Induced Occupational Bronchopulmonary Diseases: A Scoping Review" Advances in Respiratory Medicine 93, no. 4: 30. https://doi.org/10.3390/arm93040030
APA StyleGulyayev, A. E., Absattarova, K. S., Kairgeldina, S. A., Dosmagambetova, R. S., Tekebayev, K. K., Baurzhan, M. B., Sagandykova, N., & Dauletova, G. S. (2025). An Integrated Strategy for Preventing and Rehabilitating Dust-Induced Occupational Bronchopulmonary Diseases: A Scoping Review. Advances in Respiratory Medicine, 93(4), 30. https://doi.org/10.3390/arm93040030






