Abstract
Adolescence is a pivotal period of development marked by significant physiological and psychological changes, making youth particularly susceptible to mental health challenges, including depression. A growing body of research has highlighted the important role of sleep in the etiology and exacerbation of adolescent depression. Disruptions in sleep patterns, including insomnia and irregular sleep-wake cycles, are prevalent among depressed adolescents and can exacerbate depressive symptoms. In this review, we examine alterations to sleep behavior and physiology in adolescent depression. Furthermore, we introduce a theoretical model of hypersomnia in adolescent depression. This manuscript explores the intricate relationship between sleep and adolescent depression, with a focus on future directions for research and intervention.
1. Introduction
1.1. Why Study Depression in Adolescence?
Prevalence
Major depressive disorder (MDD) is a common mental illness that can limit psychosocial functioning and diminish quality of life [1]. MDD is associated with significant rates of disability, family burden, morbidity, and mortality [2,3,4]. MDD is the most prevalent mood disorder, and differential diagnosis from other mood disorders, such as bipolar disorder [5], is critical to its proper classification. As compared to unipolar depression, bipolar disorder is characterized by episodes of depression as well as at least one manic or hypomanic episode [5]. The diagnosis of bipolar depression in adolescence can be challenging because bipolar depression often starts with an episode of depression [5], and short episodes of mania or hypomania may appear later in life or be missed [5,6]. Therefore, it is not uncommon that adolescents initially diagnosed with depression may later be diagnosed with bipolar disorder. For the purposes of this review, we focus on major depressive disorder, but note that mood disorders more broadly are also associated with sleep difficulties.
In 2008, the WHO ranked major depressive disorder as the third leading cause of disease burden worldwide and predicted that the disease would rank first by 2030 [7]. An episode of major depressive disorder (MDD) is different from regular fluctuations in mood [8] and is typically characterized by diminished mood for most of the day, nearly every day for at least two weeks [1,8]. The 12-month prevalence of MDD across age groups is around 6% overall [9], while the lifetime risk is around three times higher (15–18%); [10]. When comparing high-income with low-income countries, the 12-month prevalence is similar, indicating that MDD is neither simply explained by modern-day lifestyles in highly developed countries nor by low socioeconomic status and poverty [11,12,13]. While the factors underlying the emergence of depression are complex, biological factors (e.g., genetic vulnerability) interact with environmental factors (e.g., stress, abuse, and adverse family relations) to determine the manifestation of this disorder [14]. The probability of recurrence of MDD is high and is estimated to be between 60% and 70% [15,16,17], with the risk of recurrence increasing with every additional episode [8]. In children and adolescents, self-harm and suicide are serious consequences of depression, and the risk of engaging in these behaviors increases during the teenage years [5,18,19]. Suicide is the third leading cause of death in adolescents and adolescent depression is the major risk factor for suicide [5]. Therefore, identifying risk factors early on in life is critical to reducing the personal, social, and economic burden associated with depression.
1.2. Emergence of Major Depressive Disorder
Depression typically has its first onset during the adolescent years, and thus these years present a unique opportunity for early detection and intervention. Epidemiological studies estimate that the point prevalence of major depressive disorder is around 5 to 11% in adolescence [20,21,22,23], and approximately 9 to 16% of 14- to 16-year-old adolescents experience subthreshold depression [24]. By the time they reach their 18th birthday, 15% to 20% of teens will have experienced a depressive episode [25,26,27,28]. The increased incidence of depressive symptoms and major depressive disorder [12] during adolescence is hypothesized to be due to an interaction of increased social demands and stressors, hormonal changes, and brain development [12,29,30]. Studies show that illness severity is higher, more chronic, and has a higher rate of recurrence in those who have a younger age of onset [23]. The onset of depression is often gradual but can, in some cases, be abrupt [8]. The course of depression can vary significantly between individuals and throughout life [8]. For most affected individuals, the course of illness is episodic, with episodes of relative wellbeing interspersed with acute depressive episodes [8,11]. However, the illness is unpredictable, and the trajectories in terms of episode number and duration, as well as disease patterns, are subject to pronounced interindividual variability [8]. The mean duration of depressive episodes in children and adolescents has been found to be 7 to 9 months [5,17,31,32,33]. In children and adolescents around 12 years of age or younger, major depression is more common in boys (1.3%) as compared to girls (0.8%) [34], but during adolescence and in adulthood, females are two or even three times more likely to suffer from depression than males (ratio 2:1 or 3:1) [17,20,34,35,36,37,38]. While the precise mechanisms underlying the gender gap are not understood, they are likely a combination of biological (e.g., hormones) and environmental (e.g., unequal power and status) factors that account for the higher incidence of depression among females [38].
Understanding the factors associated with the emergence and maintenance of depression is perhaps more important now than ever—the coronavirus (COVID-19) pandemic had a disastrous impact on mental health [39]. The estimated prevalence of major depressive disorders as well as anxiety disorders among the general global population increased by over 20% during 2020 [39,40]. To summarize, depression is one of the leading causes of disability worldwide and has its onset during adolescence. Therefore, detecting risk prior to the onset of a depressive episode and intervening early has the potential to alter the course of the disease and reduce burden. One such early alert of an oncoming depressive episode may be insomnia or hypersomnia.
2. Why Sleep and (Adolescent) Depression?
The links between depression and sleep are many and not well understood. In this review, we seek to elucidate what is currently known and propose future directions for research. Sleep disturbances are reported in up to 90% of individuals with depression [41,42] and are one of the diagnostic criteria of major depressive disorder [3,43,44]. These disruptions to sleep typically manifest as symptoms of insomnia, such as difficulty falling or staying asleep. While in adults with depression, early morning awakenings are also common due to the adolescent delay in the circadian system, which pushes bedtimes later (reviewed in Section 5); [45,46,47,48,49], early morning awakenings are less common in adolescents with MDD [42]. In addition to insomnia, a small minority of adults with depression report symptoms of hypersomnia, defined as prolonged sleep episodes at night or increased daytime sleepiness despite achieving recommended sleep durations. Hypersomnia represents another distinctive manifestation of sleep difficulties in adolescent MDD, being more commonly observed during the adolescent years.
Between 26 and 36% (depending on the study) of adolescents with MDD report symptoms of hypersomnia [42,50,51,52,53,54]. Though still representing a minority of adolescents with MDD, the higher prevalence of hypersomnia in depression in adolescence compared to adulthood suggests that developmental processes may account for this discrepancy. However, we note that reports of hypersomnia are based on self-reported measures of sleeping “too long” or daytime sleepiness. In the few studies that have used objective methods (e.g., polysomnography or the multiple sleep latency test for daytime sleepiness), no objective evidence of hypersomnia in adults with MDD has been found [55]. Therefore, the reports of hypersomnia are likely linked to prolonged periods spent in bed “resting” due to decreased energy associated with depression rather than prolonged sleep duration.
Theoretical Model of Hypersomnia in Adolescent Depression
We propose that reduced drive, a feature of depression, may interact with developmental changes in sleepiness and sleep propensity in adolescence to give rise to the higher incidence of hypersomnia in adolescents as compared to adult depression. Experimental studies in well slept healthy teens have shown that during the adolescent period, sleepiness and sleep propensity (measured objectively using the MSLT) increase [56,57]. In particular, recent reports indicate that the developmental decline in sleep spindles, thalamocortical oscillations in the 11 to 14 Hz range during NREM sleep, is associated with the observed increases in sleepiness across this period in healthy adolescents [56]. Sleep spindles have been shown to be diminished in adolescents with and at risk for depression (see Section 3.2). Therefore, alterations in the thalamocortical system in MDD may interact with the developmental decline in spindles, resulting in greater feelings of subjective sleepiness in adolescent MDD (Figure 1).
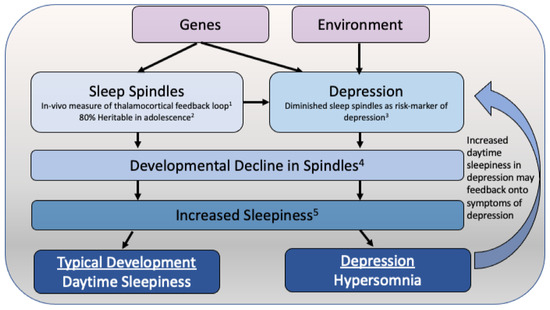
Figure 1.
Theoretical model of how hypersomnia may emerge in adolescent depression. Sleep spindles are highly heritable in adolescence, with heritability estimates of above 80% over posterior regions. A deficit in sleep spindles is found in adolescents at an increased familial risk for depression, and this risk may be driven by genetic factors. The described adolescent decline in sleep spindle activity has been associated with the increase in daytime sleepiness observed across the adolescent period. While this decline in sleep spindles may increase daytime sleepiness in typically developing teens, in adolescents with depression, the pre-existing deficit in sleep spindles may result in hypersomnia. This increased daytime sleepiness may further exacerbate depressive symptoms, particularly those related to diminished drive. (1) Contreras et al., Science, 1996 [58]; (2) Rusterholz et al., J Neurosci, 2018 [59]; (3) Lopez et al., JAACAP, 2010 [22]; (4) McClain et al., Neural Plast, 2016 [60]; (5) Campbell et al., Sleep, 2023 [56].
Taken together, self-reported insomnia and, in some individuals, hypersomnia are central features of adolescent depression. Despite this, a minority of individuals with depression do not report sleep problems—understanding why may be instrumental in treating sleep problems in depression. Indeed, objective measures of sleep have revealed a complex picture of sleep disruption in adolescent depression.
3. Sleep EEG Neurophysiology
Polysomnography is used to investigate sleep states and is considered the “gold standard” for determining vigilance states [61]. Polysomnography (PSG) typically includes electroencephalography (EEG), electrooculography (EOG), and electromyography (EMG), which together can be used to delineate sleep into five stages (Stages 1 to 5) and two states (NREM and REM sleep) [44,61]. In 2007, the American Academy of Sleep Medicine proposed a revised sleep scoring system that categorizes sleep into four stages, consolidating stages 3 and 4 into a single stage named N3 [62].
PSG studies in adolescent depression have in part confirmed the subjective reports of disrupted sleep. Several studies have shown reduced total sleep time [44,63,64] in adolescents with major depressive disorder. Moreover, as reported in multiple studies, sleep onset latency and wake after sleep onset are prolonged in adolescent depression [64,65,66,67]. Sleep efficiency (which is the ratio of total time in bed to the time asleep) has been found to be diminished in adolescents with depression [64,65,66,67,68]. Reduced non-rapid eye movement sleep (NREM), especially slow wave sleep [69,70], as well as shorter latency to rapid eye movement (REM) sleep [64,69,71,72,73] and increased REM sleep density have also been found in adolescents with depression [64,71]. These alterations are in line with those seen in adults with major depressive disorder but are likely present to a lesser extent [44,64].
However, some studies did not replicate these findings [74,75,76]. These inconsistent results might be explained by maturational factors that influence the composition of sleep stage variables across adolescent development. Among these changes is a marked 30–40% decline in slow wave sleep (SWS), a state conceptualized as deep sleep, which is replaced by a lighter form of sleep known as stage 2. The rate at which these changes occur displays a large degree of inter-individual variability, partially tied to pubertal timing [77,78,79,80]. Furthermore, the latency to REM sleep is longer in pre-adolescents as compared to post-pubertal adolescents [80,81]. As the age range in most studies of adolescent depression is broad, these maturational factors may significantly influence the findings. Moreover, there is greater heterogeneity in the spectrum of depression among adolescents [44,64]. Thus, even though adolescents with depression often complain about their sleep, the results from studies with objective measurements of sleep remain equivocal [44,63,82].
3.1. Slow Wave Activity
The mathematical quantification of slow wave sleep is called slow wave activity (SWA; also referred to as delta activity) and consists of low-frequency, high-amplitude waves between 0.5 and 4.6 Hz [83,84,85]. SWA is a measure of sleep depth and homeostasis [83,84,85]. SWA is high at the beginning of the sleep episode and dissipates across the course of sleep. Furthermore, SWA is exquisitely sensitive to prior sleep duration and increases with prolonged wakefulness in a dose-dependent manner [86,87]. Early on, it was proposed that the disruption in sleep observed in individuals with depression is attributable to abnormally low levels of sleep pressure [88] that are normalized by prolonged waking. This hypothesis was based on early studies showing diminished slow wave sleep in adults with depression [89,90] and the temporary therapeutic effect of one night of sleep deprivation in some adults with major depressive disorder [91]. Since then, polysomnographic studies in adults with major depressive disorder have shown inconsistent results—multiple studies have reported decreased slow wave activity [52,92,93,94,95], whereas others found increased slow wave activity at baseline as well as after sleep deprivation [75]. Furthermore, the decrease in slow wave activity has been shown to be particularly pronounced in the first 100 min of sleep [96]. Other factors may also influence slow wave sleep and activity in depression. For example, several studies have shown that, compared to healthy controls, SWA is diminished in adult men but not in adult women with MDD [69,92,97].
These mixed effects with regards to SWA have also been reported in children and adolescents with major depressive disorder. While some studies have reported diminished slow wave activity in adolescents with major depressive disorder [69,98,99,100], others found an increase in slow wave activity [76].
Mirroring the findings in adults, gender may strongly modulate the impact of MDD on SWA. In a large study of 104 adolescents between the ages of 13 and 18 years, of which 52 were adolescents suffering from depression and 52 were healthy controls, the accumulation of SWA in the first period of NREM sleep was only found to be lower in male adolescents but not female adolescents with MDD [100]. The dissipation of SWA over the course of the night may also be irregular in male adolescents as compared to healthy males [99]. The authors hypothesized that the known gender differences in the manifestation of depression may account for this finding [100]. However, more studies are needed to clarify this issue.
In many ways, studying SWA in adolescence is a “moving target”. For one, sleep undergoes significant changes from childhood to adolescence; one of them is a very pronounced decline in SWA [80,98,99,101]. This decline begins earlier in girls than boys, is somewhat tied to pubertal development, and shows a large degree of inter-individual variability in the degree and timing of the decline [77]. Therefore, two adolescents with the same biological age are likely at different points in their developmental trajectory of SWA. Furthermore, the discrepancy in findings with regards to SWA in both adults and adolescents may be due to other issues and highlights the difficulty of measuring SWA in individuals with major depressive disorder. For one, depression is a heterogeneous disorder with many different configurations of symptoms leading to the same diagnosis [102]. Secondly, while sleep problems are prevalent, about 20 to 40% of individuals (depending on the sample studied) do not report sleep difficulties. Furthermore, medication status may further complicate this issue [76,100]. Finally, and perhaps most importantly, measuring SWA in major depressive disorder is precarious at best since SWA is strongly dependent on prior sleep-wake history. Therefore, in most studies, individuals with major depressive disorder likely enter the study with a higher level of sleep pressure than control participants (due to short sleep duration and disrupted sleep) and often take longer to fall asleep, resulting in a higher level of sleep pressure at sleep onset [86,103,104]. Finally, individuals with major depressive disorder are also more likely to awaken throughout the night, leading to a subsequent rebound of slow wave activity in the latter part of the night [86,103]. We propose that in future studies, care should be taken in measuring SWA in depression, and prior sleep-wake history should be taken into account.
3.2. Sleep Spindles
Sleep spindles are waxing-waning oscillations (11–15 Hz) that occur as bursts of EEG activity in the sigma frequency range [105,106] and are characteristic of non-rapid eye movement (NREM) sleep measured via EEG [97,105]. There is some evidence that sleep spindles are involved in several domains, including maintaining disconnection from the external environment during sleep [107], cortical development [108], and sleep-dependent memory consolidation [109], but the precise functional role(s) of sleep spindles remains unclear. Findings in adolescents have been mixed, with some studies finding no difference [76] or a reduction [22,110] in spindle activity between adolescent participants with major depressive disorder as compared to healthy controls.
In a study that examined sleep spindles, adolescents with MDD or individuals with an elevated risk for depression based on familial history (n = 21) showed alterations in sleep spindles when compared to healthy controls with no personal or familial history of MDD (n = 21). The difference was greatest between adolescents with major depressive disorder and healthy controls, but the high-risk group had significantly lower sleep spindle activity as compared to the healthy control group [22]. The deficit in sleep spindle activity was more prominent in females as compared to males and was more often observed later in the night [22]. In another study by Hamann and colleagues, lower sleep spindle activity was associated with more depressive symptoms as measured via self-report in a non-clinical sample [111]. This difference was topographically widespread over a large cluster, including parietal, temporal, and occipital derivations [111]. A study investigating electrophysiological and microstructural features of sleep was conducted in children and adolescents aged 4 to 18 years of age [112]. The 31 participants included in the study did not have MDD, but 20 of them were born to mothers suffering from major depressive disorder (“high risk group”), and 11 children were born to mothers without a personal history of depression (“low risk group”) [112]. Even though all included participants did not have MDD, the results showed a reduction in low-frequency spindle activity and spindle spatio-temporal characteristics over frontal and central derivations in the high-risk group as compared to the low-risk group [112].
The association between sleep spindles and mental health has also been reported in younger age groups. In studies by Mikoteit and colleagues, including preschoolers, higher non-rapid eye movement spindle density was correlated with less internalizing behavior, more prosocial behavior, and a lower total problem score [113,114]. When analyzed longitudinally, a higher number of NREM spindles at the age of 5 years predicted fewer peer problems one year later [113,114]. Taken together, several studies support the role of sleep spindles in adolescent depression and at-risk states. This is somewhat in contrast to studies in adults, which typically do not report changes in sleep spindles [66,115,116]. Nevertheless, in the aforementioned studies, a significant number of participants with major depressive disorder were in remission, and several participants were taking medications [115]. Furthermore, subjects were not gender-matched, and only sleep from the first NREM sleep episode was used [115]. The fact that the groups were not sex-matched is problematic, since it is known that healthy females have greater sleep spindle activity as compared to males in adolescents and adults [117,118].
3.3. REM Sleep
Anomalies in rapid eye movement (REM) sleep are often found in adults with depression [4]. These irregularities often include shortened REM latency, which is defined as the time interval between sleep onset and the occurrence of the first REM sleep episode; increased REM sleep duration; and increased REM density, defined as the frequency of rapid eye movements per REM period [4]. The biological mechanisms that link the increased amount and intensity of REM sleep and depression are not fully understood yet [4,44], and there have only been very few studies examining this phenomenon in children and adolescents [119]. Rao and colleagues examined differences in the sleep EEG between adolescents at high risk for depression, as defined by parental depression, and a control group [120]. Adolescents at high risk showed alterations in REM sleep similar to those found in adults – shorter latency to REM sleep, a higher number of episodes, a longer duration of each episode, a higher proportion of REM sleep, as well as increased REM sleep activity and density [120].
Taken together, while subjective reports of disrupted sleep are a hallmark of depression, objective data obtained from polysomnography are mixed with regards to sleep architecture and neurophysiology. This is likely due to several of the aforementioned factors, including age, gender, medication status, and the heterogeneity of depression.
4. Sleep and the Sleep EEG as a Biomarker in Depression
The pathophysiology of depression remains unclear, and treatment outcomes are often unsatisfying, with up to 60% of patients suffering from treatment resistance [121]. For this reason, several studies have attempted to identify biomarkers that might be specific indicators of depression as well as helpful prognostic factors [122]. A National Institute of Mental Health working group has defined a biomarker as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacological responses to therapeutic intervention” [123]. Within this context, the EEG has emerged as a promising biomarker for a range of psychiatric illnesses as it is a measure of brain activity, altered in mental illness, and has a strong genetic basis [124,125,126,127].
With regards to sleep, shortened REM sleep latency and elevated REM density have been proposed as biomarkers in depression [52]. However, enthusiasm for REM sleep as a biomarker specific to depression has been diminished by observations of similar changes in other psychiatric disorders such as mania [128], schizoaffective disorder [129], schizophrenia [130], panic disorder [131], obsessive-compulsive disorder [132], and eating disorders [133]. We note that most studies on biomarkers in depression have focused on the adult population [122]. This is surprising because it is well known that early-onset depression has been associated with a worse disease course and that early interventions are more effective than interventions later in life [122]. Finding biomarkers that might be specific to depression in youth might aid diagnosis and improve treatment outcomes [122]. In one of the few biomarker studies of adolescent sleep and depression, Armitage and colleagues investigated whether sleep EEG variables could predict treatment response in adolescents with major depressive disorder [134]. Sleep EEG was measured at baseline, after six months, and after a year [134]. Significantly lower temporal coherence, a measure of connectivity, in the beta, theta, and delta frequency bands between left (C3) and right (C4) central EEG derivations in males with major depressive disorder was associated with a shorter time to recurrence, while the opposite was true in females [134]. This is yet another example of the differential impact of gender on sleep neurophysiology in adolescent depression. Given this preliminary but promising study, we posit that the sleep EEG has untapped potential as a biomarker for adolescent depression. Indeed, our group has recently shown that the sleep EEG is highly heritable (with heritability estimates above 80%) in adolescence [59,135,136]—a period of rapid change as outlined above. Future longitudinal studies are needed to explore this potential.
5. Sleep and Depression: A Bi-Directional Relationship?
While the evidence that disrupted sleep is a core feature of depression is unequivocal, it is increasingly evident that the relationship between depression and sleep is likely bi-directional. [3,44,52,137]. A series of prospective epidemiological studies have shown that adolescents with insomnia are 2 to 5 times (depending on the study) more likely to suffer from depressive symptoms or even major depressive disorder [137,138,139,140]. Furthermore, disrupted sleep is a potent risk factor for non-suicidal self-injury (NSSI) and suicidal ideation. For example, large epidemiological studies have shown adolescents who are short sleepers are twice as likely to have suicidal ideation/consideration as compared to long sleepers [141]. Furthermore, while NSSI is the best predictor of suicidality, sleep problems are nearly as important [142]. To wit, in a sample of adolescents with NSSI, more sleep disturbance was associated with more suicide attempts despite controlling for depressive symptoms, suggesting that sleep is an independent factor for suicidality [143].
The association between sleep and suicidality is particularly relevant in adolescence, as there is a developmental increase in sleep disruption, insomnia, and short sleep duration [144]. This increase may be due in part to the changes to the bioregulatory systems that control sleep during the adolescent period. Specifically, a delay in the circadian timing system during adolescence pushes bedtimes later and results in difficulties initiating sleep at an earlier clock time [145]. Concurrent to this, sleep pressure builds more slowly in late versus early pubertal adolescents, allowing older teens to stay awake longer [145]. Interacting with the maturation of the processes regulating sleep are developmental changes in brain structure/function and hormonal shifts triggered by puberty [30]. The impact of these alterations on sleep is not entirely clear, but it is likely that these modifications may in part underlie the observed maturational increase in sleep disruption. These changes to the biological systems regulating sleep interact with psychosocial pressures, such as early school start times, increased socialization with peers and academic pressure, to result in sleep that is short, ill-timed, and disrupted.
Perlis and colleagues [146] suggested a hyperarousal model of insomnia, which posits that individuals with insomnia experience heightened physiological and cognitive arousal, preventing the initiation and maintenance of sleep. According to this model, factors such as increased heart rate, elevated metabolic activity, and cognitive overactivity contribute to the difficulty of falling asleep and maintaining a restful sleep state. This model was modified by Riemann and colleagues [147], who suggested that psychosocial stress as well as inadequate problem solving and worries or rumination lead to problems with sleep onset latency as well as wakefulness after sleep onset and also reduce total sleep time [147]. Affected individuals then start worrying about their sleep and show selective attention to sleep-related stimuli [147]. Often, these individuals also show a bias toward the daytime consequences of sleep disturbances. These factors then lead to a behavioral adaptation to extended sleep opportunities [147]. These individuals often remain in bed awake or show an altered exposure to light during the sleep period [147]. These behaviors can then become conditioned and lead to depression, addiction, and anxiety disorders [147].
This model aligns with the consequences of the COVID-19 pandemic, wherein psychosocial stressors stemming from the crisis led to a deterioration in sleep among children and adolescents [148,149,150]. The long-term consequences of the COVID-19 pandemic on sleep and mental health among youth are currently unclear. Nevertheless, considering the Riemann model outlined earlier, it’s plausible that the psychosocial stress induced by the pandemic might have propelled certain young individuals into a detrimental cycle of disrupted sleep and compromised mental well-being.
Given the emerging evidence that sleep disruption is prospectively associated with poor mental health, sleep may be a powerful intervention target in adolescents to increase resilience and protect mental health. However, while the association between sleep and depression is clearly bi-directional, the precise mechanisms and the temporal sequence are unclear. Most prospective studies have used a few questions to assess sleep and have not differentiated between sleep duration and sleep disruption. Similarly, measures of mental health have been based on questionnaires. Future research in this area should carefully assess the long-term impact of sleep-based interventions on mental health. To wit, effective behavioral interventions for sleep problems exist, and a recent review highlights the use of these interventions in adolescent depression [151].
6. Sleep, Depression, Media Use and Physical Activity
The amount of time adolescents spend using digital media (including texting, social media use, electronic gaming, and general smartphone as well as computer use) is rapidly increasing [152]. Twenge and colleagues found that the time 12th graders spent online engaging in one of these activities doubled between 2006 and 2016 [153]. A national survey in the United States that was conducted with 8th, 10th, and 12th graders between 1991 and 2016 found that psychological well-being defined and measured by self-esteem, life satisfaction, and happiness suddenly decreased after 2012 [152,153,154]. A more detailed analysis showed that adolescents who spent more time on electronic devices and screens, independent of whether it was for social media use, internet use, texting, or gaming, had lower psychological well-being as compared to adolescents that engaged in more non-screen activities such as in-person social interaction, sports, homework, or attending religious services [154]. More recently, it has been suggested that some of the negative impact of digital media use on mental health may be driven by sleep disruption. An established body of literature has shown that screen media use among youth can have a negative impact on sleep [155], and studies have shown that when controlling for sleep duration and cyberbullying, the association between mental health and online digital media use is non-significant [156]. Thus, sleep may mediate the impact of digital media use on mental health.
On the other hand, physical activity (PA) is considered an effective, non-pharmacological intervention to improve sleep. The results of a large meta-analysis indicated that adolescents with higher subjective and objective physical activity levels were more likely to experience good nocturnal sleep, both subjectively and objectively [157]. In another study including 51 adolescents between the ages of 17.5 and 19.5 years, three weeks of daily morning running (30 min) were found to improve sleep in the running group as compared to the control group [158]. In the running group, objective and subjective measures of sleep improved [158], as did mood and concentration as compared to the control group [158]. However, other studies have not found an association between daily physical activity and subsequent sleep [159], suggesting that perhaps moderate exercise is necessary to see these effects. Taken together, these findings point to modifiable environmental factors that may improve sleep and, in turn, benefit mental health [154,157,158].
7. Sleep Timing and Depression
Going hand in hand with the disruptions to sleep are disruptions of the circadian system in depression [160]. These disruptions are often observed as a misalignment of the biological clock as revealed through the rhythms of hormones (e.g., melatonin) and sleep/wake timing. The social zeitgeber theory [161] and others [162] have postulated that disruptions of circadian rhythms or abnormalities in the superchiasmatic nucleus (SCN; the circadian pacemaker) underlie the alterations of circadian rhythms observed in mood disorders. For example, in humans and mice, circadian genes such as CLOCK, BMAL1, Period3, and Timeliness have been associated with unipolar and bipolar depression (reviewed [163]. Therefore, interventions targeting the circadian system, such as bright light therapy, may also improve mood and sleep in depression [164].
Another area that has garnered recent attention is the use of metrics that quantify the regularity of sleep timing, such as the sleep regularity index (SRI; [165]). Using these metrics, more variable sleep has been associated with worse mood [166,167], and a review by Becker et al. (2017) found psychopathology and greater symptom severity to be associated with greater sleep intra-individual variability [168]. The results suggested that regular sleep and wake timing might be protective of adolescent mental health [166]. Therefore, adolescents should be encouraged to maintain regular sleep times to promote mental health [166,168].
8. Conclusions
In conclusion, the role of sleep in adolescent depression is intricately intertwined with the neurobiology of the developing brain. Based on the existing literature, we conclude that sleep disturbances, such as insomnia and irregular sleep patterns, can significantly contribute to the onset and severity of depression in teenagers. Conversely, depression itself often leads to disrupted sleep, creating a vicious cycle that exacerbates both conditions. Recognizing the importance of addressing sleep-related issues in adolescents with depression is crucial for effective prevention and treatment strategies. By promoting healthy sleep habits and providing support for adolescents to manage their sleep patterns, we can make significant strides in mitigating the impact of depression during this critical stage of development. Future research should elucidate the precise neurobiological mechanisms by which changes in sleep and depressive symptoms are associated during adolescence and further explore the long-term efficacy of behavioral treatments for sleep disruption in adolescents with depression.
Author Contributions
Conceptualization, L.T. and C.E.G.C.-F.; data curation, L.T. and C.E.G.C.-F.; writing—original draft preparation, L.T. and C.E.G.C.-F.; writing—review and editing, L.T. and C.E.G.C.-F.; visualization, L.T. and C.E.G.C.-F.; supervision, L.T.; project administration, L.T.; funding acquisition, L.T. All authors have read and agreed to the published version of the manuscript.
Funding
The present study was funded by the Interfaculty Research Cooperation: Decoding Sleep (to L.T.) and the Swiss National Science Foundation Grant 32003B_184943 (to L.T.).
Acknowledgments
The authors are grateful to Claudio LA Bassetti and Fred Mast for the opportunity to be a part of the Interfaculty Research Cooperation: Decoding Sleep. The authors also thank Salome Wild for her thoughtful comments on the manuscript.
Conflicts of Interest
L.T. serves as a consultant for F. Hoffmann-La Roche Ltd., which is unrelated to this study.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2022. [Google Scholar]
- Essau, C.; Dobson, K.S. Epidemiology of depressive disorders. In Depressive Disorders in Children and Adolescents: Epidemiology, Risk Factors, and Treatment; Essau, C.A., Petermann, F., Eds.; Jason Aronson, Inc. Publishers: Lanham, MD, USA, 1999; pp. 69–103. [Google Scholar]
- Mendlewicz, J. Sleep disturbances: Core symptoms of major depressive disorder rather than associated or comorbid disorders. World J. Biol. Psychiatry 2009, 10, 269–275. [Google Scholar] [CrossRef]
- Palagini, L.; Baglioni, C.; Ciapparelli, A.; Gemignani, A.; Riemann, D. REM sleep dysregulation in depression: State of the art. Sleep Med. Rev. 2013, 17, 377–390. [Google Scholar] [CrossRef]
- Alsaad, A.J.; Azhar, Y.; Al Nasser, Y. Depression in Children; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Birmaher, B.; Axelson, D. Course and outcome of bipolar spectrum disorder in children and adolescents: A review of the existing literature. Dev. Psychopathol. 2006, 18, 1023–1035. [Google Scholar] [CrossRef]
- World Health Organization. The Global Burden of Disease: 2004 Update; World Health Organization: Geneva, Switzerland, 2008; p. 146. [Google Scholar]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Kessler, R.C.; Bromet, E.J. The epidemiology of depression across cultures. Ann. Rev. Public Health 2013, 34, 119–138. [Google Scholar] [CrossRef]
- Bromet, E.; Andrade, L.H.; Hwang, I.; Sampson, N.A.; Alonso, J.; de Girolamo, G.; de Graaf, R.; Demyttenaere, K.; Hu, C.; Iwata, N.; et al. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011, 9, 90. [Google Scholar] [CrossRef]
- Selph, S.S.; McDonagh, M.S. Depression in Children and Adolescents: Evaluation and Treatment. Am. Fam. Physician 2019, 100, 609–617. [Google Scholar]
- Thapar, A.; Collishaw, S.; Pine, D.S.; Thapar, A.K. Depression in adolescence. Lancet 2012, 379, 1056–1067. [Google Scholar] [CrossRef]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Heim, C.; Binder, E.B. Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp. Neurol. 2012, 233, 102–111. [Google Scholar] [CrossRef]
- 15 Birmaher, B.; Ryan, N.D.; Williamson, D.E.; Brent, D.A.; Kaufman, J.; Dahl, R.E.; Perel, J.; Nelson, B. Childhood and adolescent depression: A review of the past 10 years. Part I. J. Am. Acad. Child Adolesc. Psychiatry 1996, 35, 1427–1439. [Google Scholar] [CrossRef]
- Dopheide, J.A. Recognizing and treating depression in children and adolescents. Am. J. Health Syst. Pharm. 2006, 63, 233–243. [Google Scholar] [CrossRef]
- Mullen, S. Major depressive disorder in children and adolescents. Ment. Health Clin. 2018, 8, 275–283. [Google Scholar] [CrossRef]
- Hawton, K.; Saunders, K.E.A.; O’Connor, R.C. Self-harm and suicide in adolescents. Lancet 2012, 379, 2373–2382. [Google Scholar] [CrossRef]
- Morken, I.S.; Dahlgren, A.; Lunde, I.; Toven, S. The effects of interventions preventing self-harm and suicide in children and adolescents: An overview of systematic reviews. F1000Research 2019, 8, 890. [Google Scholar] [CrossRef]
- Costello, E.J.; Erkanli, A.; Angold, A. Is there an epidemic of child or adolescent depression? J. Child Psychol. Psychiatry 2006, 47, 1263–1271. [Google Scholar] [CrossRef]
- Lewinsohn, P.M.; Rohde, P.; Seeley, J.R. Major depressive disorder in older adolescents: Prevalence, risk factors, and clinical implications. Clin. Psychol. Rev. 1998, 18, 765–794. [Google Scholar] [CrossRef]
- Lopez, J.; Hoffmann, R.; Armitage, R. Reduced sleep spindle activity in early-onset and elevated risk for depression. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 934–943. [Google Scholar] [CrossRef]
- Zisook, S.; Rush, A.J.; Albala, A.; Alpert, J.; Balasubramani, G.K.; Fava, M.; Husain, M.; Sackeim, H.; Trivedi, M.; Wisniewski, S. Factors that differentiate early vs. later onset of major depression disorder. Psychiatry Res. 2004, 129, 127–140. [Google Scholar] [CrossRef]
- Bertha, E.A.; Balázs, J. Subthreshold depression in adolescence: A systematic review. Eur. Child Adolesc. Psychiatry 2013, 22, 589–603. [Google Scholar] [CrossRef]
- Kessler, R.C.; McGonagle, K.A.; Zhao, S.; Nelson, C.B.; Hughes, M.; Eshleman, S.; Wittchen, H.U.; Kendler, K.S. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch. Gen. Psychiatry 1994, 51, 8–19. [Google Scholar] [CrossRef]
- Lewinsohn, P.M.; Rohde, P.; Seeley, J.R. Psychosocial characteristics of adolescents with a history of suicide attempt. J. Am. Acad. Child Adolesc. Psychiatry 1993, 32, 60–68. [Google Scholar] [CrossRef]
- Lewinsohn, P.M.; Duncan, E.M.; Stanton, A.K.; Hautzinger, M. Age at first onset for non-bipolar depression. J. Abnorm. Psychol. 1986, 95, 378–383. [Google Scholar] [CrossRef]
- Shorey, S.; Ng, E.D.; Wong, C.H.J. Global prevalence of depression and elevated depressive symptoms among adolescents: A systematic review and meta-analysis. Br. J. Clin. Psychol. 2022, 61, 287–305. [Google Scholar] [CrossRef]
- Blakemore, S.-J. Adolescence and mental health. Lancet 2019, 393, 2030–2031. [Google Scholar] [CrossRef]
- Uccella, S.; Cordani, R.; Salfi, F.; Gorgoni, M.; Scarpelli, S.; Gemignani, A.; Geoffroy, P.A.; De Gennaro, L.; Palagini, L.; Ferrara, M.; et al. Sleep Deprivation and Insomnia in Adolescence: Implications for Mental Health. Brain Sci. 2023, 13, 569. [Google Scholar] [CrossRef]
- Kovacs, M.; Feinberg, T.L.; Crouse-Novak, M.A.; Paulauskas, S.L.; Finkelstein, R. Depressive Disorders in Childhood: I. A Longitudinal Prospective Study of Characteristics and Recovery. Arch. Gen. Psychiatry 1984, 41, 229–237. [Google Scholar] [CrossRef]
- Lewinsohn, P.M.; Clarke, G.N.; Seeley, J.R.; Rohde, P. Major depression in community adolescents: Age at onset, episode duration, and time to recurrence. J. Am. Acad. Child Adolesc. Psychiatry 1994, 33, 809–818. [Google Scholar] [CrossRef]
- McCauley, E.; Myers, K.; Mitchell, J.; Calderon, R.; Schloredt, K.; Treder, R. Depression in young people: Initial presentation and clinical course. J. Am. Acad. Child Adolesc. Psychiatry 1993, 32, 714–722. [Google Scholar] [CrossRef]
- Douglas, J.; Scott, J. A systematic review of gender-specific rates of unipolar and bipolar disorders in community studies of pre-pubertal children. Bipolar Disord. 2014, 16, 5–15. [Google Scholar] [CrossRef]
- Hyde, J.S.; Mezulis, A.H.; Abramson, L.Y. The ABCs of depression: Integrating affective, biological, and cognitive models to explain the emergence of the gender difference in de-pression. Psychol. Rev. 2008, 115, 291–313. [Google Scholar] [CrossRef] [PubMed]
- Magklara, K.; Bellos, S.; Niakas, D.; Stylianidis, S.; Kolaitis, G.; Mavreas, V.; Skapinakis, P. Depression in late adolescence: A cross-sectional study in senior high schools in Greece. BMC Psychiatry 2015, 15, 199. [Google Scholar] [CrossRef] [PubMed]
- Nolen-Hoeksema, S.; Girgus, J.S. The emergence of gender differences in depression during adolescence. Psychol. Bull. 1994, 115, 424–443. [Google Scholar] [CrossRef] [PubMed]
- Riecher-Rössler, A. Sex and gender differences in mental disorders. Lancet Psychiatry 2017, 4, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhou, F.; Hou, W.; Heybati, K.; Lohit, S.; Abbas, U.; Silver, Z.; Wong, C.Y.; Chang, O.; Huang, E.; et al. Prevalence of mental health symptoms in children and adolescents during the COVID-19 pandemic: A meta-analysis. Ann. N. Y. Acad. Sci. 2023, 1520, 53–73. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Mental Disorders Collaborators. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef] [PubMed]
- Riemann, D.; Berger, M.; Voderholzer, U. Sleep and depression—Results from psychobiological studies: An overview. Biol. Psychol. 2001, 57, 67–103. [Google Scholar] [CrossRef]
- Urrila, A.S.; Karlsson, L.; Kiviruusu, O.; Pelkonen, M.; Strandholm, T.; Marttunen, M. Sleep complaints among adolescent outpatients with major depressive disorder. Sleep Med. 2012, 13, 816–823. [Google Scholar] [CrossRef]
- Nutt, D.; Wilson, S.; Paterson, L. Sleep disorders as core symptoms of depression. Dialogues Clin. Neurosci. 2008, 10, 329–336. [Google Scholar] [CrossRef]
- Urrila, A.S.; Paunio, T.; Palomäki, E.; Marttunen, M. Sleep in adolescent depression: Physiological perspectives. Acta Physiol. 2015, 213, 758–777. [Google Scholar] [CrossRef]
- Carskadon, M.A. Sleep in adolescents: The perfect storm. Pediatr. Clin. N. Am. 2011, 58, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Crowley, S.J.; Acebo, C.; Carskadon, M.A. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007, 8, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Hagenauer, M.H.; Perryman, J.I.; Lee, T.M.; Carskadon, M.A. Adolescent changes in the homeostatic and circadian regulation of sleep. Dev. Neurosci. 2009, 31, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Hagenauer, M.H.; Lee, T.M. The neuroendocrine control of the circadian system: Adolescent chronotype. Front. Neuroendocrinol. 2012, 33, 211–229. [Google Scholar] [CrossRef] [PubMed]
- Illingworth, G. The challenges of adolescent sleep. Interface Focus 2020, 10, 20190080. [Google Scholar] [CrossRef] [PubMed]
- Kalak, N.; Lemola, S.; Brand, S.; Holsboer-Trachsler, E.; Grob, A. Sleep duration and subjective psychological well-being in adolescence: A longitudinal study in Switzerland and Norway. Neuropsychiatr. Dis. Treat. 2014, 10, 1199–1207. [Google Scholar]
- Kaplan, K.A.; Harvey, A.G. Hypersomnia across mood disorders: A review and synthesis. Sleep Med. Rev. 2009, 13, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Steiger, A.; Kimura, M. Wake and sleep EEG provide biomarkers in depression. J. Psychiatr. Res. 2010, 44, 242–252. [Google Scholar] [CrossRef]
- Williamson, D.E.; Birmaher, B.; Brent, D.A.; Balach, L.; Dahl, R.E.; Ryan, N.D. Atypical Symptoms of Depression in a Sample of Depressed Child and Adolescent Outpatients. J. Am. Acad. Child Adolesc. Psychiatry 2000, 39, 1253–1259. [Google Scholar] [CrossRef]
- Yorbik, O.; Birmaher, B.; Axelson, D.; Williamson, D.E.; Ryan, N.D. Clinical characteristics of depressive symptoms in children and adolescents with major depressive disorder. J. Clin. Psychiatry 2004, 65, 1654–1659, quiz 1760–1761. [Google Scholar] [CrossRef]
- Dauvilliers, Y.; Lopez, R.; Ohayon, M.; Bayard, S. Hypersomnia and depressive symptoms: Methodological and clinical aspects. BMC Med. 2013, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.G.; Zhang, Z.Y.; Grimm, K.J. Sleep restriction effects on sleep spindles in ado-lescents and relation of these effects to subsequent daytime sleepiness and cognition. Sleep 2023, 46, zsad071. [Google Scholar] [CrossRef] [PubMed]
- Carskadon, M.A. Patterns of sleep and sleepiness in adolescents. Pediatrician 1990, 17, 5–12. [Google Scholar] [PubMed]
- Contreras, D.; Destexhe, A.; Sejnowski, T.J.; Steriade, M. Control of Spatiotemporal Coherence of a Thalamic Oscillation by Corticothalamic Feedback. Science 1996, 274, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Rusterholz, T.; Hamann, C.; Markovic, A.; Schmidt, S.J.; Achermann, P.; Tarokh, L. Nature and Nurture: Brain Region-Specific Inheritance of Sleep Neurophysiology in Adolescence. J. Neurosci. 2018, 38, 9275–9285. [Google Scholar] [CrossRef] [PubMed]
- McClain, I.J.; Lustenberger, C.; Achermann, P.; Lassonde, J.M.; Kurth, S.; LeBourgeois, M.K. Developmental Changes in Sleep Spindle Characteristics and Sigma Power across Early Childhood. Neural Plast. 2016, 2016, 3670951. [Google Scholar] [CrossRef] [PubMed]
- Rechtschaffen, A.; Kales, A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects; United States Government Printing Office: Washington, DC, USA, 1968.
- Moser, D.; Anderer, P.; Gruber, G.; Parapatics, S.; Loretz, E.; Boeck, M.; Kloesch, G.; Heller, E.; Schmidt, A.; Danker-Hopfe, H.; et al. Sleep classification according to AASM and Rechtschaffen & Kales: Effects on sleep scoring parameters. Sleep 2009, 32, 139–149. [Google Scholar]
- Mayers, A.G.; Baldwin, D.S. The relationship between sleep disturbance and depression. Int. J. Psychiatry Clin. Pract. 2006, 10, 2–16. [Google Scholar] [CrossRef]
- Pillai, V.; Kalmbach, D.A.; Ciesla, J.A. A Meta-Analysis of Electroencephalographic Sleep in Depression: Evidence for Genetic Biomarkers. Biol. Psychiatry 2011, 70, 912–919. [Google Scholar] [CrossRef]
- Appelboom-Fondu, J.; Kerkhofs, M.; Mendlewicz, J. Depression in adolescents and young adults—Polysomnographic and neuroendocrine aspects. J. Affect. Disord. 1988, 14, 35–40. [Google Scholar] [CrossRef]
- Goetz, R.R.; Puig-Antich, J.; Ryan, N.; Rabinovich, H.; Ambrosini, P.J.; Nelson, B.; Krawiec, V. Electroencephalographic sleep of adolescents with major depression and normal controls. Arch. Gen. Psychiatry 1987, 44, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Lovato, N.; Gradisar, M. A meta-analysis and model of the relationship between sleep and depression in adolescents: Recommendations for future research and clinical practice. Sleep Med. Rev. 2014, 18, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Rao, U.; Poland, R.E. Electroencephalographic Sleep and Hypothalamic–Pituitary–Adrenal Changes from Episode to Recovery in Depressed Adolescents. J. Child Adolesc. Psychopharmacol. 2008, 18, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Armitage, R.; Emslie, G.J.; Hoffmann, R.F.; Rintelmann, J.; Rush, A.J. Delta sleep EEG in depressed adolescent females and healthy controls. J. Affect. Disord. 2001, 63, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Goetz, R.R.; Wolk, S.I.; Coplan, J.D.; Ryan, N.D.; Weissman, M.M. Premorbid poly-somnographic signs in depressed adolescents: A reanalysis of EEG sleep after longitudinal follow-up in adulthood. Biol. Psychiatry 2001, 49, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Dahl, R.E.; Puig-Antich, J.; Ryan, N.D.; Nelson, B.; Dachille, S.; Cunningham, S.L.; Trubnick, L.; Klepper, T.P. EEG sleep in adolescents with major depression: The role of suicidality and inpatient status. J. Affect. Disord. 1990, 19, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Emslie, G.J.; Rush, A.J.; Weinberg, W.A.; Rintelmann, J.W.; Roffwarg, H.P. Children with Major Depression Show Reduced Rapid Eye Movement Latencies. Arch. Gen. Psychiatry 1990, 47, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Kupfer, D.J. REM latency: A psychobiologic marker for primary depressive disease. Biol. Psychiatry 1976, 11, 159–174. [Google Scholar]
- Emslie, G.J.; Armitage, R.; Weinberg, W.A.; John Rush, A.; Mayes, T.L.; Hoffmann, R.F. Sleep polysomnography as a predictor of recurrence in children and adolescents with major depressive disorder. Int. J. Neuropsychopharmacol. 2001, 4, 159–168. [Google Scholar] [CrossRef]
- Frey, S.; Birchler-Pedross, A.; Hofstetter, M.; Brunner, P.; Götz, T.; Münch, M.; Blatter, K.; Knoblauch, V.; Wirz-Justice, A.; Cajochen, C. Young women with major depression live on higher homeostatic sleep pressure than healthy controls. Chronobiol. Int. 2012, 29, 278–294. [Google Scholar] [CrossRef]
- Tesler, N.; Gerstenberg, M.; Franscini, M.; Jenni, O.G.; Walitza, S.; Huber, R. Increased frontal sleep slow wave activity in adolescents with major depression. Neuroimage Clin. 2015, 10, 250–256. [Google Scholar] [CrossRef]
- Campbell, I.G.; Feinberg, I. Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. Proc. Natl. Acad. Sci. USA 2009, 106, 5177–5180. [Google Scholar] [CrossRef]
- Colrain, I.M.; Baker, F.C. Changes in sleep as a function of adolescent development. Neuropsychol. Rev. 2011, 21, 5–21. [Google Scholar] [CrossRef]
- Tarokh, L.; Van Reen, E.; LeBourgeois, M.; Seifer, R.; Carskadon, M.A. Sleep EEG provides evidence that cortical changes persist into late adolescence. Sleep 2011, 34, 1385–1393. [Google Scholar] [CrossRef]
- Tarokh, L.; Carskadon, M.A. Developmental changes in the human sleep EEG during early adolescence. Sleep 2010, 33, 801–809. [Google Scholar] [CrossRef]
- Feinberg, I.; Floyd, T.C. Systematic trends across the night in human sleep cycles. Psychophysiology 1979, 16, 283–291. [Google Scholar] [CrossRef]
- Augustinavicius, J.L.S.; Zanjani, A.; Zakzanis, K.K.; Shapiro, C.M. Polysomnographic features of early-onset depression: A meta-analysis. J. Affect. Disord. 2014, 158, 11–18. [Google Scholar] [CrossRef]
- Achermann, P.; Borbély, A.A. Simulation of human sleep: Ultradian dynamics of electroencephalographic slow-wave activity. J. Biol. Rhythms 1990, 5, 141–157. [Google Scholar] [CrossRef]
- Achermann, P.; Borbély, A.A. Dynamics of EEG slow wave activity during physiological sleep and after administration of benzodiazepine hypnotics. Hum. Neurobiol. 1987, 6, 203–210. [Google Scholar]
- Borbély, A.A.; Achermann, P. Sleep homeostasis and models of sleep regulation. J. Biol. Rhythms 1999, 14, 557–568. [Google Scholar]
- Borbély, A.A. A two process model of sleep regulation. Hum. Neurobiol. 1982, 1, 195–204. [Google Scholar]
- Dijk, D.-J. Regulation and Functional Correlates of Slow Wave Sleep. J. Clin. Sleep Med. 2009, 5, S6–S15. [Google Scholar] [CrossRef]
- Borbély, A.A.; Wirz-Justice, A. Sleep, sleep deprivation and depression. A hypothesis de-rived from a model of sleep regulation. Hum. Neurobiol. 1982, 1, 205–210. [Google Scholar]
- Reynolds, C.F.; Shaw, D.H.; Newton, T.F.; Coble, P.A.; Kupfer, D.J. EEG sleep in outpatients with generalized anxiety: A preliminary comparison with depressed outpatients. Psychiatry Res. 1983, 8, 81–89. [Google Scholar] [CrossRef]
- Reynolds, C.F.; Newton, T.F.; Shaw, D.H.; Coble, P.A.; Kupfer, D.J. Electroencephalographic sleep findings in depressed outpatients. Psychiatry Res. 1982, 6, 65–75. [Google Scholar] [CrossRef]
- Reynolds, C.F.; Kupfer, D.J.; Hoch, C.C.; Houck, P.R.; Stack, J.A.; Berman, S.R.; Campbell, P.I.; Zimmer, B. Sleep deprivation as a probe in the elderly. Arch. Gen. Psychiatry 1987, 44, 982–990. [Google Scholar] [CrossRef]
- Armitage, R.; Hoffmann, R.; Fitch, T.; Trivedi, M.; Rush, A.J. Temporal characteristics of delta activity during NREM sleep in depressed outpatients and healthy adults: Group and sex effects. Sleep 2000, 23, 607–617. [Google Scholar] [CrossRef]
- Armitage, R.; Hoffmann, R.; Trivedi, M.; Rush, A.J. Slow-wave activity in NREM sleep: Sex and age effects in depressed outpatients and healthy controls. Psychiatry Res. 2000, 95, 201–213. [Google Scholar] [CrossRef]
- Borbély, A.A.; Tobler, I.; Loepfe, M.; Kupfer, D.J.; Ulrich, R.F.; Grochocinski, V.; Doman, J.; Matthews, G. All-night spectral analysis of the sleep EEG in untreated depressives and normal controls. Psychiatry Res. 1984, 12, 27–33. [Google Scholar] [CrossRef]
- Kupfer, D.J.; Frank, E.; Ehlers, C.L. EEG sleep in young depressives: First and second night effects. Biol. Psychiatry 1989, 25, 87–97. [Google Scholar] [CrossRef]
- Armitage, R. Microarchitectural findings in sleep EEG in depression: Diagnostic implications. Biol. Psychiatry 1995, 37, 72–84. [Google Scholar] [CrossRef]
- Plante, D.T.; Goldstein, M.R.; Landsness, E.C.; Peterson, M.J.; Riedner, B.A.; Ferrarelli, F.; Wanger, T.; Guokas, J.J.; Tononi, G.; Benca, R.M. and sex-related differences in sleep spindles in major depressive disorder: A high-density EEG investigation. J. Affect. Disord. 2013, 146, 120–125. [Google Scholar] [CrossRef]
- Baker, F.C.; Turlington, S.R.; Colrain, I. Developmental changes in the sleep electroencephalogram of adolescent boys and girls. J. Sleep Res. 2012, 21, 59–67. [Google Scholar] [CrossRef]
- Feinberg, I.; Higgins, L.M.; Khaw, W.Y.; Campbell, I.G. The adolescent decline of NREM delta, an indicator of brain maturation, is linked to age and sex but not to pubertal stage. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1724–R1729. [Google Scholar] [CrossRef]
- Lopez, J.; Hoffmann, R.; Emslie, G.; Armitage, R. Sex Differences in Slow-wave Electro-encephalographic Activity (SWA) in Adolescent Depression. Ment. Illn. 2012, 4, e4. [Google Scholar]
- Baker, F.C.; Willoughby, A.R.; de Zambotti, M.; Franzen, P.L.; Prouty, D.; Javitz, H.; Hasler, B.; Clark, D.B.; Colrain, I.M. Age-Related Differences in Sleep Architecture and Electroencephalogram in Adolescents in the National Consortium on Alcohol and Neurodevelopment in Adolescence Sample. Sleep 2016, 39, 1429–1439. [Google Scholar] [CrossRef]
- Park, S.-C.; Kim, J.-M.; Jun, T.-Y.; Lee, M.-S.; Kim, J.-B.; Yim, H.-W.; Park, Y.C. How many different symptom combinations fulfil the diagnostic criteria for major depressive disorder? Results from the CRESCEND study. Nord. J. Psychiatry 2017, 71, 217–222. [Google Scholar] [CrossRef]
- Borbély, A. The two-process model of sleep regulation: Beginnings and outlook. J. Sleep Res. 2022, 31, e13598. [Google Scholar] [CrossRef]
- Borbély, A.A.; Daan, S.; Wirz-Justice, A.; Deboer, T. The two-process model of sleep regulation: A reappraisal. J. Sleep Res. 2016, 25, 131–143. [Google Scholar] [CrossRef]
- Fernandez, L.M.J.; Lüthi, A. Sleep Spindles: Mechanisms and Functions. Physiol. Rev. 2020, 100, 805–868. [Google Scholar] [CrossRef]
- Ricci, A.; He, F.; Younes, M.; Calhoun, S.; Fang, J.; Houser, L.; Vgontzas, A.; Liao, D.; Bixler, E.; Fernandez-Mendoza, J. Sex Differences in the Maturational Trajectories of Sleep Spindles in the Transition from Childhood to Adolescence. Sleep 2021, 44, A62. [Google Scholar] [CrossRef]
- Steriade, M. Neuronal Substrates of Sleep and Epilepsy; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Khazipov, R.; Sirota, A.; Leinekugel, X.; Holmes, G.L.; Ben-Ari, Y.; Buzsáki, G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature 2004, 432, 758–761. [Google Scholar] [CrossRef]
- Fogel, S.M.; Smith, C.T. The function of the sleep spindle: A physiological index of intelli-gence and a mechanism for sleep-dependent memory consolidation. Neurosci. Biobehav. Rev. 2011, 35, 1154–1165. [Google Scholar] [CrossRef]
- De Maertelaer, V.; Hoffman, G.; Lemaire, M.; Mendlewicz, J. Sleep spindle activity changes in patients with affective disorders. Sleep 1987, 10, 443–451. [Google Scholar] [CrossRef]
- Hamann, C.; Rusterholz, T.; Studer, M.; Kaess, M.; Tarokh, L. Association between depres-sive symptoms and sleep neurophysiology in early adolescence. J. Child Psychol. Psychiatry 2019, 60, 1334–1342. [Google Scholar] [CrossRef]
- Sesso, G.; Bat-Pitault, F.; Guyon, A.; Plancoulaine, S.; Banfi, T.; Milioli, G.; Parrino, L.; Faraguna, U.; Franco, P. Electrophysiological and microstructural features of sleep in children at high risk for depression: A preliminary study. Sleep Med. 2017, 36, 95–103. [Google Scholar] [CrossRef]
- Mikoteit, T.; Brand, S.; Perren, S.; von Wyl, A.; von Klitzing, K.; Kurath, J.; Holsboer-Trachsler, E.; Hatzinger, M. Visually detected non-rapid eye movement stage 2 sleep spindle density at age five years predicted prosocial behavior positively and hyperactivity scores negatively at age nine years. Sleep Med. 2018, 48, 101–106. [Google Scholar] [CrossRef]
- Mikoteit, T.; Brand, S.; Perren, S.; von Wyl, A.; von Klitzing, K.; Kurath, J.; Holsboer-Trachsler, E.; Hatzinger, M. Visually detected NREM Stage 2 sleep spindles in kindergarten children are associated with current and future emotional and behavioural characteristics. J. Sleep Res. 2013, 22, 129–136. [Google Scholar] [CrossRef]
- Ferrarelli, F.; Huber, R.; Peterson, M.J.; Massimini, M.; Murphy, M.; Riedner, B.A.; Watson, A.; Bria, P.; Tononi, G. Reduced sleep spindle activity in schizophrenia patients. Am. J. Psychiatry 2007, 164, 483–492. [Google Scholar] [CrossRef]
- Reynolds, C.F.; Kupfer, D.J.; Taska, L.S.; Hoch, C.C.; Spiker, D.G.; Sewitch, D.E.; Zimmer, B.; Marin, R.S.; Nelson, J.P.; Martin, D. EEG sleep in elderly depressed, demented, and healthy subjects. Biol. Psychiatry 1985, 20, 431–442. [Google Scholar] [CrossRef]
- Huupponen, E.; Himanen, S.-L.; Värri, A.; Hasan, J.; Lehtokangas, M.; Saarinen, J. A study on gender and age differences in sleep spindles. Neuropsychobiology 2002, 45, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Markovic, A.; Kaess, M.; Tarokh, L. Gender differences in adolescent sleep neurophysiology: A high-density sleep EEG study. Sci. Rep. 2020, 10, 15935. [Google Scholar] [CrossRef] [PubMed]
- Zajkowska, Z.; Walsh, A.; Zonca, V.; Gullett, N.; Pedersen, G.A.; Kieling, C.; Swartz, J.R.; Karmacharya, R.; Fisher, H.L.; Kohrt, B.A.; et al. A systematic review of the association between biological markers and environmental stress risk factors for adolescent depression. J. Psychiatr. Res. 2021, 138, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Rao, U.; Hammen, C.L.; Poland, R.E. Risk Markers for Depression in Adolescents: Sleep and HPA Measures. Neuropsychopharmacology 2009, 34, 1936–1945. [Google Scholar] [CrossRef]
- Fava, M. Diagnosis and definition of treatment-resistant depression. Biol. Psychiatry 2003, 53, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Zwolińska, W.; Dmitrzak-Węglarz, M.; Słopień, A. Biomarkers in Child and Adolescent Depression. Child Psychiatry Hum. Dev. 2023, 54, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Dharmadhikari, A.S.; Tandle, A.L.; Jaiswal, S.V.; Sawant, V.A.; Vahia, V.N.; Jog, N. Frontal Theta Asymmetry as a Biomarker of Depression. East Asian Arch. Psychiatry 2018, 28, 17–22. [Google Scholar]
- McVoy, M.; Lytle, S.; Fulchiero, E.; Aebi, M.E.; Adeleye, O.; Sajatovic, M. A systematic review of quantitative EEG as a possible biomarker in child psychiatric disorders. Psychiatry Res. 2019, 279, 331–344. [Google Scholar] [CrossRef]
- Verrusio, W.; Ettorre, E.; Vicenzini, E.; Vanacore, N.; Cacciafesta, M.; Mecarelli, O. The Mozart Effect: A quantitative EEG study. Conscious Cogn. 2015, 35, 150–155. [Google Scholar] [CrossRef]
- Wichniak, A.; Wierzbicka, A.; Jernajczyk, W. Sleep as a biomarker for depression. Int. Rev. Psychiatry 2013, 25, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.I.; Lipinski, J.F.; Frankenburg, F.R.; Grochocinski, V.J.; Kupfer, D.J. Electroencephalographic sleep in mania. Arch. Gen. Psychiatry 1988, 45, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Reich, L.; Weiss, B.L.; Coble, P.; McPartland, R.; Kupfer, D.J. Sleep disturbance in schizophrenia. A revisit. Arch. Gen. Psychiatry 1975, 32, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Zarcone, V.P.; Benson, K.L.; Berger, P.A. Abnormal rapid eye movement latencies in schizophrenia. Arch. Gen. Psychiatry 1987, 44, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Uhde, T.W.; Roy-Byrne, P.; Gillin, J.C.; Mendelson, W.B.; Boulenger, J.P.; Vittone, B.J.; Post, R.M. The sleep of patients with panic disorder: A preliminary report. Psychiatry Res. 1984, 12, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Insel, T.R.; Gillin, J.C.; Moore, A.; Mendelson, W.B.; Loewenstein, R.J.; Murphy, D.L. The sleep of patients with obsessive-compulsive disorder. Arch. Gen. Psychiatry 1982, 39, 1372–1377. [Google Scholar] [CrossRef]
- Katz, J.L.; Kuperberg, A.; Pollack, C.P.; Walsh, B.T.; Zumoff, B.; Weiner, H. Is there a relationship between eating disorder and affective disorder? New evidence from sleep recordings. Am. J. Psychiatry 1984, 141, 753–759. [Google Scholar]
- Armitage, R.; Hoffmann, R.F.; Emslie, G.J.; Weinberg, W.A.; Mayes, T.L.; Rush, A.J. Sleep microarchitecture as a predictor of recurrence in children and adolescents with depression. Int. J. Neuropsychopharmacol. 2002, 5, 217–228. [Google Scholar] [CrossRef]
- Markovic, A.; Kaess, M.; Tarokh, L. Heritability of REM sleep neurophysiology in adolescence. Transl. Psychiatry 2022, 12, 399. [Google Scholar] [CrossRef]
- Markovic, A.; Achermann, P.; Rusterholz, T.; Tarokh, L. Heritability of Sleep EEG Topography in Adolescence: Results from a Longitudinal Twin Study. Sci. Rep. 2018, 8, 7334. [Google Scholar] [CrossRef]
- Roberts, R.E.; Duong, H.T. Depression and insomnia among adolescents: A prospective perspective. J. Affect. Disord. 2013, 148, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Alvaro, P.K.; Roberts, R.M.; Harris, J.K.; Bruni, O. The direction of the relationship between symptoms of insomnia and psychiatric disorders in adolescents. J. Affect. Disord. 2017, 207, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Sivertsen, B.; Harvey, A.G.; Lundervold, A.J.; Hysing, M. Sleep problems and depression in adolescence: Results from a large population-based study of Norwegian adolescents aged 16–18 years. Eur. Child Adolesc. Psychiatry 2014, 23, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Roane, B.M.; Taylor, D.J. Adolescent insomnia as a risk factor for early adult depression and substance abuse. Sleep 2008, 31, 1351–1356. [Google Scholar]
- Joseph, V.A.; Kreski, N.T.; Keyes, K.M. Sleep deprivation and suicide risk among minoritized US adolescents. BMC Psychiatry 2023, 23, 638. [Google Scholar] [CrossRef]
- Haghish, E.F.; Nes, R.B.; Obaidi, M.; Qin, P.; Stänicke, L.I.; Bekkhus, M.; Laeng, B.; Czajkowski, N. Unveiling Adolescent Suicidality: Holistic Analysis of Protective and Risk Factors Using Multiple Machine Learning Algorithms. J. Youth Adolesc. 2023. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Lerch, S.; Maggetti, A.; Reichl, C.; Tarokh, L.; Kaess, M. The relationship between sleep disturbance and self-harming behaviours in high-risk clinical adolescents. J. Psychiatr. Res. 2023, 158, 81–87. [Google Scholar] [CrossRef]
- Tarokh, L.; Saletin, J.M.; Carskadon, M.A. Sleep in adolescence: Physiology, cognition and mental health. Neurosci. Biobehav. Rev. 2016, 70, 182–188. [Google Scholar] [CrossRef]
- Crowley, S.J.; Wolfson, A.R.; Tarokh, L.; Carskadon, M.A. An update on adolescent sleep: New evidence informing the perfect storm model☆. J. Adolesc. 2018, 67, 55–65. [Google Scholar] [CrossRef]
- Perlis, M.L.; Giles, D.E.; Mendelson, W.B.; Bootzin, R.R.; Wyatt, J.K. Psychophysiological insomnia: The behavioural model and a neurocognitive perspective. J. Sleep Res. 1997, 6, 179–188. [Google Scholar] [CrossRef]
- Riemann, D.; Spiegelhalder, K.; Feige, B.; Voderholzer, U.; Berger, M.; Perlis, M.; Nissen, C. The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Med. Rev. 2010, 14, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, F.; Argente, D.; Lippi, F. A Simple Planning Problem for COVID-19 Lock-down, Testing, and Tracing. Am. Econ. Rev. Insights 2021, 3, 367–382. [Google Scholar] [CrossRef]
- Richter, S.A.; Ferraz-Rodrigues, C.; Schilling, L.B.; Camargo, N.F.; Nunes, M.L. Effects of the COVID-19 pandemic on sleep quality in children and adolescents: A systematic review and meta-analysis. J. Sleep Res. 2023, 32, e13720. [Google Scholar] [CrossRef] [PubMed]
- da Silva, B.B.L.; de Melo, M.C.F.; Studart-Pereira, L.M. Adolescents’ sleep quality during the COVID-19 pandemic. Sleep Sci. 2022, 15, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Gradisar, M.; Kahn, M.; Micic, G.; Short, M.; Reynolds, C.; Orchard, F.; Bauducco, S.; Bartel, K.; Richardson, C. Sleep’s role in the development and resolution of adolescent depression. Nat. Rev. Psychol. 2022, 1, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Twenge, J.M.; Blake, A.B.; Haidt, J.; Campbell, W.K. Commentary: Screens, Teens, and Psychological Well-Being: Evidence from Three Time-Use-Diary Studies. Front. Psychol. 2020, 11, 181. [Google Scholar] [CrossRef] [PubMed]
- Twenge, J.M.; Martin, G.N.; Spitzberg, B.H. Trends in U.S. Adolescents’ media use, 1976–2016: The rise of digital media, the decline of TV, and the (near) demise of print. Psychol. Pop. Media Cult. 2019, 8, 329–345. [Google Scholar] [CrossRef]
- Twenge, J.M.; Martin, G.N.; Campbell, W.K. Decreases in psychological well-being among American adolescents after 2012 and links to screen time during the rise of smartphone technology. Emotion 2018, 18, 765–780. [Google Scholar] [CrossRef]
- Hale, L.; Kirschen, G.W.; LeBourgeois, M.K.; Gradisar, M.; Garrison, M.M.; Montgomery-Downs, H.; Kirschen, H.; McHale, S.M.; Chang, A.-M.; Buxton, O.M. Youth screen media habits and sleep: Sleep-friendly screen-behavior recommendations for clinicians, educators, and parents. Child Adolesc. Psychiatr. Clin. N. Am. 2018, 27, 229–245. [Google Scholar] [CrossRef]
- Kerr, S.; Kingsbury, M. Online digital media use and adolescent mental health. Health Rep. 2023, 34, 17–28. [Google Scholar]
- Lang, C.; Kalak, N.; Brand, S.; Holsboer-Trachsler, E.; Pühse, U.; Gerber, M. The relationship between physical activity and sleep from mid adolescence to early adulthood. A systematic review of methodological approaches and meta-analysis. Sleep Med. Rev. 2016, 28, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Kalak, N.; Gerber, M.; Kirov, R.; Mikoteit, T.; Yordanova, J.; Pühse, U.; Holsboer-Trachsler, E.; Brand, S. Daily morning running for 3 weeks improved sleep and psychological functioning in healthy adolescents compared with controls. J. Adolesc. Health 2012, 51, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Castiglione-Fontanellaz, C.E.G.; Timmers, T.T.; Lerch, S.; Hamann, C.; Kaess, M.; Tarokh, L. Sleep and physical activity: Results from a long-term actigraphy study in adolescents. BMC Public Health 2022, 22, 1328. [Google Scholar] [CrossRef] [PubMed]
- De Leeuw, M.; Verhoeve, S.I.; van der Wee, N.J.A.; van Hemert, A.M.; Vreugdenhil, E.; Coomans, C.P. The role of the circadian system in the etiology of depression. Neurosci. Biobehav. Rev. 2023, 153, 105383. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, C.L.; Frank, E.; Kupfer, D.J. Social zeitgebers and biological rhythms. A unified approach to understanding the etiology of depression. Arch. Gen. Psychiatry 1988, 45, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Czeisler, C.A.; Kronauer, R.E.; Mooney, J.J.; Anderson, J.L.; Allan, J.S. Biologic Rhythm Disorders, Depression, and Phototherapy: A New Hypothesis. Psychiatr. Clin. 1987, 10, 687–709. [Google Scholar]
- Claudio, A.; Andrea, F. Circadian neuromarkers of mood disorders. J. Affect. Disord. Rep. 2022, 10, 100384. [Google Scholar] [CrossRef]
- Ballard, R.; Parkhurst, J.; Julian, K.; Pasetes, L.N.; Fawcett, A.; Li, A.; Goel, N.; Sit, D.K. Light Therapy for Adolescent Depression: A Scoping Review. Curr. Psychiatry Rep. 2023, 25, 373–386. [Google Scholar] [CrossRef]
- Phillips, A.J.K.; Clerx, W.M.; O’Brien, C.S.; Sano, A.; Barger, L.K.; Picard, R.W.; Lockley, S.W.; Klerman, E.B.; Czeisler, C.A. Irregular sleep/wake patterns are associated with poorer academic performance and delayed circadian and sleep/wake timing. Sci. Rep. 2017, 7, 3216. [Google Scholar] [CrossRef]
- Castiglione-Fontanellaz, C.E.G.; Schaufler, S.; Wild, S.; Hamann, C.; Kaess, M.; Tarokh, L. Sleep regularity in healthy adolescents: Associations with sleep duration, sleep quality, and mental health. J. Sleep Res. 2023, 32, e13865. [Google Scholar] [CrossRef]
- Mathew, G.M.; Reichenberger, D.A.; Master, L.; Buxton, O.M.; Chang, A.-M.; Hale, L. Actigraphic Sleep Variability is Associated with Lower Positive Mood in Adolescents. J. Adolesc. Health 2023, 73, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.P.; Sidol, C.A.; Van Dyk, T.R.; Epstein, J.N.; Beebe, D.W. Intraindividual variability of sleep/wake patterns in relation to child and adolescent functioning: A systematic review. Sleep Med. Rev. 2017, 34, 94–121. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).