Abstract
Surfactants derived from renewable sources such as plants are an ecological alternative to synthetic surfactants. Aqueous solutions of natural surfactants extracted from soapnuts obtained from two plants, Sapindus mukorossi and Sapindus trifoliatus, were studied. Their properties in terms of surface tension reduction and wettability were examinated. The natural surfactants show the ability to reduce the surface tension and increase the wettability of the hydrophobic polytetrafluoroethylene surface. These nuts can be used repeatedly for washing also in hard water. Crude extracts from Sp. trifoliatus exhibit better surface properties than those from Sp. mukorossi. This makes these soapnuts a good potential source of biosurfactants for household use.
1. Introduction
Synthetic surfactants are used in large amounts in a wide range of applications in industry and households. In 2019 the size of the global surfactants market was 41.3 billion USD and it is projected to reach 58.5 billion USD by 2027, reaching a Compound Annual Growth Rate of 5.3% from 2020 to 2027 [1]. In 2006 the worldwide production of surfactants was about 12.5 million tons [2]. The properties of low price and easy availability are the features of synthetic surfactants that make them extensively used as detergents in households. Taking into account the type of surfactants, anionic and non-ionic surfactants are most often produced [1]. Surface active compounds are emitted to waters, the atmosphere and soil, and can accumulate in living organisms [3]. It was shown that around 60% of all produced surfactants end up in the aquatic environment [4].
Synthetic surfactants are not easily biodegradable, affect the environment, may affect the biological activity and can even be toxic or irritant to microorganisms, humans, flora and fauna [3,5]. The accumulation of surfactants has a negative effect on the ecosystem and this effect depends on the type of surfactant and its physicochemical properties, environmental conditions and various biotic factors [5]. In aquatic systems, the biological impact of surface active compounds is determined by both their sorption and ability to penetrate through biological membranes [6]. Moreover, anionic surfactants were found to be less toxic than cationic ones [5].
In nature, surface active compounds are produced by bacteria, fungi, animals and plants [4,7,8,9,10,11,12,13]. Surfactants produced by living matter are called biosurfactants, although this name is often used to describe surfactants produced by microorganisms [10,14]. As they are obtained from renewable sources, they are non-toxic or show a low toxicity and they are biodegradable. Thus biosurfactants are an ecological alternative to synthetic surfactants [10,11,13].
Saponins are one of the popular surface active compounds found in different species of plants, among others in species of the genus Sapindaceae [4,11,12,15,16]. The nuts (called soapnuts or reetha) produced by trees belong to the family Sapindeae growing in tropical and sub-tropical regions, mainly in India and Pakistan [9,12] and contain from 6 to 10 weight% of saponin [17]. The pericarp of the fruits of these trees is used for cleaning woolen fabrics and as household detergents, e.g., as shampoo. In traditional Indian medicine, saponins extracted from these pericarps are used as anti-inflammatory, antipruritic and antifertility agents [16]. The research most frequently reported in literature refers to saponins of natural origin including saponins from Sapindus mukorossi [4,8,9,11,12,15,18].
Saponins are amphiphilic compounds containing glycosides with non-sugar aglycone and belong to the glycoside derivatives of steroids or polycyclic triterpenes [12,19]. Saponins produced from soapnuts are mostly of the triterpenoid type [20]. Different critical micelle concentrations were reported in literature, depending on the extraction method and type of the natural source [4,9,11,12,15].
Saponins extracted from reetha exhibit a surface tension reduction down to values below 40 mN/m [4,9,11] and have an excellent emulsification activity [4,9,15] and foaming ability [8,21]. Schmitt [11] proved the ability of crude saponin extract to stabilize an emulsion polymerization reaction leading to monodisperse latexes. The extracts of Sapindus mukorossi were also found to exhibit antibacterial activity against H. pylori [18]. Yang et al. [8] showed its effectiveness as a preservative against Staphylococcus aureus ATCC 6538.
The aim of this study was to test the ability of crude extracts from soapnuts to lower the surface tension of water in respect to their possible use as detergent. Hence, aqueous solutions in doubly distilled deionized water and tap water were tested. Natural surfactants extracted from two plants, Sapindus mukorossi and Sapindus trifoliatus, were used. While the interfacial properties of Sp. mukkorosi are frequently studied in the literature, to the best of our knowledge there are very scarce reports on Sp. trifoliatus. In this work also the possibility of using the same nuts several times was also investigated, which had not been done so far. Recent studies in literature include only studies with distilled water, which is far from practical in application. Our goal was also to test the properties in tap water reflecting the conditions of practical use at home.
2. Materials and Methods
2.1. Sample Preparation
We used well dried commercially available fruit pericarp of Sapindus mukorossi and Sapindus trifoliatus (Bio Company Pulst J.M, Siemianowice Śl., Poland) (Figure 1).

Figure 1.
Sapindus mucorossi and Sapindus trifoliatus fruit pericarps.
The ratio of fruit pericarp to water was selected according to the manufacturer’s recommendation. To prepare a water extract 4 g of fruit pericarp (without grinding) was placed in 500 mL of Milli-Q water and incubated in a shaker (100 rpm) for 15 min at 25 °C. The fruit pericarp was placed in sterile gauze which facilitated their easy removal from the solution. The aqueous solutions obtained in this way were prepared just before measurements and diluted for further experiments. The aqueous solutions were also prepared by extending the contact time of the fruit pericarp in water to 30, 45, 60, 75 and 120 min. To check the properties under the conditions of practical use, aqueous solutions were also prepared in tap water (with a conductivity of 115.6 µS/cm) under the same conditions as for Milli-Q water. As the surfactant content in the investigated soapnuts is unknown, the concentration was later expressed as the mass of fruit pericarp per water volume. The initial nut concentration for the extraction process was 8 g/L, then the extract was diluted.
In order to investigate the possibility of using the same fruit pericarp multiple times, after extraction, they were dried at room temperature and used again to prepare an aqueous solution. These operations were repeated five times using the same batch of nuts extracted for 15 min at 25 °C. For the determination of effect of extraction temperature on the surface tension, the extraction was conducted over 15 min in water at temperatures of 25 °C, 40 °C, 50 °C and 60 °C, respectively. Surface tension measurements were done at 20 °C.
The surface tension and conductivity of solutions prepared in the given way were measured using the automatic KSV Sigma 700 (KSV, Espoo, Finland) ring tensiometer and a multiparametric system ELMETRON CX-731, respectively at 20 ± 2 °C. Before the measurements, each solution was thermostated at 20 °C. Each sample was measured several times and the presented results are averaged values.
2.2. Contact Angles Measurements
The advancing contact angles of aqueous solution of both fruits pericarp extracts were measured on polytetrafluoroethylene (PTFE) (Nitrogen Industrial Plant in Tarnow, Poland) using the sessile drop method using the contact angle meter equipped with a video-camera system (GBX, Bourg-de-Peage, France) at 20 ± 2 °C. The single drop volume for the contact angle measurements was 4 µL.
3. Results and Discussion
Saponins are considered as weakly acidic due to the hydrolysis of glycosides [22]. The pH values of Sp. mukorossi and Sp. trifoliatu solutions at an amount of 8 g/L were respectively 4.6 and 4.8 in distilled water and 7.1 in tap water. Figure 2 shows the surface tension changes as a function of crude reetha concentration in distilled and tap water. The surface tension (γ) reduction with increasing extract concentration is slightly higher in distilled water. The minimum surface tension values in distilled water were ca. 52 mN/m and 47 mN/m for Sp. mukorossi and Sp. trifoliatus, respectively. In tap water this value tends to 48 mN/m for both tested solutions. Similar minimal value of γ of 51 mN/m for Sp. mukorossi was obtained in [12] after 3h extraction. An extraction lasting overnight led to a surface tension lowering to 38 mN/m [9] and 35.3 mN/m [4], whereas in [11] a value of 36 mN/m after extraction with 5 min microwave irradiation was reported.
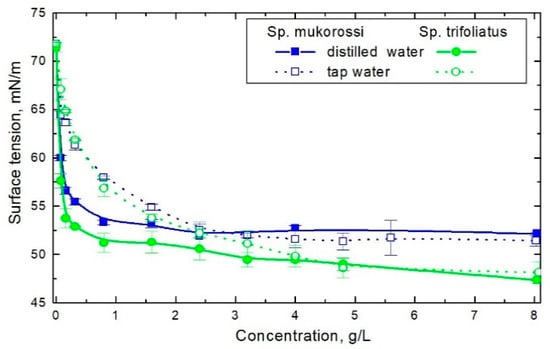
Figure 2.
Changes of the surface tension of aqueous solutions of investigated fruit pericarps prepared using Milli-Q and tap water, respectively.
The critical micelle concentration (cmc) obtained from the surface tension isotherms in deionized water are 0.32 g/L and 0.24 g/L for Sp. mukorossi and Sp. trifoliatus, respectively. This refers to 0.032 wt% and 0.024 wt% of crude extract. These values are similar to 0.045 wt% obtained for Sp. mukorossi in [15] and 0.03 wt% Sp. trifoliatus reported in [23]. Muntaha and Khan [12] found the cmc of Sp. mukorossi to be 0.13 wt% and Ghagi et al. [9] as 1.7%. However, they used solutions extracted from soapnuts over 3 h and overnight. Schmitt et al. [11] determined the cmc of crude Sp. mukorossi extract to be 0.005% mass fraction in distilled water.
In tap water, the cmc value was 2.4 g/L for both soapnuts. It was found that the cmc of crude soapnut extracts increase with the pH and water hardness [15]. Schmitt et al. [11] found that the cmc of Sp. mukorossi at pH 7.9 and in 0.12 M NaCl amounts to 0.07% which is about 10 times higher than in distilled water.
In the next step, the impact of the extraction time on the surface tension changes of solutions prepared with 8 g/L reetha in deionized water were checked. As can be seen in Figure 3 the surface tension values decreased with increasing extraction time up to 1 h. Further prolongation of the extraction time did not significantly change the γ value. The obtained surface tension changes correlate with changes in the conductivity.
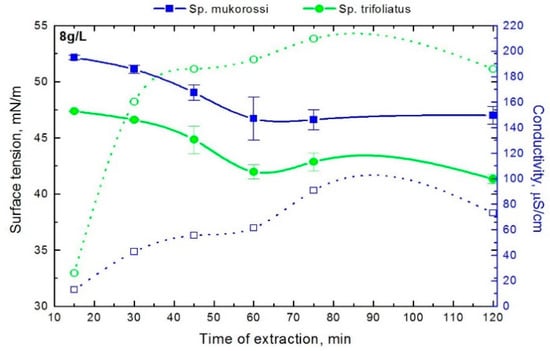
Figure 3.
Dependence of surface tension (filled symbols) and conductivity (opened symbols) of aqueous solutions of the investigated fruit pericarp extracts as a function of the extraction time. The amount of fruit pericarps was 8g/L in Milli-Q purified water.
Figure 4 shows the change in surface tension of the solutions obtained by 15 min extraction of soapnuts at an amount of 8 g/L as a function of the extraction temperature. The increase in temperature affects the efficiency of extraction to a greater extent in the case of Sp. mukorossi soapnuts. The deterioration of the surface activity with increasing temperatures is not large and shows that the soapnut extraction works more effectively at room temperature. This is consistent with results reported by Góral and Wojciechowski [13] showing a lowering of the surface activity of the saponin plant extracts in hot water.
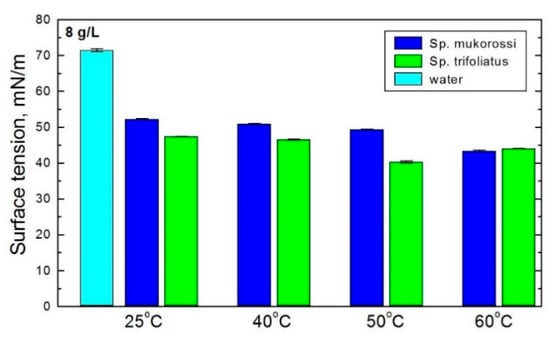
Figure 4.
Surface tension of aqueous solution of investigated fruit pericarp extracts prepared at different temperatures. The amount of fruit pericarps was 8g/L.
According to the producers, soapnuts can be used several times. Figure 5 shows the dynamic surface tensions of aqueous solutions of soapnuts extracted up to five times using the same nuts. It can be seen that for both types of nuts the largest and fastest reduction in surface tension was obtained after the first extraction. Each subsequent extraction of the same batch of nuts leads to less and slower decreasing γ values. With extraction times longer than 15 min, the effectiveness of the repeated use of the same nuts decreases. However, the obtained values of surface tension indicate that it is still reasonable to use the same nuts a few more times, especially during shorter contact times with the solution.
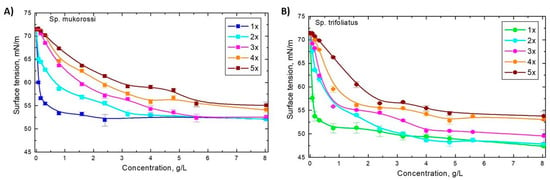
Figure 5.
Surface tension of aqueous solutions of: (A) Sp. Mukorossi and (B) Sp. trifoliatus after repetitive 15 min extraction of the same pericarps at 25 °C.
Wetting of solids by liquids plays a crucial role, among others, during washing. It is known that the addition of surfactants to water improves the wetting of hydrophobic surfaces such as PTFE [24]. In Figure 6 the advancing contact angle of aqueous extract solutions of the investigated soapnuts on PTFE surfaces is depicted. The contact angle decreases with increasing surfactant concentration. Sp. trifoliatus shows much lower contact angles as compared to Sp. mukorossi indicating its better wetting ability. The contact angle values at a soapnut amount of 8 g/L for Sp. mukorossi and Sp. trifiliatus are 102.6° and 73°, respectively. Paria et al. [25] found for Sp. mukorossi 109.88° as the minimum contact angle. In [26] the minimum contact angle of aqueous solution of saponins extracted from Saponaria officinalis on a PTFE surface was 82°. For comparison with synthetic non-ionic surfactants, the contact angles values on PTFE were 68° for TX-100 and 78° for TX-165 [24]. This shows that the surfactant solutions obtained from Sp. trifoliatus have only a moderate wettability.
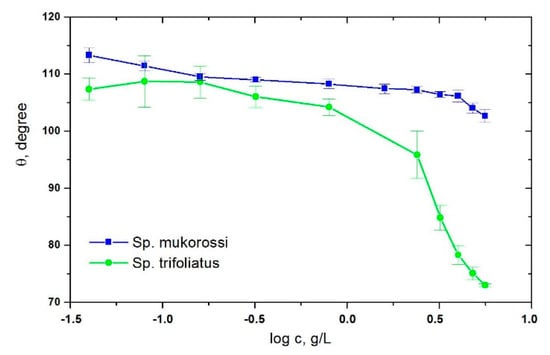
Figure 6.
Contact angle vs. logarithm of the amount of fruit pericarp.
The obtained results show that the tested extracts of nuts exhibit surface activity and moderate wetting properties. According to the manufacturer, they can be used several times and do not lose their activity in tap water. This makes them promising green biosurfactants for household use.
4. Conclusions
In this study we investigated the surface activity and wettability of surface active components extracted from the soapnuts Sp. mukorossi and Sp. trifoliatus. The extracts were used as obtained via a mild extraction in water without subsequent separation and purification of particular components. The extracts of both tested soapnuts show a decrease in surface tension value and an increase in wettability of the hydrophobic polytetrafluoroethylene surface. Crude soapnuts do not lose their properties in tap water and their surface activity depends to a small extent on the extraction temperature. Better properties have been shown for Sp. trifoliatus. These green surfactants derived from natural sources can be used instead of synthetic and non-biodegradable synthetic surfactants.
Author Contributions
Conceptualization, A.S. and R.M.; methodology, A.S. and L.H.; validation, A.S., R.M. and L.H.; formal analysis, P.W. and M.S.; investigation, P.W. and M.S.; writing—original draft preparation, A.S.; writing—review and editing, R.M. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Available online: https://www.alliedmarketresearch.com/surfactant-market (accessed on 26 October 2020).
- Edser, C. Latest market analysis. Focus Surfactants 2006, 5, 1–2. [Google Scholar] [CrossRef]
- Olkowska, E.; Rumanand, M.; Polkowska, Ż. Occurrence of Surface Active Agents in the Environment. J. Anal. Methods Chem. 2014, 769708. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.; Bhattacharyya, A. Quest for an eco-friendly alternative surfactant: Surface and foam characteristics of natural surfactants. J. Clean. Prod. 2017, 150, 127–134. [Google Scholar] [CrossRef]
- Masakorala, K.; Turner, A.; Brown, M.T. Toxicity of Synthetic Surfactants to the Marine Macroalga, Ulva lactuca. Water Air Soil Pollut. 2011, 218, 283–291. [Google Scholar] [CrossRef]
- Rosen, M.J.; Li, F.; Morrall, S.W.; Versteeg, D.J. The Relationship between the interfacial properties of surfactants and their toxicity to aquatic organisms. Environ. Sci. Technol. 2001, 35, 954–959. [Google Scholar] [CrossRef]
- Cooper, A.; Kennedy, M.W. Biofoams and natural protein surfactants. Biophys. Chem. 2010, 151, 96–104. [Google Scholar] [CrossRef]
- Yang, C.-H.; Huang, Y.-C.; Chen, Y.-F.; Chang, M.-H. Foam Properties, Detergent Abilities and Long-term Preservative Efficacy of the Saponins from Sapindus mukorossi. J. Food Drug Anal. 2010, 18, 155–164. [Google Scholar] [CrossRef]
- Ghagi, R.; Satpute, S.K.; Chopade, B.A.; Banpurkar, A.G. Study of functional properties of Sapindus mukorossi as a potential bio-surfactant. Indian J. Sci. Technol. 2011, 4, 531–533. [Google Scholar] [CrossRef]
- Sachdev, D.P.; Cameotra, S.S. Biosurfactants in agriculture. Appl. Microbiol. Biotechnol. 2013, 97, 1005–1016. [Google Scholar] [CrossRef]
- Schmitt, C.; Grassl, B.; Lespes, G.; Desbrières, J.; Pellerin, V.; Reynaud, S.; Gigault, J.; Hackley, V.A. Saponins: A Renewable and Biodegradable Surfactant From Its Microwave-Assisted Extraction to the Synthesis of Monodisperse Lattices. Biomacromolecules 2014, 15, 856–862. [Google Scholar] [CrossRef]
- Muntaha, S.-T.; Khan, M.N. Natural surfactant extracted from Sapindus mukurossi as an eco-friendly alternate to synthetic surfactant e a dye surfactant interaction study. J. Clean. Prod. 2015, 93, 145–150. [Google Scholar] [CrossRef]
- Góral, J.; Wojciechowski, K. Surface activity and foaming properties of saponin-reach plants extracts. Adv. Colloid Interf. 2020, 279, 102145. [Google Scholar] [CrossRef] [PubMed]
- Ron, E.Z.; Rosenberg, E. Natural roles of biosurfactant. Environ. Microbiol. 2001, 3, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, S.; Varughese, S.; Deshpande, A.P. Micellar characterisation of saponin from Sapindus mukorossi. Tenside Surfactants Deterg. 2006, 43, 262–268. [Google Scholar] [CrossRef]
- Raja, M.; Suresh, M. Evaluation of Larvicidal Activity of Sapindus Emargintus (Family: Sapindaceae) Leaf Extracts against the Housefly Larvae (Musca domestica) LINN. Int. J. Sci. Res. 2015, 6, 200–204. [Google Scholar]
- Kommalpatti, R.R.; Valsaraj, K.T.; Constant, W.D.; Roy, D. Soil flushing using CGA suspensions generated from a plant-based surfactant. J. Hazard. Mater. 1998, 60, 73–87. [Google Scholar] [CrossRef]
- Ibrahim, M.; Khan, A.A.; Tiwari, S.K.; Habeeb, M.A.; Khaja, M.N.; Habibullah, C.M. Antimicrobial activity of Sapindus Mukorossi and Rheum emodi extracts against H. pylori: In vitro and in vivo studies. World J. Gastroenterol. 2004, 12, 7136–7142. [Google Scholar] [CrossRef]
- Vincken, J.-P.; Heng, L.; Groot, A.; Gruppen, H. Saponins, classification and occurence in the plant kongdom. Phytochemistry 2007, 68, 275–297. [Google Scholar] [CrossRef]
- Li, R.; Wu, Z.L.; Wang, Y.J.; Li, L.L. Separation of total saponins from the pericarp of Sapindus mukorossi Gaerten by foam fractionation. Ind. Crop. Prod. 2013, 51, 163–170. [Google Scholar] [CrossRef]
- Santini, E.; Jarek, E.; Ravera, F.; Liggieri, L.; Warszynski, P.; Krzan, M. Surface properties and foamability of saponin and saponin-chitosan systems. Colloid Surf. B 2019, 181, 198–206. [Google Scholar] [CrossRef]
- Hong, K.; Tokunaga, S.; Kajiuchi, T. Evaluation of remediation process with plant-derived iosurfactant for recovery of heavy metals from contaminated soils. Chemosphere 2002, 49, 379–387. [Google Scholar] [CrossRef]
- Grover, R.K.; Roy, A.D.; Roy, R.; Joshi, S.K.; Srivastava, V.; Arora, S.K. Complete 1H and 13C NMR assignments of six saponins from Sapindus trifoliatus. Magn. Reson. Chem. 2005, 43, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Szymczyk, K.; Zdziennicka, A.; Krawczyk, J.; Jańczuk, B. Wettability, adhesion, adsorption and interface tension in the polymer/surfactant aqueous solution system. I. Critical surface tension of polymer wetting and its surface tension. Colloid Surf. A 2012, 402, 132–138. [Google Scholar] [CrossRef]
- Paria, S.; Biswal, N.R.; Chaudhuri, R.G. Surface Tension, Adsorption, and Wetting Behaviors of Natural Surfactants on a PTFE Surface. AIChE J. Soft Matter Synth. Process. Prod. 2015, 61, 655–663. [Google Scholar] [CrossRef]
- Rekiel, E.; Smułek, W.; Zdziennicka, A.; Kaczorek, E.; Jańczuk, B. Wetting properties of Saponaria officinalis saponins. Colloid Surf. A 2020, 584, 123980. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).