Abstract
Bismuth vanadate (BiVO4) has been regarded as a valuable semiconductor material for photocatalytic decomposition of organic pollutants thanks to its narrow band gap and environmental friendliness. However, its practical application is restricted by its small specific surface area, severe photo-generated carrier recombination, and low photocatalytic degradation efficiency. Herein, a microemulsion method followed by a hydrothermal process is developed to prepare a flower-like BiVO4 microsphere constituted of thin nanosheets. Because of increase in reactive sites, facilitation of photo-induced carrier transfer, and generation of high-activity superoxygen (•O2−) and hydroxyl (•OH) radicals, the photocatalytic degradation efficiency of the flower-like BiVO4 microparticle (synthesized with a hydrothermal duration of 6 h) for Congo red reaches 86.2% with a high degradation rate constant of 0.0134 min−1. Moreover, the cyclic degradation test proves the reasonable photocatalytic stability of the flower-like BiVO4 microparticle, showing its great application potential for photocatalytic degradation of organic pollutants.
1. Introduction
Sewage discharge poses a significant threat to both the environment and living organisms. There are a large number of organic pollutants in sewage. Synthetic dyes such as Congo red (CR) discharged from the textile industry exert acute toxicity on aquatic organisms and impede the penetration of sunlight into water bodies; even at trace concentrations, antibiotics can induce bacterial resistance and trigger ecological imbalance [1,2]. Compared to commonly used wastewater treatment technologies such as physical separation and phase transformation, photocatalytic technology utilizes green, environmentally friendly and renewable solar energy, effectively addressing the issue of high cost. Meanwhile, the photo-induced carriers in the photocatalytic materials, upon being excited by sunlight, can effectively degrade organic pollutants into less toxic or non-toxic products.
Bismuth vanadate (BiVO4) has been regarded as a promising photocatalyst for degrading organic pollutants, owing to its superior chemical stability, high safety, non-toxicity, and a relatively narrow band gap of 2.4 eV that enables visible-light utilization [3,4,5,6,7]. However, it is necessary to exploit novel BiVO4 photocatalysts with extended visible-light response, efficient photo-generated carrier separation and fast charge carrier transfer for efficient decomposition of pollutants.
Reducing the particle size of photocatalytic materials can improve the photocatalytic degradation efficiency because of the increase in the specific surface area and active site number in the degradation reactions [8,9]. However, when the average free pathway of electrons is close to the size of photocatalytic nanomaterials, it would be affected by quantum confinement effect [10], resulting in an increase in the recombination probability of photo-generated electrons and holes and a deterioration of the photocatalytic degradation performance [11,12,13]. Moreover, photocatalysts with different morphologies exhibit significant differences in their photocatalytic properties due to the exposure of different crystal planes. Therefore, it needs to adjust the particle size and shape of BiVO4 for increasing the active sites and facilitating the photo-induced carrier transfer.
There are a few methods for preparing BiVO4, such as hydrothermal method, microemulsion method, high-temperature solid-state method, co-precipitation method, sol–gel method, and electrospinning method [14]. Among them, the microemulsion method is widely used for preparing nanomaterials due to its short reaction time, simple preparation process, and precise adjustment of the morphology [15,16]. In this study, a flower-like BiVO4 microsphere composed of thin nanosheets is prepared by a microemulsion technology followed by a hydrothermal process. The morphology can be easily tuned by changing the preparation condition. Various characterizations also confirm the efficient photocatalytic degradation of CR by the flower-like BiVO4 microparticle, and the degradation mechanism is also disclosed.
2. Materials and Methods
2.1. Materials
Sorbitan oleate (Span-80), cyclohexane, n-butanol, and bismuth nitrate pentahydrate (Bi(NO3)3·5H2O) were purchased from InnoChem Science & Technology Co., Ltd., Beijing, China. Concentrated nitric acid was purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. Ethylenediaminetetraacetic acid (EDTA) was purchased from Xilong Chemical Co., Ltd., Shantou, China. Ammonium metavanadate (NH4VO3) was purchased from Macklin Biochemical Co., Ltd., Shanghai, China.
2.2. Synthesis of BiVO4
The synthesis process of flower-like BiVO4 microparticle contained the preparation of the oil phase and aqueous phase [17]. Figure 1 shows the synthesis process. Two identical groups of emulsions were prepared as the oil phase of the system, with the preparation method as follows: Span-80 and OP-10 were mixed at a volume ratio of 6.5:3.5 (9.75 mL Span-80 and 5.25 mL OP-10) to obtain a mixture with a total volume of 15 mL (Figure S1), followed by mixing cyclohexane, n-butanol and the above mixture at a volume ratio of 8:1:2. These two groups of emulsions were labeled as Emulsion A and Emulsion B, respectively.
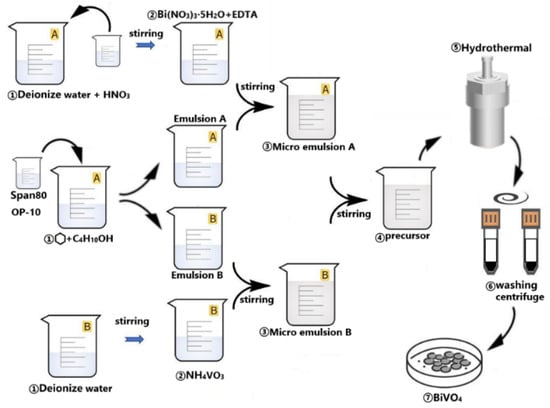
Figure 1.
Flow chart for the synthesis of BiVO4.
A total of 3 mmol of Bi(NO3)3·5H2O and 3 mmol of EDTA were added into 3 mL of deionized water, stirred for 30 min under heating conditions at 60 °C, then 1 mL of concentrated nitric acid was added dropwise to the solution and stirred well. The above solution was labeled as Solution A. Then, 3 mmol of NH4VO3 was added into 4 mL of deionized water and stirred for 30 min under heating conditions at 60 °C. The above solution was labeled as Solution B.
Solution A was slowly added dropwise to Emulsion A and stirred for 30 min to obtain Microemulsion A. Solution B was slowly added dropwise to Emulsion B and stirred for 30 min to obtain Microemulsion B. Microemulsion B was slowly added dropwise to Microemulsion A and stirred for 30 min to obtain the microemulsion precursor.
The microemulsion precursor was transferred into a hydrothermal autoclave with a polytetrafluoroethylene liner (Changyi Instrument & Equipment Co., Ltd., Xi’an, China), and the hydrothermal reaction was carried out at 180 °C. After the reaction, the BiVO4 powder was obtained through centrifugation, washing, centrifugation and drying. The BiVO4 samples subjected to hydrothermal treatment at 180 °C for 2, 4, 6, and 8 h were denoted as BVO-2h, BVO-4h, BVO-6h, and BVO-8h, respectively.
2.3. Materials’ Characterizations
A SmartLab SE X-ray diffractometer (XRD) (Rigaku Corporation, Tokyo, Japan) with a Cu target was utilized to characterize the phase composition and crystallization of the BiVO4 powders at a scanning rate of 12° min−1 and a step size of 0.01° (high XRD scan rate may limit the resolution of fine structural features and cause peak broadening). A Sigma 300 scanning electron microscope (SEM) (Carl Zeiss AG, Oberkochen, Germany) was employed for characterizing the microstructure and morphology of the nanomaterials. A F-4600 photoluminescence (PL) spectroscope (Hitachi High-Tech Co., Ltd., Tokyo, Japan) coupled with a 375 nm high-pass filter (Shanghai Mega-9 Optoelectronic Technology Co., Ltd., Shanghai, China) was employed to detect the recombination rate of the photo-generated electrons and holes in a range of 300−800 nm at a scanning rate of 1200 nm min−1, with an excitation wavelength of 397 nm and slit widths of 5 nm for both excitation and emission.
2.4. Photocatalytic Degradation Test
A photocatalytic dye degradation experiment was conducted by adding 50 mg of BiVO4 catalyst sample in 100 mL CR solution (10 mg L−1) at 25 °C with a pH value of 7. The BiVO4 solution was firstly stirred in a dark room for 30 min, and then the light of 500 W xenon lamp (Guangzhou Xingchuang Electronics Co., Ltd., Guangzhou, China) was turned on for the CR degradation. The xenon lamp was positioned at a distance of 15 cm above the reaction vessel, and a 420 nm cutoff filter was used to simulate visible light irradiation conditions. During the degradation process, the solution was collected every 30 min for determining the CR concentration. The CR degradation efficiency (η) was calculated according to Equation (1) following Lambert–Beer law:
where A0 and At represented the absorbance of the CR solution during the degradation process for 0 and t minutes, and C0 and Ct represented the concentration of the CR solution after 0 and t minutes of the degradation process, respectively.
η = (A0 − At)/A0 = (C0 − Ct)/C0
Generally, the degradation reaction kinetics of pollutants usually followed a Langmuir–Hinshelwood kinetic model, as shown in Equation (2):
where Ceq and Ct represented the equilibrium concentration of the CR solution after the dark reaction for 30 min and the concentration of the CR solution after t minutes of degradation process, respectively, and k represented the surface reaction rate constant during the photocatalytic process.
−ln(Ct/Ceq) = kt
2.5. Photocatalytic Stability Test of Catalyst
To examine the photocatalytic stability of the BiVO4 catalyst, the catalyst powders were recovered and then the degradation experiment was carried out by using fresh dye solutions again. The specific operation process was described as follows. The suspension after the photocatalytic process was centrifuged to collect the BiVO4 powders. After that, the BiVO4 powders were cleaned by ethanol and water 3 times, respectively, and then desiccated in an oven for 12 h at 80 °C to remove the solvent. Then, the CR photo-degradation experiment was conducted until the photocatalytic degradation efficiency was around 80% of the pristine degradation efficiency. The degradation efficiency of a different degradation process (or cyclic number) was also calculated and recorded.
2.6. Free Radical Quenching Test
To explore the main active substances in the CR photo-degradation process, ammonium oxalate (AO), tert-butyl alcohol (TBA), and p-benzoquinone (BQ) were utilized in a molar ratio of 5:1 of BiVO4 as free radical quenchers of h+, •OH, and •O2−, respectively. The specific operation process was described as follows. The BiVO4 catalyst was put in the CR solutions and then the abovementioned free radical quenchers were added in the solutions with an adjusted pH value of 7, respectively. The CR photocatalytic degradation experiment under normal conditions (without the free radical quenchers) was set as a blank control. The operation process of the photocatalytic degradation experiments was the same as the process in Section 2.4. The impact of active groups and a possible CR degradation mechanism in the photo-degradation process were disclosed by analyzing the CR degradation efficiencies.
3. Results and Discussion
3.1. Microstructure
XRD measurements confirm the successful synthesis of monoclinic-phase BiVO4 with typical lattice planes of (121), (040), (200) and (211) (Figure 2). With the increase in the hydrothermal time from 2 to 8 h, the intensity of the peak at ~29° gradually decreases, while the half-height width first increases and then decreases. In particular, the BVO-8h sample shows low crystallinity, compared to other samples. The Scherrer Equation (3) allows for calculating the average crystallite sizes (D) of the samples [18]:
where K is the Scherrer constant (0.94), λ is the wavelength of the X-ray sources (0.15406 nm), β is the full-width at half maximum of the X-ray peak, and θ is the peak position. According to Equation (3), the as-synthesized BVO-2h, BVO-4h and BVO-6h have average D values of 23.16, 23.88 and 31.74 nm, respectively, indicating the increase in the D value with the hydrothermal reaction time. In contrast, BVO-8h shows an almost amorphous structure according to the XRD pattern.
D = (Kλ)/(βcosθ)
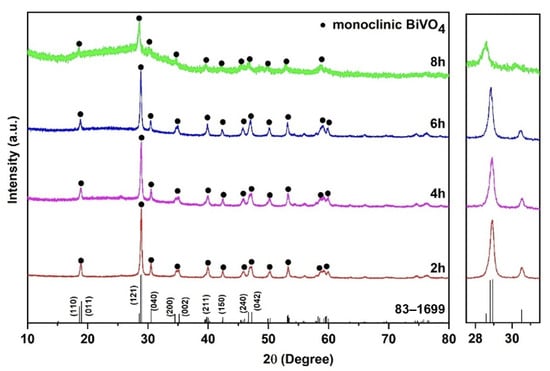
Figure 2.
XRD patterns of BiVO4 with various hydrothermal times of 2, 4, 6 and 8 h.
SEM was also utilized to observe the microstructure of BiVO4 synthesized with different hydrothermal times (Figure 3 and Figure S2). All the BiVO4 samples are flower-like hierarchical microspheres, which are constituted of thin nanosheets of 100−500 nm in size. In particular, the BVO-6h sample is more loose with a uniform distribution of nanosheets, which is beneficial for increasing the reactive sites for efficient photocatalytic degradation of organic dyes.

Figure 3.
SEM images of BiVO4 with hydrothermal time of (a,b) 4, (c,d) 6, and (e,f) 8 h.
The exploration of the effect of aqueous phase volume on the microstructure of BiVO4 synthesized by the microemulsion method can be found in Figures S3 and S4. This explains why the aqueous phase volume was chosen to be 4 mL for synthesizing flower-like BiVO4 microspheres constituted of high-crystallinity nanosheets.
3.2. Optical Properties
Figure 4 presents the PL spectra of BiVO4 prepared with various hydrothermal times. The PL peak intensity decreases with the hydrothermal time increase from 4 to 6 h, while the peak intensity increases with the hydrothermal time increase from 6 to 8 h. Commonly, the lower the peak intensity, the less photo-generated the carrier recombination. Therefore, the separation capability of the photo-generated carriers of BVO-6h is the strongest, which is helpful for the carrier transfer and the photocatalytic degradation of CR. Please note that the PL intensity comparison is qualitative, because emission collection is also affected by light-scattering properties of particle morphology. Figure S5 presents the UV-vis DRS absorption spectrum of BVO-6h and the corresponding Tauc plot. The band gap of BVO-6h is calculated to be 2.4 eV, which is consistent with the report [19].
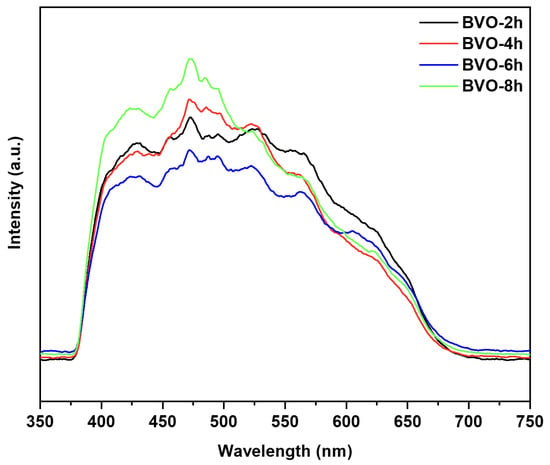
Figure 4.
Photoluminescence spectra of BiVO4 with different hydrothermal time.
3.3. Photocatalytic Degradation Performance
The CR degradation experiment was carried out by applying a xenon lamp of 500 W coupled with a UV filter to evaluate the photocatalytic degradation efficiency of the BiVO4 samples under visible light condition. Figure 5a,b show the photocatalytic results of the BiVO4 samples with different hydrothermal times. The CR degradation efficiencies of the BVO-2h, BVO-4h, BVO-6h, and BVO-8h samples in 120 min are 30.5%, 56.7%, 86.2%, and 42.9%, respectively. The BVO-6h sample also has a high degradation rate constant of 0.0134 min−1. But it deviates from ideal first-order kinetics (R2 = 0.84), likely due to surface saturation or rapid initial adsorption. Based on the aforesaid analyses, the high degradation efficiency of the BVO-6h sample should be attributed to the high crystallization of BVO-6h (Figure 2) and the hierarchical flower-like morphology composed of nanosheets (Figure 3c,d), which is beneficial for increasing the photocatalytic active sites and facilitating the photo-generated carrier transfer (Figure 4). The dye adsorption rates of all the samples after a 30 min dark reaction can also be found in Figure S6.
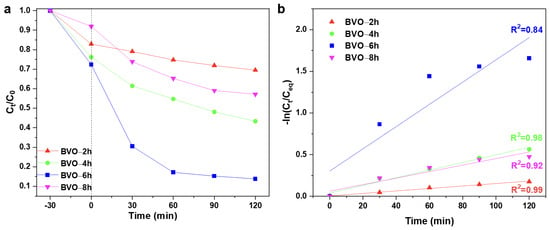
Figure 5.
(a) CR photocatalytic degradation test and (b) first-order reaction kinetic analysis results of the BiVO4 samples with different hydrothermal time.
Table 1 summarizes the CR degradation performance of BiVO4 and BiVO4-based composite/heterojunction photocatalysts in the recent literature. Notably, the CR degradation efficiency of the BiVO4 prepared in this work under its corresponding light source and reaction time is comparable to those of many BiVO4-based composite/heterojunction materials which typically feature more elaborate structural modifications. Although direct comparison is limited due to differences in light source intensity (xenon vs. sunlight) and reaction conditions, the BiVO4 synthesized herein still exhibits competitive catalytic activity within the BiVO4-based photocatalytic system.

Table 1.
Comparison of CR photo-degradation ability of BiVO4.
3.4. Photocatalytic Stability
The photocatalytic stability of the BVO-6h sample was also evaluated by eight consecutive photocatalytic degradation experiments (Figure 6a). The CR degradation efficiency almost maintained its pristine value of ~86% during the first three photocatalytic degradation processes, and then decreased to 66.3% after eight photocatalytic degradation processes with a loss of 23.1%.
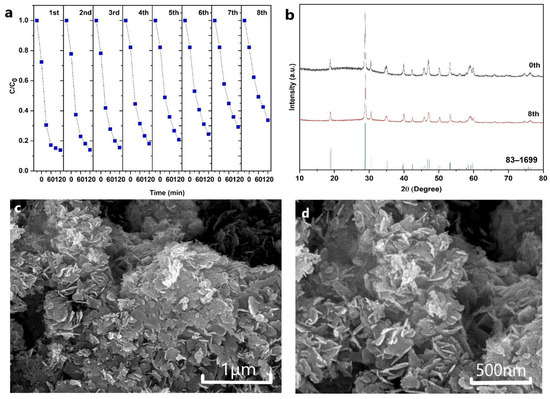
Figure 6.
(a) CR degradation stability test of the BVO-6h sample, (b) XRD patterns of the BVO-6h sample before and after eight photocatalytic degradation experiments, and (c,d) SEM image of the BVO-6h sample after eight photocatalytic degradation experiments.
To disclose the reason why the BVO-6h sample kept the high photocatalytic stability, XRD and SEM measurements were then conducted. The XRD patterns show that the BVO-6h sample maintains the typical characteristic peaks of the monoclinic BiVO4 even after the eight consecutive photocatalytic degradation experiments (Figure 6b), indicating the high structure stability of the sample during the degradation process. The SEM image in Figure 6c,d further shows that the BVO-6h sample still displays the flower-like microstructure, which is constituted of nanosheets without destruction. The well-maintained crystal structure and micromorphology is helpful for offering more active sites for the efficient photo-degradation of dyes.
3.5. Photocatalytic Degradation Mechanism
In order to clarify the contribution mechanism of active substances in the photocatalytic degradation of CR by BiVO4, free radical quenching experiments were also conducted to uncover the role of holes (h+), hydroxyl radicals (•OH), electron (e−) and superoxide radicals (•O2−) (Figure 7a). The CR degradation efficiency of BVO-6h without the capture agents was 86.2%, while the CR disintegration efficiency of BVO-6h with the added capture agents of AO, TBA, AgNO3 and BQ decreased to 53.4%, 42.2%, 21.7% and 15.7%, respectively, indicating that h+ and •OH participated in the CR decomposition process and both e− and •O2− are the key active species that dominated the degradation progress. Please note that the use of AgNO3 may result in Ag deposition and influence the observed degradation rates independent of electron scavenging.
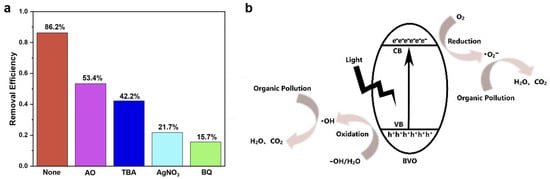
Figure 7.
(a) CR photocatalytic degradation efficiency by BiVO4 with different agents for capturing free radicals, and (b) photocatalytic degradation mechanism of BiVO4.
Based on the abovementioned results of the free radical capture experiment, the proposed photocatalytic disintegration mechanism of CR by BiVO4 is described in Figure 7b and Equations (4)–(7). The electrons on the valence band (VB) of BiVO4 are excited to move in conduction band (CB) under visible light irradiation, leaving holes in the VB [28,29]. Then the photo-generated holes and electrons move to the BiVO4 catalyst surface and react with H2O and O2 to form free radicals of •OH and •O2−. After that, the free radicals would participate in the following CR degradation process [30]: (1) cleavage of sulfonate groups from aromatic rings after being attacked by •O2− radicals; (2) decomposition of azo double bonds (-N=N-); (3) dissociation of benzene rings, especially the dissociation of side benzene rings; and (4) disintegration of the C-C and C-N bonds of chromophores into smaller molecules (e.g., H2O and CO2) [23,31,32,33].
4. Conclusions
In this study, flower-like BiVO4 microspheres constituted of thin nanosheets were synthesized by developing a microemulsion technology and a subsequent hydrothermal procedure of 6 h. The hierarchical microstructure of BiVO4 with abundant active sites was beneficial for the fast transport of photo-induced electrons and holes and the generation of high-activity •O2− and •OH radicals. As a result, the CR degradation efficiency of the flower-like BiVO4 microparticles reached 86.2%, and the degradation rate constant reached 0.0134 min−1. Moreover, the flower-like BiVO4 microparticles exhibited reasonable photocatalytic stability over eight consecutive CR degradation cycles, which was attributed to the preservation of its crystalline structure and nanosheet-based morphology throughout the reaction process.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/colloids10010011/s1, Figure S1: Test chart of optimal water solubilization capacity; Figure S2: SEM images of BVO-2h; Figure S3: XRD patterns of BiVO4 with various aqueous volume of 2, 4, 6 and 8 mL; Figure S4: SEM images of BiVO4 with aqueous volume of (a,b) 2, (c,d) 4 and (e,f) 6 mL; Figure S5: (a) UV-vis DRS absorption spectrum of BVO-6h and (b) the corresponding Tauc plot; Figure S6: Adsorption rate after 30 min of dark reaction.
Author Contributions
Conceptualization, C.S. and J.Z. (Junmou Zhou); methodology, C.S.; software, J.Z. (Junmou Zhou); validation, C.S., J.Z. (Junmou Zhou) and Z.W.; formal analysis, J.Z. (Jinkui Zhang); investigation, J.M.; resources, L.L.; data curation, C.S.; writing—original draft preparation, C.S.; writing—review and editing, J.Z. (Junmou Zhou); visualization, J.Z. (Junmou Zhou); supervision, L.L.; project administration, L.L.; funding acquisition, L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (52272200) and the Fundamental Research Funds for the Central Universities (2025MS040).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotox. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Duan, J.-L.; Ma, J.-Y.; Sun, X.-D.; Liu, X.-Y.; Wang, Y.; Du, L.; Xia, P.-F.; Yuan, X.-Z. Bubbles Expand the Dissemination of Antibiotic Resistance in the Aquatic Environment. Environ. Sci. Technol. 2023, 57, 10079–10088. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic degradation of dyes using semiconductor photocatalysts to clean industrial water pollution. J. Ind. Eng. Chem. 2021, 97, 111–128. [Google Scholar] [CrossRef]
- Sudarshan, S.; Harikrishnan, S.; RathiBhuvaneswari, G.; Alamelu, V.; Aanand, S.; Rajasekar, A.; Govarthanan, M. Impact of textile dyes on human health and bioremediation of textile industry effluent using microorganisms: Current status and future prospects. J. Appl. Microbiol. 2023, 134, lxac064. [Google Scholar] [CrossRef]
- Harja, M.; Buema, G.; Bucur, D. Recent advances in removal of Congo Red dye by adsorption using an industrial waste. Sci. Rep. 2022, 12, 6087. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, Z.H.; Okab, A.A.; Graimed, B.H.; Issa, M.A.; Ammar, S.H. Photocatalytic destruction of Congo red dye in wastewater using a novel Ag2WO4/Bi2S3 nanocomposite decorated g-C3N4 nanosheet as ternary S-scheme heterojunction: Improving the charge transfer efficiency. Diam. Relat. Mater. 2023, 133, 109711. [Google Scholar] [CrossRef]
- Wang, M.; Han, J.; Zhang, Y.; You, M.-Y.; Yang, G.-J.; Chai, T.-Y.; Zhu, T. Effects of Y Doping Content on Visible-light Photocatalytic Property of N-BiVO4. J. Mater. Eng. 2018, 46, 36–42. [Google Scholar] [CrossRef]
- Hübner, U.; Spahr, S.; Lutze, H.; Wieland, A.; Rüting, S.; Gernjak, W.; Wenk, J. Advanced oxidation processes for water and wastewater treatment—Guidance for systematic future research. Heliyon 2024, 10, e30402. [Google Scholar] [CrossRef]
- Wary, R.R.; Baglari, S.; Brahma, D.; Gautam, U.K.; Kalita, P.; Baruah, M.B. Synthesis, characterization, and photocatalytic activity of ZnO nanoparticles using water extract of waste coconut husk. Environ. Sci. Pollut. Res. 2022, 29, 42837–42848. [Google Scholar] [CrossRef]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Recent progress in g-C3N4, TiO2 and ZnO based photocatalysts for dye degradation: Strategies to improve photocatalytic activity. Sci. Total Environ. 2021, 767, 144896. [Google Scholar] [CrossRef]
- Wary, R.R.; Brahma, D.; Nikhil, S.K.; Nair, R.G.; Kalita, P.; Baruah, M.B. Surface decoration of spherical Ag3PO4 with Ag embedded nano-cocoon BiPO4 nano-heterojunction photocatalyst with improved photodegradation of tetracycline hydrochloride and industrial dyes. Colloids Surf. A Physicochem. Eng. Asp. 2023, 669, 131541. [Google Scholar] [CrossRef]
- Lotfi, S.; Ouardi, M.E.; Ahsaine, H.A.; Assani, A. Recent progress on the synthesis, morphology and photocatalytic dye degradation of BiVO4 photocatalysts: A review. Catal. Rev. 2024, 66, 214–258. [Google Scholar] [CrossRef]
- Lin, Y.; Pan, D.; Luo, H. Hollow direct Z−Scheme CdS/BiVO4 composite with boosted photocatalytic performance for RhB degradation and hydrogen production. Mat. Sci. Semicon. Proc. 2021, 121, 105453. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, H.-Y.; Yang, Z.-X.; Gao, X.-Y.; Dong, Z.-X.-W.; Luo, Y.; Chen, Y. Effect of pH Value on Photocatalytic Properties of BiVO4 Prepared by Ethylene Glycol Sol-gel. J. Mater. Eng. 2015, 43, 6–11. [Google Scholar] [CrossRef]
- Yu, J.; Dong, J.; Zhao, Y.; Zhao, F.; Ge, B.; Pu, X.; Zhang, D. The morphology control and full-spectrum photodegradation tetracycline performance of microwave-hydrothermal synthesized BiVO4:Yb3+,Er3+ photocatalyst. J. Fuel Chem. Technol. 2025, 53, 348–359. [Google Scholar] [CrossRef]
- Cao, B.; Gong, S.; Zubairu, S.M.; Liu, L.; Xu, Y.; Guo, L.; Dang, R.; Zhu, G. Fabrication of Er3+/Yb3+ Co-Doped Bi5O7I Microsphere With Upconversion Luminescence and Enhanced Photocatalytic Activity for Bisphenol A Degradation. Front. Chem. 2020, 8, 773. [Google Scholar] [CrossRef]
- Yao, X.; Jiang, X.; Zhang, D.; Lu, S.; Wang, M.; Pan, S.; Pu, X.; Liu, J.; Cai, P. Achieving improved full-spectrum responsive 0D/3D CuWO4/BiOBr:Yb3+,Er3+ photocatalyst with synergetic effects of up-conversion, photothermal effect and direct Z-scheme heterojunction. J. Colloid Interf. Sci. 2023, 644, 95–106. [Google Scholar] [CrossRef]
- O’Connell, J.H.; Lee, M.E.; Yagoub, M.Y.A.; Swart, H.C.; Coetsee, E. Characterization of crystallite morphology for doped strontium fluoride nanophosphors by TEM and XRD. Phys. B 2016, 480, 169–173. [Google Scholar] [CrossRef]
- Heckel, S.; Wittmann, M.; Reid, M.; Villa, K.; Simmchen, J. An Account on BiVO4 as Photocatalytic Active Matter. Acc. Mater. Res. 2024, 5, 400–412. [Google Scholar] [CrossRef]
- Kato, H.; Hori, M.; Konta, R.; Shimodaira, Y.; Kudo, A. Construction of Z-Scheme Type Heterogeneous Photocatalysis Systems for Water Splitting into H2 and O2 Under Visible Light Irradiation. Chem. Lett. 2004, 33, 1348–1349. [Google Scholar] [CrossRef]
- Abou Taleb, M.F.; Jabeen, A.; Albalwi, H.A.; El Fadl, F.I.A.; Anwar, M.; Ibrahim, M.M. Fabrication of an efficient MXene based ternary nanocomposite of bismuth vanadate-bismuth sulfide as photocatalyst for the degradation of harmful industrial effluents. FlatChem 2023, 42, 100561. [Google Scholar] [CrossRef]
- Narzary, M.; Wary, R.R.; Brahma, B.B.; Kalita, P.; Baruah, M.B. Oxygen vacancy mediated Zn3V2O8/BiVO4 type-I heterojunction: An efficient photocatalyst for the degradation of organic dye. Colloids Surf. A Physicochem. Eng. Asp. 2025, 719, 137057. [Google Scholar] [CrossRef]
- Zhou, T.; Liu, J.; Zhan, H.; Wang, P.; Chao, K.; Chen, M.; Zheng, J.; Fu, B. Facile preparation of BiVO4/Bi-MOF composites for photocatalytic dye removal. J. Phys. Chem. Solids 2024, 188, 111917. [Google Scholar] [CrossRef]
- Shekardasht, M.B.; Givianrad, M.H.; Gharbani, P.; Mirjafary, Z.; Mehrizad, A. Preparation of a novel Z-scheme g-C3N4/RGO/Bi2Fe4O9 nanophotocatalyst for degradation of Congo Red dye under visible light. Diam. Relat. Mater. 2020, 109, 108008. [Google Scholar] [CrossRef]
- Senapati, D.; Swain, J.; Priyadarshini, A.; Hajra, S.; Kim, H.J.; Samantaray, R.; Sinha, J.K.; Sahu, R. Photocatalytic removal of congo red dye using ZIF-8@BiVO4: Impact of catalyst design and operational parameters. J. Mater. Sci-Mater. Electron. 2025, 36, 667. [Google Scholar] [CrossRef]
- Wang, X.; Sun, F.; Xu, Q.; Yue, B.; Xia, Y.; Liu, Q.; Luo, C.; Shao, H.; Yu, W.; Dong, X. FeVO4//BiVO4 Janus nanofiber S-scheme tandem heterojunction photo-Fenton catalyst with double Fenton effects for efficient degradation of organic contaminants. J. Environ. Chem. Eng. 2025, 13, 116344. [Google Scholar] [CrossRef]
- Sankeetha, S.; Muralidharan, R.; Abirami, N.; Leelavathi, H.; Tamizharasan, S.; Kumarasamy, A.; Arulmozhi, R. Interaction of BiVO4 anchored 2D hexagonal boron nitride nanocomposite for photocatalytic water pollutants degradation and phytotoxicity assessment. Colloids Surf. A Physicochem. Eng. Asp. 2023, 675, 132024. [Google Scholar] [CrossRef]
- Kukreti, K.; Roy, A.; Biswas, R.; Adhikari, S.D.; Himanshu, M.; Singh, R.P.; Dutta, A.; Sharma, M.P.; Yadav, K.L.; Yadav, K. Role of oxygen vacancy enriched m-BiVO4/t-BiVO4 isotype heterojunction for enhanced photocatalytic degradation of rhodamine B dye and in ferroelastic to paraelastic phase transition. Surf. Interfaces 2024, 52, 104932. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Xiao, N.; Wang, G. Preparation and photoelectrochemical properties of WO3/BiVO4 composite films. J. Mater. Eng. 2023, 51, 124–130. [Google Scholar] [CrossRef]
- Mulani, S.R.; Bimli, S.; Choudhary, E.; Bunkar, R.; Kshirsagar, U.A.; Devan, R.S. Cationic and anionic cross-assisted synergistic photocatalytic removal of binary organic dye mixture using Ni-doped perovskite oxide. Chemosphere 2023, 340, 139890. [Google Scholar] [CrossRef]
- Güy, N.; Çakar, S.; Özacar, M. Comparison of palladium/zinc oxide photocatalysts prepared by different palladium doping methods for congo red degradation. J. Colloid Interf. Sci. 2016, 466, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, T.S.; Natarajan, K.; Bajaj, H.C.; Tayade, R.J. Energy Efficient UV-LED Source and TiO2 Nanotube Array-Based Reactor for Photocatalytic Application. Ind. Eng. Chem. Res. 2011, 50, 7753–7762. [Google Scholar] [CrossRef]
- Erdemoğlu, S.; Aksu, S.K.; Sayılkan, F.; İzgi, B.; Asiltürk, M.; Sayılkan, H.; Frimmel, F.; Güçer, Ş. Photocatalytic degradation of Congo Red by hydrothermally synthesized nanocrystalline TiO2 and identification of degradation products by LC–MS. J. Hazard. Mater. 2008, 155, 469–476. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.