Abstract
Hemodialysis (HD) is a life-sustaining membrane-based therapy that is essential for managing kidney failure. However, it can have significant physical and psychological effects on patients due to chronic or acute consequences related to membrane bioincompatibility. End-stage renal disease (ESRD) patients on hemodialysis have a high incidence of psychiatric illness, particularly depression and anxiety disorders, and poor quality of life has been observed. Dialysis can also lead to physical symptoms of its own, such as fatigue, loss of appetite, anemia, low blood pressure, and fluid overload, in addition to the symptoms associated with kidney failure. Therefore, this critical review aims to comprehensively understand the impact of dialysis membrane bioincompatibility and the use of varying molecular weight cut-off membranes on the physical and psychological symptoms experienced by dialysis patients. We analyzed the latest research on the correlation between major inflammatory biomarkers released in patients’ blood due to membrane incompatibility, as well as the critical influence of low levels of hemoglobin and vital proteins such as human serum albumin due to the use of high-cut-off membranes and correlated these factors with the physical and psychological symptoms experienced by dialysis patients. Furthermore, our study aims to provide valuable insights into the impact of dialysis on critical symptoms, higher hospitalization rates, and the quality of life of First Nations, as well as child and youth dialysis patients, in addition to diabetic dialysis patients. Our goal is to identify potential interventions aiming to optimize the dialysis membrane and minimize its negative effects on patients, ultimately improving their well-being and long-term outcomes.
1. Introduction
Hemodialysis (HD) is a life-sustaining extracorporeal blood purifying treatment for end-stage renal disease (ESRD) patients. However, this membrane-based therapy is associated with acute side effects, life-threatening chronic conditions, and unacceptably high morbidity and mortality rates. Dialysis is linked to many physical symptoms and complications such as infection, thrombosis, and cardiovascular disease [1]. Inflammation is a common side effect of dialysis and can lead to muscle wasting, fatigue, and weakness in patients [2]. The accumulation of uremic toxins and presence of biomarkers, including Interleukin-6 (IL-6), C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), and fibrinogen adsorption to the membrane surface indicate inflammation [3,4,5]. Anemia and low serum albumin levels are also prevalent in ESRD patients and may contribute to further physical symptoms, including fatigue, weakness, and swelling [6]. Furthermore, changes in Antithrombin III (ATIII) and Von Willebrand factor (vWF) levels can affect blood coagulation and increase the risk of thrombosis [7,8,9].
In addition to physical symptoms, it is well-established in the literature that ESRD patients have a high prevalence of psychiatric illness, in particular, depression and anxiety [10,11,12]. The high rates of depression and anxiety in ESRD patients may be due to various psychosocial factors, such as isolation, lack of autonomy, and restricted activity resulting from rigid management of symptoms and frequent dialysis sessions. Depression in ESRD patients undergoing hemodialysis is associated with poor quality of life (QOL), poor treatment adherence, lower odds of transplantation, higher mortality rates, and increased rates of hospitalization [10,13,14,15,16,17]. Some inflammatory biomarkers, including IL-6, CRP, and serum albumin level, have been associated with depressive symptoms in ESRD patients [18]. Anemia may also contribute to depression and sleeping disorders in this population [19]. Further research needs to be conducted to explore how other biomarkers, such as activation of the complement system (C3a and C5a), ATIII, and vWF, may influence psychological symptoms in ESRD patients.
High-risk populations encompass individuals who face a greater likelihood of adverse health outcomes due to various factors such as age, race, and pre-existing conditions. Racial disparities can impact health outcomes, with individuals from certain racial or ethnic groups facing a higher risk for specific diseases or complications [20]. Additionally, individuals with pre-existing conditions such as diabetes, heart disease, or respiratory disorders may be more susceptible to complications and poorer health outcomes [21,22].
Our in-depth investigation of HD membranes available in Canadian hospitals [23,24,25,26,27,28,29] explored the key reasons behind blood activation and unstable cytokine levels, which were found to be associated with poor membrane biocompatibility and morphological characteristics. We highlighted the main reasons related to membrane chemistry and morphology. Our research group has conducted extensive studies on hemodialysis membrane modification to improve its compatibility [30,31,32,33,34,35,36,37,38,39,40,41]. However, it is crucial to correlate the key factors of membrane compatibility and its morphological structures with the aim of enhancing the quality of life of dialysis patients. Therefore, the objective of this critical review is to gain a comprehensive understanding of the impact of dialysis membrane bioincompatibility and the clinical practice of using different molecular weight cut-off membranes on the physical and psychological symptoms experienced by dialysis patients.
2. Challenges of Dialysis Membrane Bioincompatibility and Clinical Practices
Dialysis membrane bioincompatibility poses significant challenges in clinical practices for patients undergoing hemodialysis. The most common clinical manifestation of membrane bioincompatibility is chronic inflammation, which is associated with increased morbidity and mortality in dialysis patients. In addition, the activation of the complement system and other pathways can lead to adverse reactions such as anemia, hypotension, and cardiovascular complications.
When blood comes into contact with a foreign surface such as a dialysis membrane during hemodialysis, a series of events are triggered that can lead to inflammation. One of the initial events is protein adsorption, where plasma proteins such as fibrinogen, albumin, and immunoglobulin bind to the surface of the membrane. This binding can initiate coagulation and thrombotic responses, leading to changes in the behavior of the membrane.
Additionally, complement pathway components, specifically C3a and C5a, can be triggered by antigen–antibody complexes or bound C-reactive protein (CRP) and bind to the membrane as presented in Figure 1 [42,43,44]. This reaction is caused by proinflammatory cytokines, such as Interleukin-1β (IL-1β), Tumor necrosis factor-α (TNF-α), and Interleukin-6 (IL-6), and involves the interaction between different receptors and ligands on the leukocytes. Activated leukocytes can also release reactive oxygen species (ROS), which are overproduced in HD patients. This is catalyzed by 5-Lipoxygenase (5-LO), which leads to ROS formation and the release of lipoxins and leukotrienes. Activation of the 5-lipoxygenase pathways has been observed in uremia and HD. Heat shock proteins (Hsp70) can also play a role in inflammation [45]. Hsp70 can act as molecular chaperones and cytokines and intervene in antigen processing and presentation. Recent studies have shown that Hsp70 has high binding capabilities in relation to artificial surfaces, such as the dialysis membrane. Figure 1 illustrates the primary reactions that occur in blood due to biomaterial surfaces, with a focus on protein adsorption, cell rupture, complement system activation, and coagulation pathways. The fibrin network that forms as a result of coagulation pathway activation is also depicted. Additionally, the figure highlights the appearance of complement proteins on the biomaterial interface, indicating both the classical and alternative complement pathways. The eicosanoid biosynthesis pathways from arachidonic acid are also shown, with the involvement of various coagulation factors, interleukins, interferons, immunoglobulin G, lipoxygenase, and cyclooxygenase.
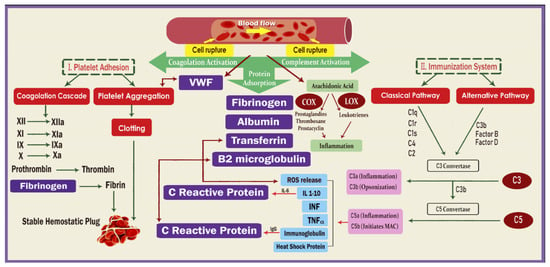
Figure 1.
Blood–membrane interactions: key reactions and pathways.
Finally, membrane incompatibility can lead to the accumulation of beta-2 microglobulin (β2M), a protein that belongs to the major histocompatibility class I family. In patients relying on long-term hemodialysis, higher concentrations of circulating β2M can accumulate into amyloid fibers and cause dialysis-related amyloidosis. Taken together, these events can lead to inflammation in patients undergoing hemodialysis and may contribute to long-term complications associated with chronic kidney disease.
Serpins, also known as serine protease inhibitors, play a key role in regulating blood coagulation. Antithrombin III (ATIII) is a serpin that inhibits the activity of several coagulation factors, including thrombin and factor Xa. In dialysis patients, levels of ATIII can be reduced, potentially leading to an increased risk of thrombosis and other clotting disorders. The von Willebrand factor (vWF) is a glycoprotein involved in the process of blood clotting. In dialysis patients, vWF levels can be increased, which can also contribute to a pro-thrombotic state. Therefore, the levels of both ATIII and vWF can have a significant impact on blood coagulation and clotting disorders in dialysis patients [46].
Furthermore, one of the major challenges in managing membrane bioincompatibility is determining the optimal type of membrane for individual patients. There are many different types of membranes available, each with its own advantages and disadvantages. For example, high-flux membranes can lead to an increase in the removal of red blood cells during dialysis due to their larger pore size and greater permeability. This can exacerbate anemia in patients with pre-existing anemia or lead to anemia in patients with normal blood cell counts. In some cases, a reduction in the dose of anticoagulants or a switch to a different type of membrane may be necessary to minimize the risk of anemia. High-flux membranes can also lead to a greater loss of albumin during dialysis due to their greater permeability [47,48,49]. Albumin is an important protein that helps to maintain blood volume and blood pressure and a loss of albumin can lead to fluid shifts and hypotension. On the other hand, low-flux membranes have smaller pore sizes and lower permeability, which may reduce the risk of inflammation and other adverse reactions but may not be as effective at removing toxins. Furthermore, high-cut-off membranes have an average pore size near 100 nm, which is twice bigger in comparison with high-flux membranes, which leads to further complications related to hemoglobin and albumin loss [48].
4. Influence of Dialysis Membrane Hemocompatibility and Clinical Practices on Physical Symptoms
There are several factors related to dialysis that can affect physical symptoms in patients. The composition of the dialysate, including electrolyte concentrations, pH, and temperature, can impact physical symptoms in patients. For example, a high dialysate sodium concentration can lead to hypertension and fluid overload, while a low dialysate temperature can cause hypotension and shivering. The blood flow rate during hemodialysis can affect physical symptoms such as headache, muscle cramps, and nausea. A high blood flow rate such as 500 mL/min can lead to hypotension, while a low blood flow rate can cause clotting and ineffective dialysis [94,95]. The frequency and duration of dialysis sessions can also impact physical symptoms. Longer and more frequent dialysis sessions have been shown to improve physical symptoms such as fatigue, which affects around 70–97% of dialysis patients, and muscle cramps, which affects 30–60% of dialysis patients. Furthermore, complications related to vascular access, such as infection or thrombosis, can cause pain, swelling, and other physical symptoms [1,96,97]. In addition, patients with comorbid conditions such as diabetes or cardiovascular disease may experience more physical symptoms during dialysis due to their underlying health status.
Inflammation is a common complication among dialysis patients and it can contribute to a range of physical symptoms. Chronic inflammation in the body can lead to muscle wasting, fatigue, and weakness, which can significantly impact a patient’s ability to perform daily activities. Inflammation can also lead to bone and joint pain, shortness of breath, weakness, nausea, vomiting, poor appetite, constipation, sore mouth, drowsiness, poor mobility, itching, difficulty sleeping, restless legs, skin changes and rashes, other skin problems, diarrhea, infections, muscle cramps, fatigue, and lethargy or low energy [2]. One of the most common causes of inflammation in dialysis patients is the presence of infections, which can cause a fever and increase inflammatory markers in the body. Inflammation can also be caused by other factors, such as the accumulation of toxins that are not adequately cleared by the dialysis process or the presence of other underlying medical conditions.
There are several biomarkers that can indicate the presence of inflammation and may be associated with the physical symptoms experienced by dialysis patients. Some commonly measured biomarkers include C-reactive protein (CRP), Interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) [3,4,5]. In addition, fibrinogen can also be produced in response to inflammation and may contribute to the development of cardiovascular disease [89]. CRP can contribute to the development of cardiovascular disease and it has also been linked to anemia, bone disease, and muscle wasting. CRP can also affect the function of the immune system, making patients more susceptible to infections and other illnesses [73,74]. IL-6 and TNF- α can contribute to the development of anemia, bone disease, and muscle wasting [75,76,83]. It can also affect the function of the cardiovascular and respiratory systems, leading to shortness of breath and other symptoms. Elevated levels of these biomarkers may indicate the presence of inflammation in dialysis patients and may be associated with the physical symptoms experienced by these patients. Furthermore, elevated levels of β2M have been associated with a range of physical symptoms in dialysis patients, including bone disease, joint pain, and muscle wasting. This is because β2M can form deposits in joints and bones, leading to inflammation and tissue damage [84]. On the other hand, increased levels of IS and PCS are associated with a higher risk of cardiovascular mortality in CKD patients [85,86,87,88].
In addition, anemia and low levels of albumin are both common among dialysis patients who use high-flux and high MWCO and can contribute to physical symptoms [47,48]. Anemia, which is a condition characterized by a low red blood cell count or hemoglobin level, which affects around 40–60% of dialysis patients, can cause fatigue, weakness, and shortness of breath. This is because red blood cells are responsible for carrying oxygen throughout the body and a low red blood cell count can lead to a decreased supply of oxygen to the tissues and organs. Low levels of albumin, which is a protein found in the blood, can also contribute to physical symptoms in dialysis patients. Albumin helps to maintain fluid balance in the body and plays a role in transporting important molecules such as hormones and medications. A low level of albumin can lead to fluid accumulation in the tissues, which can cause swelling and edema. Additionally, low albumin levels can impair the immune system and contribute to malnutrition and muscle wasting.
In addition, changes in ATIII and vWF levels can affect blood coagulation and increase the risk of thrombosis, which can lead to physical symptoms such as pain, swelling, and organ damage [81,82]. Thrombosis can also be life-threatening in some cases, such as when it causes a heart attack or stroke. The physical symptoms of inflammation can significantly affect the quality of life of dialysis patients. Patients may experience a loss of appetite, weight loss, and difficulty sleeping, which can further contribute to fatigue and weakness [98]. Joint pain and stiffness can make it challenging to move around and perform daily activities, while skin rashes and other skin problems can cause discomfort and affect a patient’s self-esteem.
5. Correlation between Physical and Psychological Symptoms
There is a well-established correlation between psychological and physical symptoms, particularly among patients with chronic medical conditions such as end-stage renal disease (ESRD) who require dialysis. Chronic medical conditions can lead to significant physical symptoms that can impact a patient’s quality of life, such as fatigue, pain, and difficulty sleeping [99]. However, these physical symptoms can also contribute to psychological symptoms such as anxiety, depression, and stress. Psychological symptoms can in turn exacerbate physical symptoms, creating a cycle of negative symptoms and poor health outcomes. Research has shown that dialysis patients with higher levels of depression and anxiety report more physical symptoms and lower overall quality of life. Additionally, stress and other psychological factors have been shown to contribute to inflammation in the body, which can worsen physical symptoms in patients with ESRD. For example, fatigue can be exacerbated by psychological factors, making it difficult to maintain motivation and energy levels. Pain perception can also be intensified by stress and anxiety, making it more difficult to manage. Sleep disturbances can be caused by medical conditions but psychological factors can also disrupt sleep and contribute to poor sleep quality. Anemia may also be related to the high prevalence of sleep disorders in ESRD patients. A study of 245 ESRD patients undergoing hemodialysis found that 74.4% of the patients reported sleep disturbance [19]. Patients with poor sleep also scored higher on the Beck Depression Inventory; thus, anemia and depression were predictors of lower sleep quality and insomnia in ESRD patients [100,101].
Changes in appetite and nutritional status can also be influenced by psychological factors such as depression and anxiety, leading to poor dietary choices and decreased overall health. Itching is a common physical symptom among dialysis patients, which affects 30–70% of dialysis patients; stress and anxiety can also contribute to itching by triggering the release of histamines [1,96,97]. Fluid overload can be caused by excess fluid in the body but anxiety and depression can also contribute by triggering the release of stress hormones, which can lead to fluid retention and increased blood pressure [102]. Dialysis patients are at increased risk for cardiovascular disease and while this is often attributed to factors such as high blood pressure and inflammation, psychological factors such as depression and stress have also been shown to increase the risk of cardiovascular disease.
Muscle weakness can be caused by malnutrition and inflammation but psychological factors such as depression and anxiety can also contribute by causing decreased physical activity and muscle mass [103]. Restless leg syndrome is primarily caused by physical factors but stress and anxiety can exacerbate symptoms by interfering with sleep and triggering the release of dopamine. Cognitive impairment can be caused by anemia, inflammation, and cerebral small vessel disease but depression and anxiety can also contribute by interfering with attention, memory, and other cognitive processes. Sexual dysfunction can be caused by hormonal imbalances and nerve damage but depression and anxiety can also contribute by decreasing libido and interfering with sexual performance [104].
Infections can be caused by immunosuppression and the use of catheters for vascular access but stress and anxiety can also weaken the immune system and make the body more susceptible to infections. Nausea and vomiting can be caused by gastrointestinal issues and medication side effects but stress and anxiety can also contribute by disrupting digestion and causing nausea [105]. Skin problems such as dryness, itching, and rashes can be caused by dehydration and high levels of phosphorus in the blood but stress and anxiety can also weaken the immune system and increase inflammation. Elevated levels of vWF, a marker of vascular endothelial dysfunction, are associated with cardiovascular disease, which is prevalent in hemodialysis patients and can significantly impact their quality of life [106].
In conclusion, there is a clear correlation between psychological and physical symptoms in dialysis patients. While many physical symptoms can be directly attributed to underlying medical conditions, psychological factors such as stress, anxiety, and depression can also play a significant role in exacerbating or even causing these symptoms. Conversely, physical symptoms such as pain, fatigue, and anemia can have a negative impact on patients’ psychological well-being, leading to depression, anxiety, and other psychological symptoms. As a result, the effective management of dialysis patients requires a holistic approach that addresses both physical and psychological factors. Healthcare providers should be aware of the potential interactions between physical and psychological symptoms and tailor their treatment plans accordingly, incorporating both medical treatments and psychological interventions as appropriate. By addressing both physical and psychological symptoms, patients can achieve improved outcomes and a better overall quality of life. Table 1 examines the complex relationships between various inflammatory biomarkers and their effects on both physical and psychological symptoms in dialysis patients. The biomarkers discussed include loss of hemoglobin (anemia), low serum albumin levels, high C-reactive protein (CRP), high Interleukin-6 (IL-6), high complement components C3a and C5a, high von Willebrand Factor (vWF) and serpin/antithrombin III (ATIII), high tumor necrosis factor-alpha (TNF-α), β2 microglobulin, the buildup of uremic toxins such as indoxyl sulfate (IS) and p-cresol sulfate (PCS), and fibrinogen activation. Table 1 also connects the biomarkers to the resultant physical outcomes and psychological symptoms observed in dialysis patients, emphasizing the profound impact of inflammatory processes on the overall physical and mental well-being of these individuals.
Figure 2 provides a comprehensive overview of the various factors affecting dialysis patients, summarizing the issues related to membrane hemocompatibility and dialysis practices and detailing both physical symptoms and psychosocial symptoms. These factors collectively contribute to a reduced QOL for patients undergoing dialysis. It is worth noting that the visual elements depicted in Figure 2 do not necessarily have an equal influence or effect on the quality of life of dialysis patients.
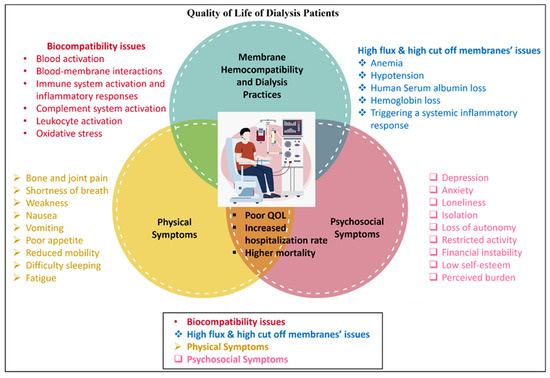
Figure 2.
Interplay of hemocompatibility and physical and psychosocial factors affecting quality of life in dialysis patients.
6. Case Studies: Extra Challenges and Impacts of Dialysis on High-Risk Populations in Canada
This section specifically focuses on the extra challenges and impacts of dialysis on high-risk populations in Canada. These case studies illuminate universal challenges faced in dialysis care and underscore innovative solutions that hold the potential for adaptation in diverse global contexts. Understanding the complex interplay of age, race, and pre-existing conditions is crucial for tailoring healthcare interventions that effectively address the unique needs of these populations and improve their overall well-being.
6.1. First Nations Dialysis Patients
It is important to note that the Canadian healthcare system provides comprehensive medical coverage for all ESRD patients, including those from First Nations communities [107]. However, specific challenges hinder the accessibility and effectiveness of these services for First Nations patients. Many reside in remote areas, requiring lengthy travel to dialysis centers, often compounded by severe weather conditions and unreliable transportation infrastructure [108]. Furthermore, the frequent need for transportation, sometimes multiple times per week, introduces substantial costs and logistical difficulties [109]. Strategies such as mobile health services would support patients in rural areas, ensuring equitable care delivery across all populations [110]. First Nations patients undergoing dialysis face a myriad of challenges that can have both physical and psychological implications.
Nevertheless, it is important to acknowledge that there are other options available to adapt to these barriers, such as peritoneal dialysis (PD) options, smaller HD centers close to some communities, and home HD. These alternative approaches aim to increase accessibility and improve the quality of care for patients, particularly those in remote or underserved areas. By offering a range of dialysis modalities tailored to individual needs and circumstances, healthcare providers can better address the challenges faced by high-risk populations like First Nations communities. Integrating these options into healthcare delivery strategies can enhance patient outcomes and promote health equity across diverse populations. In addition In the United States, efforts to enhance ESRD outreach for patients in remote areas include the adoption of peritoneal dialysis (PD), the establishment of smaller hemodialysis (HD) centers, and the utilization of home HD supported by trained traveling healthcare teams. PD offers a viable and economical home-based therapy option, particularly beneficial in geographically vast areas, thus mitigating accessibility issues associated with traditional in-center HD treatments [111,112]. Moreover, smaller decentralized HD centers and home HD services facilitated by mobile healthcare professionals are crucial for providing continuous and integrated care, further reducing the travel burden and enhancing treatment compliance [113,114]. These strategies collectively play a critical role in improving access to renal care services and enhancing the quality of life of patients in remote settings.
According to a recent report by the Canadian Institute for Health Information, Indigenous kidney disease patients have a 20% higher likelihood of hospitalization due to complications from dialysis treatment and a lower patient survival rate compared to non-Indigenous patients. The report also highlights that Indigenous patients are 30% more likely to be hospitalized specifically for dialysis-related infections [115]. The report underscores the need for further research to understand the underlying reasons and to develop targeted interventions that address these specific needs, thereby improving the overall psychological and physical well-being of First Nations patients.
6.2. Child and Youth Dialysis Patients
Furthermore, youth and children with ESRD are at high risk of developing psychiatric disorders. Children and adolescents with CKD demonstrate a high prevalence of depression; approximately 35% of pediatric CKD patients exhibit depressive symptoms [116]). Depression in youth with ESRD may be a result of medication use, difficulty coping with the loss of renal function and physical symptoms, inability to attend school, and low self-esteem related to loss of sexual function, delayed puberty, and short stature [116]. The management of ESRD has a significant impact on pediatric patients’ quality of life, as frequent hospitalizations can affect school attendance, restrict participation in activities, and hinder socialization [117]. Pediatric ESRD patients described feeling alienated, abandoned, and isolated; they also reported a lack of autonomy in daily decision making, such as what food to eat [118]. Children and youth may be especially vulnerable to developing mental health issues, such as depression, anxiety, and feelings of hopelessness, due to a lower capacity for managing stress and poorer coping skills compared to adults. One study found that adolescent patients with ESRD were more likely than older patients to view their diagnosis as a loss or challenge [119]. Children and youth with ESRD may also be less likely to adhere to treatment recommendations, thus increasing their risk of poor health outcomes [117].
Child and youth patients undergoing dialysis encounter a myriad of challenges that significantly impact their physical well-being and overall quality of life. The treatment process itself poses physical demands and time constraints, requiring regular visits to dialysis centers and strict adherence to medical regimens. This disrupts their daily routines, including school attendance, extracurricular activities, and social interactions, which are crucial for their development and overall physical health. Additionally, dialysis can give rise to various physical symptoms, such as fatigue, weakness, and discomfort, further affecting their overall physical well-being. Furthermore, dialysis has specific physical effects on child and youth patients, which can have long-term consequences. These effects encompass fluid and electrolyte imbalances, anemia, growth and developmental challenges, bone and mineral disorders, cardiovascular complications, and potential complications related to vascular access [120]. Child and youth patients may exhibit symptoms such as edema, high blood pressure, fatigue, weakness, pale skin, delayed growth, bone pain, skeletal deformities, and an increased risk of fractures [120]. These physical manifestations can significantly impact their day-to-day activities, mobility, and overall physical functioning [121].
Moreover, child and youth patients face unique challenges related to their developmental stage. Navigating the complex physical, emotional, and social changes associated with growing up can be particularly challenging for them and the need for ongoing medical interventions adds an additional layer of complexity. These challenges may contribute to feelings of frustration, isolation, and limitations on their activities, potentially impacting their mental well-being as well.
Addressing the physical challenges faced by child and youth patients undergoing dialysis requires a comprehensive approach that encompasses medical management, individualized care plans, and support systems tailored to their specific needs.
6.3. Diabetic Dialysis Patients
Diabetic patients who require dialysis are more likely to experience severe physical symptoms compared to non-diabetic patients on dialysis. Diabetic patients often experience a range of characteristic psychological symptoms, including but not limited to depression, anxiety, and diabetes-related distress. Depression can manifest as persistent feelings of sadness, hopelessness, and a loss of interest in previously enjoyable activities. Anxiety may manifest as excessive worry, restlessness, and fear about diabetes management and complications. Diabetes-related distress encompasses emotional reactions to the daily demands of diabetes, such as frustration, guilt, or fear of hypoglycemia. These psychological symptoms can profoundly affect a patient’s quality of life, self-care behaviors, and glycemic control, underscoring the importance of their early identification and management in diabetes care.
The combination of diabetes and kidney failure can lead to various complications that can worsen their overall health status [122]. The inflammatory markers such as C-reactive protein (CRP), Interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) are frequently found at higher levels in diabetic patients on dialysis compared to non-diabetic dialysis patients [123]. The presence of inflammation in diabetic dialysis patients can have significant implications for their health. According to studies, diabetic patients on dialysis have a higher prevalence of cardiovascular complications compared to non-diabetic patients on dialysis, with an increased risk of heart attacks, strokes, and peripheral artery disease. In fact, research shows that diabetic dialysis patients have a two to four times higher risk of cardiovascular mortality than non-diabetic dialysis patients [124,125].
Another factor contributing to the severity of symptoms in diabetic dialysis patients is neuropathy. Diabetic neuropathy, a nerve damage condition associated with diabetes, can affect various parts of the body. When combined with the uremic neuropathy that can occur in kidney failure, the symptoms can be more severe and debilitating. Studies have shown that around 60–70% of diabetic patients with ESRD experience neuropathy symptoms [126,127].
Poor wound healing is also a concern for diabetic dialysis patients. Diabetes can impair the body’s ability to heal wounds due to reduced blood flow and compromised immune function. In the presence of kidney failure, wounds may take longer to heal, putting diabetic dialysis patients at a higher risk of infections and complications. The risk of infections is further increased due to the weakened immune system associated with both diabetes and kidney failure.
Metabolic imbalances are common in diabetic dialysis patients. Diabetes and kidney failure can disrupt the body’s normal metabolic processes, leading to electrolyte imbalances, fluid retention, and metabolic acidosis. These imbalances can cause symptoms such as muscle cramps, weakness, fatigue, and altered mental status [22,128]. Proper management of blood sugar levels, fluid balance, and electrolyte levels becomes crucial in alleviating these symptoms. In summary, diabetic dialysis patients are at a higher risk of experiencing severe physical symptoms due to the combined effects of diabetes and kidney failure. Cardiovascular complications, neuropathy, poor wound healing, increased infection risk, and metabolic imbalances contribute to the severity of symptoms in this population.
7. Outlook
Dialysis is a life-saving treatment for patients with end-stage renal disease (ESRD) but it can also have a significant impact on their psychosocial well-being and quality of life. Research studies have shown that dialysis patients often experience psychosocial symptoms such as depression, anxiety, stress, and social isolation. These symptoms can be caused by a variety of factors, including the chronic nature of the illness, the demands of the treatment, changes in lifestyle, and the uncertainty of the future. In addition, the physical side effects of dialysis can also impact a patient’s quality of life. Patients may experience fatigue, sleep disturbances, loss of appetite, and a reduced ability to participate in activities they previously enjoyed.
While dialysis can be challenging for patients, a multidisciplinary approach that includes medical, psychological, and social support can help improve the psychosocial symptoms and quality of life of dialysis patients. Depression, anxiety, stress, and other psychological factors can exacerbate physical symptoms and contribute to the development of other health complications. Dialysis patients should receive social support from family, friends, and healthcare providers; education and counseling about the illness and treatment; and access to resources such as support groups and mental health services. Moreover, technological advancements and innovations would improve dialysis therapy, providing patients with more options and flexibility, allowing them to maintain their daily routines and continue participating in activities they enjoy.
Enhancing membrane hemocompatibility and developing new materials that are less likely to trigger an immune response can potentially reduce inflammation and physical and psychological symptoms by improving the biocompatibility of dialysis membranes, which can reduce the activation of the immune system and the release of pro-inflammatory cytokines. Reducing inflammation can also have a positive impact on the psychological well-being of patients. Chronic inflammation has been associated with depression, anxiety, and cognitive dysfunction as well as reducing inflammation, which may help to alleviate these symptoms and improve the overall quality of life for dialysis patients.
Furthermore, understanding and addressing the impact of dialysis on psychosocial symptoms in youth patients holds promising prospects. While research in this area is limited, studies have shed light on the emotional distress, reduced quality of life, and social challenges experienced by youth patients undergoing dialysis. However, by recognizing the importance of social support, promoting positive coping strategies, and involving multidisciplinary care teams, we can work toward improving the psychosocial well-being of these patients. Further research is needed to deepen our understanding of the specific challenges faced by youth patients and develop targeted interventions to address their unique needs. By focusing on enhancing psychosocial support and implementing comprehensive care approaches, we can strive to improve the overall quality of life for youth patients undergoing dialysis. Continued efforts in research, collaboration, and patient-centered care can make a positive impact on the psychosocial well-being of youth patients and help them navigate the challenges of dialysis more effectively.
In addition, the outlook for managing the physical symptoms experienced by child and youth patients undergoing dialysis is focused on comprehensive care and multidisciplinary approaches. By implementing regular monitoring, medication management, and nutritional support, healthcare teams can work toward minimizing fluid and electrolyte imbalances, managing anemia, and addressing growth and developmental challenges. Additionally, efforts to optimize dialysis treatment strategies and prevent complications related to bone and mineral disorders and cardiovascular complications are crucial. Continued research, collaboration, and patient-centered care are essential in improving the overall well-being and long-term outcomes of child and youth patients undergoing dialysis.
The impact of dialysis on critical symptoms, hospitalization, and quality of life in First Nations patients is a complex and multifaceted issue. Limited research specifically focused on this population makes it challenging to draw definitive conclusions. Quality of life in First Nations patients undergoing dialysis may be influenced by factors such as cultural and social support, access to healthcare resources, and the management of comorbidities. Further research is needed to gain a deeper understanding of the specific impact of dialysis on critical symptoms, hospitalization, and quality of life in First Nations patients, in order to develop targeted interventions and improve overall outcomes.
Dialysis presents unique challenges for patients with diabetes, as the combination of these conditions can lead to various complications. Diabetic dialysis patients often experience more severe physical symptoms, such as cardiovascular complications, neuropathy, poor wound healing, increased infection risk, and metabolic imbalances. By addressing the specific needs of diabetic dialysis patients in addition to improving membrane compatibility, we can improve their quality of life and overall well-being.
In summary, improving membrane hemocompatibility and optimizing clinical practices can be a promising strategy to reduce inflammation and its associated consequences, thereby improving the physical and psychological symptoms of dialysis patients. However, further research is necessary to fully comprehend the effectiveness of these interventions in enhancing patient outcomes.
Funding
This research was funded by Saskatchewan Research Health Foundation (SHRF).
Acknowledgments
The authors would like to acknowledge the generous funding support provided by the Saskatchewan Research Health Foundation (SHRF). The authors are also grateful to the University of Saskatchewan (U of S) for providing all the necessary facilities to conduct this research in collaboration with the Saskatchewan Transplant Program at St. Paul’s Hospital in Saskatoon.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gadaen, R.J.R.; Kooman, J.P.; Cornelis, T.; van der Sande, F.M.; Winkens, B.J.; Broers, N.J.H. The Effects of Chronic Dialysis on Physical Status, Quality of Life, and Arterial Stiffness: A Longitudinal Study in Prevalent Dialysis Patients. Nephron 2021, 145, 44–54. [Google Scholar] [CrossRef] [PubMed]
- de Virgilio, C.; Frank, P.; Grigorian, A. (Eds.) Question Sets and Answers. In Surgery; Springer: New York, NY, USA, 2015; pp. 591–699. [Google Scholar] [CrossRef]
- Jhamb, M.; Weisbord, S.D.; Steel, J.L.; Unruh, M. Fatigue in Patients Receiving Maintenance Dialysis: A Review of Definitions, Measures, and Contributing Factors. Am. J. Kidney Dis. 2008, 52, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.T.; Ahmed, F.A.; Hamm, L.L.; Teran, F.J.; Chen, C.-S.; Liu, Y.; Shah, K.; Rifai, N.; Batuman, V.; Simon, E.E.; et al. Association of C-reactive protein, tumor necrosis factor-alpha, and interleukin-6 with chronic kidney disease. BMC Nephrol. 2015, 16, 77. [Google Scholar] [CrossRef]
- Meuwese, C.L.; Boulaksil, M.; van Dijk, J.; Polad, J.; Meijburg, H.W. Transient left ventricular outflow tract obstruction with systolic anterior motion of the mitral valve: A stunning cause. Echocardiography 2017, 34, 1089–1091. [Google Scholar] [CrossRef] [PubMed]
- Walters, B.A.J.; Hays, R.D.; Spritzer, K.L.; Fridman, M.; Carter, W.B. Health-related quality of life, depressive symptoms, anemia, and malnutrition at hemodialysis initiation. Am. J. Kidney Dis. 2002, 40, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- El-Hady, H.A.; Kora, M.A.; El-Zorkany, K.M.; Montaser, B.A.; Yousuf, A.M. Plasma levels of von Willebrand factor in maintenance hemodialysis patients: Its relation to vascular access thrombosis. Menoufia Med. J. 2019, 32, 345–351. [Google Scholar]
- Kirmizis, D.; Tsiandoulas, A.; Pangalou, M.; Koutoupa, E.; Rozi, P.; Protopappa, M.; Barboutis, K. Validity of plasma fibrinogen, D-dimer, and the von Willebrand factor as markers of cardiovascular morbidity in patients on chronic hemodialysis. Med. Sci. Monit. 2006, 12, CR55–CR62. [Google Scholar] [CrossRef] [PubMed]
- Ocak, G.; Roest, M.; Verhaar, M.C.; Rookmaaker, M.B.; Blankestijn, P.J.; Bos, W.J.; Fijnheer, R.; Péquériaux, N.C.; Dekker, F.W. Von Willebrand factor, ADAMTS13 and mortality in dialysis patients. BMC Nephrol. 2021, 22, 222. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-K.; Tsai, Y.-C.; Hsu, H.-J.; Wu, I.-W.; Sun, C.-Y.; Chou, C.-C.; Lee, C.-C.; Tsai, C.-R.; Wu, M.-S.; Wang, L.-J. Depression and Suicide Risk in Hemodialysis Patients with Chronic Renal Failure. Psychosomatics 2010, 51, 528–528.e6. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.; Vecchio, M.; Craig, J.C.; Tonelli, M.; Johnson, D.W.; Nicolucci, A.; Pellegrini, F.; Saglimbene, V.; Logroscino, G.; Fishbane, S.; et al. Prevalence of depression in chronic kidney disease: Systematic review and meta-analysis of observational studies. Kidney Int. 2013, 84, 179–191. [Google Scholar] [CrossRef]
- Wang, L.-J.; Chen, C.-K. The Psychological Impact of Hemodialysis on Patients with Chronic Renal Failure. In Renal Failure—The Facts; Polenakovic, M., Ed.; InTech: Shanghai, China, 2012; pp. 217–236. ISBN 978-953-51-0630-2. [Google Scholar]
- Chan, L.; Tummalapalli, S.L.; Ferrandino, R.; Poojary, P.; Saha, A.; Chauhan, K.; Nadkarni, G.N. The Effect of Depression in Chronic Hemodialysis Patients on Inpatient Hospitalization Outcomes. Blood Purif. 2017, 43, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Elhadad, A.A.; Ragab, A.Z.; Atia, S.A. Psychiatric comorbidity and quality of life in patients undergoing hemodialysis. Middle East Curr. Psychiatry 2020, 27, 9. [Google Scholar] [CrossRef]
- Farrokhi, F.; Abedi, N.; Beyene, J.; Kurdyak, P.; Jassal, S.V. Association Between Depression and Mortality in Patients Receiving Long-term Dialysis: A Systematic Review and Meta-analysis. Am. J. Kidney Dis. 2014, 63, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, P.L.; Peterson, R.A.; Weihs, K.L.; Simmens, S.J.; Alleyne, S.; Cruz, I.; Veis, J.H. Multiple measurements of depression predict mortality in a longitudinal study of chronic hemodialysis outpatients. Kidney Int. 2000, 57, 2093–2098. [Google Scholar] [CrossRef] [PubMed]
- Szeifert, L.; Bragg-Gresham, J.L.; Thumma, J.; Gillespie, B.W.; Mucsi, I.; Robinson, B.M.; Pisoni, R.L.; Disney, A.; Combe, C.; Port, F.K. Psychosocial variables are associated with being wait-listed, but not with receiving a kidney transplant in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol. Dial. Transplant. 2011, 27, 2107–2113. [Google Scholar] [CrossRef] [PubMed]
- Gregg, L.P.; Carmody, T.; Le, D.; Martins, G.; Trivedi, M.; Hedayati, S.S. A Systematic Review and Meta-Analysis of Depression and Protein–Energy Wasting in Kidney Disease. Kidney Int. Rep. 2020, 5, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.-F.; Hsu, S.-P.; Yang, S.-Y.; Ho, T.-I.; Lai, C.-F.; Peng, Y.-S. Sleep Disturbance in Chronic Hemodialysis Patients: The Impact of Depression and Anemia. Ren. Fail. 2007, 29, 673–677. [Google Scholar] [CrossRef]
- Foley, R.N.; Sexton, D.J.; Drawz, P.; Ishani, A.; Reule, S. Race, Ethnicity, and End-of-Life Care in Dialysis Patients in the United States. J. Am. Soc. Nephrol. 2018, 29, 2387–2399. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Mangano, M.; Stucchi, A.; Ciceri, P.; Conte, F.; Galassi, A. Cardiovascular disease in dialysis patients. Nephrol. Dial. Transplant. 2018, 33 (Suppl. 3), iii28–iii34. [Google Scholar] [CrossRef]
- Rhee, C.M.; Leung, A.M.; Kovesdy, C.P.; Lynch, K.E.; Brent, G.A.; Kalantar-Zadeh, K. Updates on the Management of Diabetes in Dialysis Patients. Semin. Dial. 2014, 27, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Abdelrasoul, A.; Shoker, A. WCN23-0020 impact of hydration shell of hemodialysis membranes on activation of surrogate biomarkers in the uremic serum of dialysis patients. Kidney Int. Rep. 2023, 8, S303. [Google Scholar] [CrossRef]
- Eduok, U.; Abdelrasoul, A.; Shoker, A.; Doan, H. Recent developments, current challenges and future perspectives on cellulosic hemodialysis membranes for highly efficient clearance of uremic toxins. Mater. Today Commun. 2021, 27, 102183. [Google Scholar] [CrossRef]
- Mollahosseini, A.; Argumeedi, S.; Abdelrasoul, A.; Shoker, A. A case study of poly (aryl ether sulfone) hemodialysis membrane interactions with human blood: Molecular dynamics simulation and experimental analyses. Comput. Methods Programs Biomed. 2020, 197, 105742. [Google Scholar] [CrossRef] [PubMed]
- Saadati, S.; Eduok, U.; Westphalen, H.; Abdelrasoul, A.; Shoker, A.; Choi, P.; Doan, H.; Ein-Mozaffari, F.; Zhu, N. Assessment of polyethersulfone and polyacrylonitrile hemodialysis clinical membranes: In situ synchrotron-based imaging of human serum proteins adsorption, interaction analyses, molecular docking and clinical inflammatory biomarkers investigations. Mater. Today Commun. 2021, 29, 102928. [Google Scholar] [CrossRef]
- Sishi, Z.; Bahig, J.; Kalugin, D.; Shoker, A.; Zhu, N.; Abdelrasoul, A. Influence of clinical hemodialysis membrane morphology and chemistry on protein adsorption and inflammatory biomarkers released: In-situ synchrotron imaging, clinical and computational studies. Biomed. Eng. Adv. 2023, 5, 100070. [Google Scholar] [CrossRef]
- Westphalen, H.; Abdelrasoul, A.; Shoker, A.; Zhu, N. Assessment of hemodialysis clinical practices using polyaryl ether sulfone-polyvinylpyrrolidone (PAES: PVP) clinical membrane: Modeling of in vitro fibrinogen adsorption, in situ synchrotron-based imaging, and clinical inflammatory biomarkers investigations. Sep. Purif. Technol. 2021, 259, 118136. [Google Scholar] [CrossRef]
- Westphalen, H.; Saadati, S.; Eduok, U.; Abdelrasoul, A.; Shoker, A.; Choi, P.; Doan, H.; Ein-Mozaffari, F. Case studies of clinical hemodialysis membranes: Influences of membrane morphology and biocompatibility on uremic blood-membrane interactions and inflammatory biomarkers. Sci. Rep. 2020, 10, 14808. [Google Scholar] [CrossRef] [PubMed]
- Abdelrasoul, A.; Shoker, A. Induced hemocompatibility of polyethersulfone (PES) hemodialysis membrane using polyvinylpyrrolidone: Investigation on human serum fibrinogen adsorption and inflammatory biomarkers released. Chem. Eng. Res. Des. 2022, 177, 615–624. [Google Scholar] [CrossRef]
- Abdelrasoul, A.; Shoker, A. Influence of hydration shell of hemodialysis clinical membranes on surrogate biomarkers activation in uremic serum of dialysis patients. Biomed. Eng. Adv. 2022, 4, 100049. [Google Scholar] [CrossRef]
- Abdelrasoul, A.; Kalugin, D.; Shoker, A. Recent Developments and Current Challenges of Heparin-Grafted Hemodialysis Membranes. J. Compos. Sci. 2022, 6, 244. [Google Scholar] [CrossRef]
- Abdelrasoul, A.; Shoker, A. WCN23-0021 polyvinylpyrrolidone (pvp) enhances the hemocompatibility of polyethersulfone (pes) hemodialysis membranes. Kidney Int. Rep. 2023, 8, S303. [Google Scholar] [CrossRef]
- Eduok, U.; Camara, H.; Abdelrasoul, A.; Shoker, A. Influence of UV-irradiation intensity and exposure duration on the hemobiocompatibility enhancement of a novel synthesized phosphobetaine zwitterions polyethersulfone clinical hemodialysis membranes. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 110, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Mollahosseini, A.; Abdelrasoul, A. Novel insights in hemodialysis: Most recent theories on membrane hemocompatibility improvement. Biomed. Eng. Adv. 2022, 3, 100034. [Google Scholar] [CrossRef]
- Mollahosseini, A.; Abdelrasoul, A.; Shoker, A. A critical review of recent advances in hemodialysis membranes hemocompatibility and guidelines for future development. Mater. Chem. Phys. 2020, 248, 122911. [Google Scholar] [CrossRef]
- Mollahosseini, A.; Abdelrasoul, A.; Shoker, A. Latest advances in zwitterionic structures modified dialysis membranes. Mater. Today Chem. 2020, 15, 100227. [Google Scholar] [CrossRef]
- Nazari, S.; Abdelrasoul, A. Impact of Membrane Modification and Surface Immobilization Techniques on the Hemocompatibility of Hemodialysis Membranes: A Critical Review. Membranes 2022, 12, 1063. [Google Scholar] [CrossRef] [PubMed]
- Nazari, S.; Abdelrasoul, A. Surface zwitterionization of hemodialysismembranesfor hemocompatibility enhancement and protein-mediated anti-adhesion: A critical review. Biomed. Eng. Adv. 2022, 3, 100026. [Google Scholar] [CrossRef]
- Saadati, S.; Eduok, U.; Westphalen, H.; Abdelrasoul, A.; Shoker, A.; Choi, P.; Doan, H.; Ein-Mozaffari, F.; Zhu, N. In situ synchrotron imaging of human serum proteins interactions, molecular docking and inflammatory biomarkers of hemocompatible synthesized zwitterionic polymer coated-polyvinylidene fluoride (PVDF) dialysis membranes. Surf. Interfaces 2021, 27, 101505. [Google Scholar] [CrossRef]
- Saadati, S.; Westphalen, H.; Eduok, U.; Abdelrasoul, A.; Shoker, A.; Choi, P.; Doan, H.; Ein-Mozaffari, F.; Zhu, N. Biocompatibility enhancement of hemodialysis membranes using a novel zwitterionic copolymer: Experimental, in situ synchrotron imaging, molecular docking, and clinical inflammatory biomarkers investigations. Mater. Sci. Eng. C 2020, 117, 111301. [Google Scholar] [CrossRef]
- Craddock, P.R.; Fehr, J.; Brigham, K.L.; Kronenberg, R.S.; Jacob, H.S. Complement and Leukocyte-Mediated Pulmonary Dysfunction in Hemodialysis. N. Engl. J. Med. 1977, 296, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Merle, N.S.; Noe, R.; Halbwachs-Mecarelli, L.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part II: Role in Immunity. Front. Immunol. 2015, 6, 257. [Google Scholar] [CrossRef] [PubMed]
- Woulfe, D.; Yang, J.; Brass, L. ADP and platelets: The end of the beginning. J. Clin. Investig. 2001, 107, 1503–1505. [Google Scholar] [CrossRef] [PubMed]
- Koga, Y.; Fujieda, H.; Meguro, H.; Ueno, Y.; Aoki, T.; Miwa, K.; Kainoh, M. Biocompatibility of Polysulfone Hemodialysis Membranes and Its Mechanisms: Involvement of Fibrinogen and Its Integrin Receptors in Activation of Platelets and Neutrophils. Artif. Organs 2018, 42, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-J.; Wei, R.; Wang, Y.; Su, T.; Di, P.; Li, Q.; Yang, X.; Li, P.; Chen, X. Blood coagulation system in patients with chronic kidney disease: A prospective observational study. BMJ Open 2017, 7, e014294. [Google Scholar] [CrossRef] [PubMed]
- Fushimi, F.; Nakayama, M.; Nishimura, K.; Hiyoshi, T. Platelet Adhesion, Contact Phase Coagulation Activation, and C5a Generation of Polyethylene Glycol Acid-Grafted High Flux Cellulosic Membrane with Varieties of Grafting Amounts. Artif. Organs 1998, 22, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Gondouin, B.; Hutchison, C.A. High Cut-off Dialysis Membranes: Current Uses and Future Potential. Adv. Chronic Kidney Dis. 2011, 18, 180–187. [Google Scholar] [CrossRef]
- Villa, G.; Zaragoza, J.J.; Sharma, A.; Neri, M.; De Gaudio, A.R.; Ronco, C. Cytokine Removal with High Cut-Off Membrane: Review of Literature. Blood Purif. 2014, 38, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-J.; Yen, C.-H.; Chen, C.-K.; Wu, I.-W.; Lee, C.-C.; Sun, C.-Y.; Chang, S.-J.; Chou, C.-C.; Hsieh, M.-F.; Chen, C.-Y.; et al. Association between uremic toxins and depression in patients with chronic kidney disease undergoing maintenance hemodialysis. Gen. Hosp. Psychiatry 2013, 35, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, P.L. Psychosocial factors in dialysis patients. Kidney Int. 2001, 59, 1599–1613. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Chao, H.-H.; Huang, W.-T.; Chen, S.C.-C.; Yang, H.-Y. Psychiatric disorders risk in patients with iron deficiency anemia and association with iron supplementation medications: A nationwide database analysis. BMC Psychiatry 2020, 20, 216. [Google Scholar] [CrossRef] [PubMed]
- Couch, S. Psychiatric Problems Faced by Patients on Dialysis: The Missing Element. Clin. J. Am. Soc. Nephrol. 2019, 14, 1275–1276. [Google Scholar] [CrossRef] [PubMed]
- Drayer, R.A.; Piraino, B.; Reynolds, C.F.; Houck, P.R.; Mazumdar, S.; Bernardini, J.; Shear, M.K.; Rollman, B.L. Characteristics of depression in hemodialysis patients: Symptoms, quality of life and mortality risk. Gen. Hosp. Psychiatry 2006, 28, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, I.; Valderrábano, F.; Fort, J.; Jofré, R.; López-Gómez, J.M.; Moreno, F.; Sanz-Guajardo, D. Psychosocial Factors and Health-Related Quality of Life in Hemodialysis Patients. Qual. Life Res. 2005, 14, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Abbas Tavallaii, S.; Ebrahimnia, M.; Shamspour, N.; Assari, S. Effect of depression on health care utilization in patients with end-stage renal disease treated with hemodialysis. Eur. J. Intern. Med. 2009, 20, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, S.S. Association Between Major Depressive Episodes in Patients With Chronic Kidney Disease and Initiation of Dialysis, Hospitalization, or Death. JAMA 2010, 303, 1946. [Google Scholar] [CrossRef] [PubMed]
- Murtagh, F.E.M.; Addington-Hall, J.; Higginson, I.J. The Prevalence of Symptoms in End-Stage Renal Disease: A Systematic Review. Adv. Chronic Kidney Dis. 2007, 14, 82–99. [Google Scholar] [CrossRef] [PubMed]
- Cukor, D.; Ver Halen, N.; Fruchter, Y. Anxiety and Quality of Life in ESRD. Semin. Dial. 2013, 26, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Keskin, G.; Engin, E. The evaluation of depression, suicidal ideation and coping strategies in haemodialysis patients with renal failure. J. Clin. Nurs. 2011, 20, 2721–2732. [Google Scholar] [CrossRef] [PubMed]
- Kurella, M.; Kimmel, P.L.; Young, B.S.; Chertow, G.M. Suicide in the United States End-Stage Renal Disease Program. J. Am. Soc. Nephrol. 2005, 16, 774–781. [Google Scholar] [CrossRef]
- Flythe, J.E.; Hilliard, T.; Castillo, G.; Ikeler, K.; Orazi, J.; Abdel-Rahman, E.; Pai, A.B.; Rivara, M.B.; St. Peter, W.L.; Weisbord, S.D.; et al. Symptom Prioritization among Adults Receiving In-Center Hemodialysis: A Mixed Methods Study. Clin. J. Am. Soc. Nephrol. 2018, 13, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Schick-Makaroff, K.; Wozniak, L.A.; Short, H.; Davison, S.N.; Klarenbach, S.; Buzinski, R.; Walsh, M.; Johnson, J.A. Burden of mental health symptoms and perceptions of their management in in-centre hemodialysis care: A mixed methods study. J. Patient-Rep. Outcomes 2021, 5, 111. [Google Scholar] [CrossRef] [PubMed]
- Abdelrasoul, A.; Westphalen, H.; Saadati, S.; Shoker, A. Hemodialysis biocompatibility mathematical models to predict the inflammatory biomarkers released in dialysis patients based on hemodialysis membrane characteristics and clinical practices. Nat. Sci. Rep. 2021, 11, 23080. [Google Scholar] [CrossRef] [PubMed]
- Macleod, A.M.; Campbell, M.; Cody, J.D.; Daly, C.; Donaldson, C.; Grant, A.; Khan, I.; Rabindranath, K.S.; Vale, L.; Wallace, S. Cellulose, modified cellulose and synthetic membranes in the haemodialysis of patients with end-stage renal disease. Cochrane Database Syst. Rev. 2005, 2005, CD003234. [Google Scholar] [CrossRef] [PubMed]
- Uchiumi, N.; Sakuma, K.; Sato, S.; Matsumoto, Y.; Kobayashi, H.; Toriyabe, K.; Hayashi, K.; Kawasaki, T.; Watanabe, T.; Itohisa, A.; et al. The clinical evaluation of novel polymethyl methacrylate membrane with a modified membrane surface: A multicenter pilot study. Ren Replace Ther 2018, 4, 32. [Google Scholar] [CrossRef]
- Satoh, M.; Yamasaki, Y.; Nagake, Y.; Kasahara, J.; Hashimoto, M.; Nakanishi, N.; Makino, H. Oxidative stress is reduced by the long-term use of vitamin E-coated dialysis filters. Kidney Int. 2001, 59, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- Kalugin, D.; Bahig, J.; Shoker, A.; Abdelrasoul, A. Heparin-Immobilized Polyethersulfone for Hemocompatibility Enhancement of Dialysis Membrane: In Situ Synchrotron Imaging, Experimental, and Ex Vivo Studies. Membranes 2023, 13, 718. [Google Scholar] [CrossRef] [PubMed]
- Hidese, S.; Saito, K.; Asano, S.; Kunugi, H. Association between iron-deficiency anemia and depression: A web-based Japanese investigation. Psychiatry Clin. Neurosci. 2018, 72, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Onder, G.; Penninx, B.W.; Cesari, M.; Bandinelli, S.; Lauretani, F.; Bartali, B.; Gori, A.M.; Pahor, M.; Ferrucci, L. Anemia Is Associated With Depression in Older Adults: Results From the InCHIANTI Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2005, 60, 1168–1172. [Google Scholar] [CrossRef] [PubMed]
- Ambrus, L.; Westling, S. Inverse association between serum albumin and depressive symptoms among drug-free individuals with a recent suicide attempt. Nord. J. Psychiatry 2019, 73, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Qiu, W.; Yu, Y.; Li, N.; Wu, H.; Chen, Z. The association between serum albumin and depression in chronic liver disease may differ by liver histology. BMC Psychiatry 2022, 22, 5. [Google Scholar] [CrossRef] [PubMed]
- Charytan, D.; Fishbane, S.; Małyszko, J.; McCullough, P.; Goldsmith, D. Cardiorenal Syndrome and the Role of the Bone-Mineral Axis and Anemia. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2015, 66, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A. Nutritional problems in adult patients with chronic kidney disease. Clin. Queries Nephrol. 2012, 1, 222–235. [Google Scholar] [CrossRef]
- Pelosi, L.; Berardinelli, M.; Forcina, L.; Ascenzi, F.; Rizzuto, E.; Sandri, M.; Benedetti, F.; Scicchitano, B.; Musarò, A. Sustained Systemic Levels of IL-6 Impinge Early Muscle Growth and Induce Muscle Atrophy and Wasting in Adulthood. Cells 2021, 10, 1816. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Gupta, R.; Guerra, A.; Veigh, P.; Gardenghi, S.; Casu, C.; Vinchi, F.; Rivella, S. Lack of IL6 Improves Recovery from Anemia of Inflammation Which Gets Hampered in Presence of Excess Iron. Blood 2021, 138, 3072. [Google Scholar] [CrossRef]
- Crider, A.; Feng, T.; Pandya, C.D.; Davis, T.; Nair, A.; Ahmed, A.O.; Baban, B.; Turecki, G.; Pillai, A. Complement component 3a receptor deficiency attenuates chronic stress-induced monocyte infiltration and depressive-like behavior. Brain Behav. Immun. 2018, 70, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Hattori, K.; Miyakawa, T.; Watanabe, K.; Hidese, S.; Sasayama, D.; Ota, M.; Teraishi, T.; Hori, H.; Yoshida, S.; et al. Increased cerebrospinal fluid complement C5 levels in major depressive disorder and schizophrenia. Biochem. Biophys. Res. Commun. 2018, 497, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Poppelaars, F.; Faria, B.; Gaya da Costa, M.; Franssen, C.F.; van Son, W.J.; Berger, S.P.; Daha, M.R.; Seelen, M.A. The Complement System in Dialysis: A Forgotten Story? Front. Immunol. 2018, 9, 71. [Google Scholar] [CrossRef]
- Reginia, A.; Kucharska-Mazur, J.; Jabłoński, M.; Budkowska, M.; Dołȩgowska, B.; Sagan, L.; Misiak, B.; Ratajczak, M.Z.; Rybakowski, J.K.; Samochowiec, J. Assessment of Complement Cascade Components in Patients with Bipolar Disorder. Front. Psychiatry 2018, 9, 614. [Google Scholar] [CrossRef] [PubMed]
- Ebbesen, L.; Olesen, S.; Kruhøffer, M.; Ingerslev, J.; Ørntoft, T. Folate deficiency induced hyperhomocysteinemia changes the expression of thrombosis-related genes. Blood Coagul. Fibrinolysis 2006, 17, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Siegel, A.; Siegel, A.; Stec, J.; Lipinska, I.; Cott, E.; Lewandrowski, K.; Ridker, P.; Ridker, P.; Tofler, G. Effect of marathon running on inflammatory and hemostatic markers. Am. J. Cardiol. 2001, 88, 918–920. [Google Scholar]
- Reid, M.; Li, Y. Tumor necrosis factor-α and muscle wasting: A cellular perspective. Respir. Res. 2001, 2, 269–272. [Google Scholar] [CrossRef]
- Portales-Castillo, I.; Yee, J.; Tanaka, H.; Fenves, A.Z. Beta-2 Microglobulin Amyloidosis: Past, Present, and Future. Kidney360 2020, 1, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Bammens, B.; Evenepoel, P.; Keuleers, H.; Verbeke, K.; Vanrenterghem, Y. Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int. 2006, 69, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Gryp, T.; Vanholder, R.; Vaneechoutte, M.; Glorieux, G. p-Cresyl Sulfate. Toxins 2017, 9, 52. [Google Scholar] [CrossRef]
- Liu, W.-C.; Tomino, Y.; Lu, K.-C. Impacts of Indoxyl Sulfate and p-Cresol Sulfate on Chronic Kidney Disease and Mitigating Effects of AST-120. Toxins 2018, 10, 367. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.; Wu, P.; Lin, Y.; Chiu, Y.; Hwang, S.; Huang, J.; Chen, S.; Lee, S.; Liang, S. P1286THE relationship between protein-bound uremic toxins and target cardiovascular proteins in hemodialysis patients. Nephrol. Dial. Transplant. 2020, 35, gfaa142.P1286. [Google Scholar] [CrossRef]
- Maat, M.; Pietersma, A.; Kofflard, M.; Sluiter, W.; Kluft, C. Association of plasma fibrinogen levels with coronary artery disease, smoking and inflammatory markers. Atherosclerosis 1996, 121, 185–191. [Google Scholar] [PubMed]
- Lee, D.; Haase, M.; Haase-Fielitz, A.; Paizis, K.; Goehl, H.; Bellomo, R. A Pilot, Randomized, Double-Blind, Cross-Over Study of High Cut-Off versus High-Flux Dialysis Membranes. Blood Purif. 2009, 28, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Atoh, K.; Itoh, H.; Haneda, M. Serum indoxyl sulfate levels in patients with diabetic nephropathy: Relation to renal function. Diabetes Res. Clin. Pract. 2009, 83, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Pillai, A. Chronic stress and complement system in depression. Braz. J. Psychiatry 2022, 44, 366–367. [Google Scholar] [CrossRef] [PubMed]
- Melchior, P.; Erlenkötter, A.; Zawada, A.M.; Delinski, D.; Schall, C.; Stauss-Grabo, M.; Kennedy, J.P. Complement activation by dialysis membranes and its association with secondary membrane formation and surface charge. Artif. Organs 2021, 45, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.Y.; Kim, S.-H.; Kim, Y.O.; Jin, D.C.; Song, H.C.; Choi, E.J.; Kim, Y.-L.; Kim, Y.-S.; Kang, S.-W.; Kim, N.-H.; et al. The impact of blood flow rate during hemodialysis on all-cause mortality. Korean J. Intern. Med. 2016, 31, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Mc Causland, F.R.; Brunelli, S.M.; Waikar, S.S. Dialysis Dose and Intradialytic Hypotension: Results from the HEMO Study. Am. J. Nephrol. 2013, 38, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Filipčič, T.; Bogataj, Š.; Pajek, J.; Pajek, M. Physical Activity and Quality of Life in Hemodialysis Patients and Healthy Controls: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 18, 1978. [Google Scholar] [CrossRef] [PubMed]
- Motedayen, Z.; Nehrir, B.; Tayebi, A.; Ebadi, A.; Eynollahi, B. The Effect of the Physical and Mental Exercises During Hemodialysis on Fatigue: A Controlled Clinical Trial. Nephro-Urol. Mon. 2014, 6, e14686. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.-Y.; Chou, Y.-H.; Lin, Y.-H.; Huang, W.-L. Sleep and emotional disturbance in patients with non-dialysis chronic kidney disease. J. Formos. Med. Assoc. 2019, 118, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, A.; Dervisoglu, E.; Kutlu, A. Sleep quality and its correlates in patients on continuous ambulatory peritoneal dialysis. Scand. J. Urol. Nephrol. 2012, 46, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, H.-Y.; Liu, J.-W.; Pappalardo, M.; Cheng, M.-H. The Impacts of Lymph on the Adipogenesis of Adipose-Derived Stem Cells. Plast. Reconstr. Surg. 2022, 151, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Turkmen, K.; Erdur, F.M.; Guney, I.; Gaipov, A.; Turgut, F.; Altintepe, L.; Saglam, M.; Tonbul, H.Z.; Abdel-Rahman, E.M. Sleep quality, depression, and quality of life in elderly hemodialysis patients. Int. J. Nephrol. Renov. Dis. 2012, 135, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Lopez, T.; Banerjee, D. Management of fluid overload in hemodialysis patients. Kidney Int. 2021, 100, 1170–1173. [Google Scholar] [CrossRef] [PubMed]
- Johansen, K.L.; Shubert, T.; Doyle, J.; Soher, B.; Sakkas, G.K.; Kent-Braun, J.A. Muscle atrophy in patients receiving hemodialysis: Effects on muscle strength, muscle quality, and physical function. Kidney Int. 2003, 63, 291–297. [Google Scholar] [CrossRef]
- El-Assmy, A. Erectile dysfunction in hemodialysis: A systematic review. World J. Nephrol. 2012, 1, 160. [Google Scholar] [CrossRef] [PubMed]
- Asgari, M.R.; Asghari, F.; Ghods, A.A.; Ghorbani, R.; Hoshmand Motlagh, N.; Rahaei, F. Incidence and severity of nausea and vomiting in a group of maintenance hemodialysis patients. J. Ren. Inj. Prev. 2016, 6, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Borawski, J.; Naumnik, B.; Pawlak, K.; Myśliwiec, M. Endothelial dysfunction marker von Willebrand factor antigen in haemodialysis patients: Associations with pre-dialysis blood pressure and the acute phase response. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2001, 16, 1442–1447. [Google Scholar] [CrossRef] [PubMed]
- Health Canada. Overview of the Canadian Healthcare System; Government of Canada: Ottawa, ON, Canda, 2019.
- Conway, J.; Lawn, S.; Crail, S.; McDonald, S. Indigenous patient experiences of returning to country: A qualitative evaluation on the Country Health SA Dialysis bus. BMC Health Serv. Res. 2018, 18, 1010. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Subhan, F.B.; Williams, K.; Chan, C.B. Barriers and Mitigating Strategies to Healthcare Access in Indigenous Communities of Canada: A Narrative Review. Healthcare 2020, 8, 112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Savaşer, S.K.; Kara, B.Y. Mobile healthcare services in rural areas: An application with periodic location routing problem. OR Spectr. 2022, 44, 875–910. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nayak, A.; Karopadi, A.; Antony, S.; Sreepada, S.; Nayak, K. Use of a Peritoneal Dialysis Remote Monitoring System in India. Perit. Dial. Int. 2012, 32, 200–204. [Google Scholar] [CrossRef]
- Yu, X.; Yang, X. Remote Patient Management for Emerging Geographical Areas. Contrib. Nephrol. 2019, 197, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, L.; Kirk, R.; Cuttitta, T.; Bryant, N.; Fox, K.; McCall, M.; Perry, E.; Swartz, J.; Restovic, Y.; Jeter, A.; et al. Remote Management for Peritoneal Dialysis: A Qualitative Study of Patient, Care Partner, and Clinician Perceptions and Priorities in the United States and the United Kingdom. Kidney Med. 2019, 1, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, N.; Suh, H.; Cabralda, T.; Sokol, E.; Sokunbi, D.; Solomon, M. Peritoneal dialysis with trained home nurses in elderly and disabled end-stage renal disease patients. Adv. Perit. Dialysis. Conf. Perit. Dial. 1993, 9, 130–133. [Google Scholar]
- Cram, S. (2 February 2017). CBC News. Available online: https://www.cbc.ca/news/indigenous/indigenous-dialysis-patients-infection-1.3952078 (accessed on 24 May 2023).
- Rodriguez Cuellar, C.I.; García de la Puente, S.; Hernández Moraria, J.; Bojórquez Ochoa, A.; Filler, G.; Zaltzman Grishevich, S. High depression rates among pediatric renal replacement therapy patients: A cross-sectional study. Pediatr. Transplant. 2019, 23, e13591. [Google Scholar] [CrossRef] [PubMed]
- Pai, A.L.; McGrady, M. Systematic Review and Meta-Analysis of Psychological Interventions to Promote Treatment Adherence in Children, Adolescents, and Young Adults with Chronic Illness. J. Pediatr. Psychol. 2014, 39, 918–931. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, A.M.; Richards, C.; Szarka, J.; McFarland, L.V.; Showalter, W.; Vig, E.K.; Sudore, R.L.; Crowley, S.T.; Trivedi, R.; Taylor, J.S. Emotional Impact of Illness and Care on Patients with Advanced Kidney Disease. Clin. J. Am. Soc. Nephrol. 2018, 13, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Laudański, K.; Nowak, Z.; Niemczyk, S. Age-related differences in the quality of life in end-stage renal disease in patients enrolled in hemodialysis or continuous peritoneal dialysis. Med. Sci. Monit. 2013, 19, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Nehus, E.; Mitsnefes, M.M. When to Initiate Dialysis in Children and Adolescents: Is Waiting Worthwhile? Am. J. Kidney Dis. 2019, 73, 762–764. [Google Scholar] [CrossRef] [PubMed]
- Splinter, A.; Tjaden, L.A.; Haverman, L.; Adams, B.; Collard, L.; Cransberg, K.; van Dyck, M.; Van Hoeck, K.J.; Hoppe, B.; Koster-Kamphuis, L.; et al. Children on dialysis as well as renal transplanted children report severely impaired health-related quality of life. Qual. Life Res. 2018, 27, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Eldehni, M.T.; Crowley, L.E.; Selby, N.M. Challenges in Management of Diabetic Patient on Dialysis. Kidney Dial. 2022, 2, 553–564. [Google Scholar] [CrossRef]
- Hofherr, A.; Williams, J.; Gan, L.-M.; Söderberg, M.; Hansen, P.B.; Woollard, K.J. Targeting inflammation for the treatment of Diabetic Kidney Disease: A five-compartment mechanistic model. BMC Nephrol. 2022, 23, 208. [Google Scholar] [CrossRef] [PubMed]
- Pálsson, R.; Patel, U.D. Cardiovascular Complications of Diabetic Kidney Disease. Adv. Chronic Kidney Dis. 2014, 21, 273–280. [Google Scholar] [CrossRef]
- Soleymanian, T.; Kokabeh, Z.; Ramaghi, R.; Mahjoub, A.; Argani, H. Clinical outcomes and quality of life in hemodialysis diabetic patients versus non-diabetics. J. Nephropathol. 2016, 6, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Arnold, R.; Issar, T.; Krishnan, A.V.; Pussell, B.A. Neurological complications in chronic kidney disease. JRSM Cardiovasc. Dis. 2016, 5, 204800401667768. [Google Scholar] [CrossRef] [PubMed]
- Pop-Busui, R.; Roberts, L.; Pennathur, S.; Kretzler, M.; Brosius, F.C.; Feldman, E.L. The Management of Diabetic Neuropathy in CKD. Am. J. Kidney Dis. 2010, 55, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Sasaki, T.; Yamamoto, S.; Hayashi, H.; Ako, S.; Tanaka, Y. Effects of exercise on kidney and physical function in patients with non-dialysis chronic kidney disease: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 18195. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
