Using Drone Footage to Analyze the Effect of Diver Presence on Juvenile Manta Ray Behavior
Highlights
- Diver presence significantly influenced juvenile manta ray behavior in a nursery habitat, leading to an increase in avoidance behaviors and a higher likelihood of having their cephalic fins unfurled.
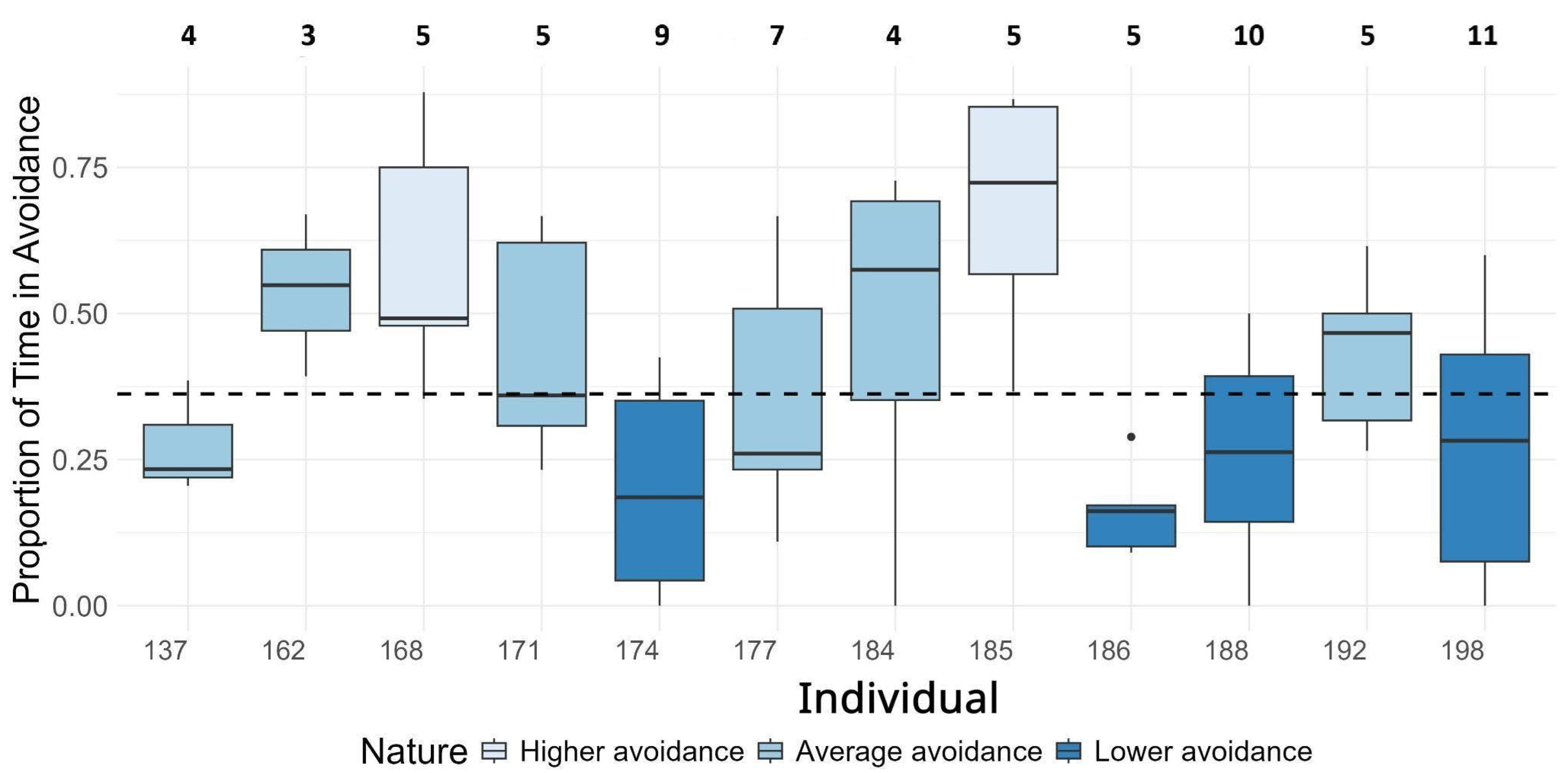
- Individual mantas showed a significant difference in behavioral response to diver presence.
- These findings indicate that marine tourism could have a negative effect on juvenile manta rays by increasing the energetic and time costs of mantas performing avoidance behaviors in the presence of divers and reducing energy acquisition for natural behaviors such as feeding, transiting, and socializing.
- Our results show that quantifying the behavioral responses and energetic costs of juvenile manta rays to human presence is critical for developing sustainable management practices, particularly for populations that may be more vulnerable to the effects of marine tourism activity.
Abstract
1. Introduction
2. Methods
2.1. Study Site
2.2. Video Collection
2.3. Video Analysis
3. Results
Data Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- O’Malley, M.P.; Lee-Brooks, K.; Medd, H.B. The Global Economic Impact of Manta Ray Watching Tourism. PLoS ONE 2013, 8, e65051. [Google Scholar] [CrossRef]
- Healy, T.J.; Hill, N.J.; Barnett, A.; Chin, A. A Global Review of Elasmobranch Tourism Activities, Management and Risk. Mar. Policy 2020, 118, 103964. [Google Scholar] [CrossRef]
- Burgess, G. Diving with Elasmobranchs: A Call for Restraint. Shark News 1998, 11, 1–16. Available online: https://www.iucnssg.org/uploads/5/4/1/2/54120303/sn11_july1998.pdf (accessed on 20 August 2025).
- Pérez Tadeo, M.; Gammell, M.; O’Brien, J. Assessment of Anthropogenic Disturbances Due to Ecotourism on a Grey Seal (Halichoerus Grypus) Colony in the Blasket Islands SAC, Southwest Ireland and Recommendations on Best Practices. Aquat. Mamm. 2021, 47, 268–282. [Google Scholar] [CrossRef]
- Montero-Quintana, A.N.; Vázquez-Haikin, J.A.; Merkling, T.; Blanchard, P.; Osorio-Beristain, M. Ecotourism Impacts on the Behaviour of Whale Sharks: An Experimental Approach. Oryx 2020, 54, 270–275. [Google Scholar] [CrossRef]
- Laute, A.; Grove, T.; Rasmussen, M.; Smith, A.; Loisa, O.; Fournet, M. Impact of Whale-Watching Vessels on Humpback Whale Calling Behavior on an Icelandic Foraging Ground during the Covid-19 Pandemic. Mar. Ecol. Prog. Ser. 2022, 701, 159–173. [Google Scholar] [CrossRef]
- Stack, S.H.; Sprogis, K.R.; Olson, G.L.; Sullivan, F.A.; Machernis, A.F.; Currie, J.J. The Behavioural Impacts of Commercial Swimming with Whale Tours on Humpback Whales (Megaptera novaeangliae) in Hervey Bay, Australia. Front. Mar. Sci. 2021, 8, 696136. [Google Scholar] [CrossRef]
- Kassamali-Fox, A.; Christiansen, F.; May-Collado, L.J.; Ramos, E.A.; Kaplin, B.A. Tour Boats Affect the Activity Patterns of Bottlenose Dolphins (Tursiops Truncatus) in Bocas Del Toro, Panama. PeerJ 2020, 8, e8804. [Google Scholar] [CrossRef]
- Shawky, A.M.; Christiansen, F.; Ormond, R. Effects of Swim-with-dolphin Tourism on the Behaviour of Spinner Dolphins, at Samadai Reef in the Egyptian Red Sea. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 1373–1384. [Google Scholar] [CrossRef]
- Ribaric, D.; Clarkson, J. Nautical Tourism Affects Common Bottlenose Dolphin (Tursiops Truncatus M.) Foraging Success in a NATURA 2000 Site, North-Eastern Adriatic Sea. Mediterr. Mar. Sci. 2021, 22, 285–296. [Google Scholar] [CrossRef]
- Dans, S.L.; Degrati, M.; Pedraza, S.N.; Crespo, E.A. Effects of Tour Boats on Dolphin Activity Examined with Sensitivity Analysis of Markov Chains. Conserv. Biol. 2012, 26, 708–716. [Google Scholar] [CrossRef]
- Lusseau, D. Effects of Tour Boats on the Behavior of Bottlenose Dolphins: Using Markov Chains to Model Anthropogenic Impacts. Conserv. Biol. 2003, 17, 1785–1793. [Google Scholar] [CrossRef]
- Meissner, A.M.; Christiansen, F.; Martinez, E.; Pawley, M.D.M.; Orams, M.B.; Stockin, K.A. Behavioural Effects of Tourism on Oceanic Common Dolphins, Delphinus Sp., in New Zealand: The Effects of Markov Analysis Variations and Current Tour Operator Compliance with Regulations. PLoS ONE 2015, 10, e0116962. [Google Scholar] [CrossRef]
- Gayford, J.H.; Pearse, W.D.; De La Parra Venegas, R.; Whitehead, D.A. Quantifying the Behavioural Consequences of Shark Ecotourism. Sci. Rep. 2023, 13, 12938. [Google Scholar] [CrossRef] [PubMed]
- Villegas-Amtmann, S.; Schwarz, L.K.; Sumich, J.L.; Costa, D.P. A Bioenergetics Model to Evaluate Demographic Consequences of Disturbance in Marine Mammals Applied to Gray Whales. Ecosphere 2015, 6, 1–19. [Google Scholar] [CrossRef]
- Armstrong, A.O.; Stevens, G.M.W.; Townsend, K.A.; Murray, A.; Bennett, M.B.; Armstrong, A.J.; Uribe-Palomino, J.; Hosegood, P.; Dudgeon, C.L.; Richardson, A.J. Reef Manta Rays Forage on Tidally Driven, High Density Zooplankton Patches in Hanifaru Bay, Maldives. PeerJ 2021, 9, e11992. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.; Garrud, E.; Ender, I.; Lee-Brooks, K.; Atkins, R.; Lynam, R.; Arnold, K.; Roberts, C.; Hawkins, J.; Stevens, G. Protecting the Million-Dollar Mantas; Creating an Evidence-Based Code of Conduct for Manta Ray Tourism Interactions. J. Ecotourism 2020, 19, 132–147. [Google Scholar] [CrossRef]
- Setyawan, E.; Erdmann, M.V.; Mambrasar, R.; Ambafen, O.; Hasan, A.W.; Izuan, M.; Mofu, I.; Putra, M.I.H.; Sianipar, A.B.; Constantine, R.; et al. Spatial Connectivity of Reef Manta Rays across the Raja Ampat Archipelago, Indonesia. R. Soc. Open Sci. 2024, 11, 230895. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Pardo, S.A.; Simpfendorfer, C.A.; Carlson, J.K. Diagnosing the Dangerous Demography of Manta Rays Using Life History Theory. PeerJ 2014, 2, e400. [Google Scholar] [CrossRef]
- Venables, S.K.; Rohner, C.A.; Flam, A.L.; Pierce, S.J.; Marshall, A.D. Persistent Declines in Sightings of Manta and Devil Rays (Mobulidae) at a Global Hotspot in Southern Mozambique. Environ. Biol. Fishes 2025, 108, 749–765. [Google Scholar] [CrossRef]
- Stewart, J.D.; Cronin, M.R.; Largacha, E.; Lezama-Ochoa, N.; Lopez, J.; Hall, M.; Hutchinson, M.; Jones, E.G.; Francis, M.; Grande, M.; et al. Get Them off the Deck: Straightforward Interventions Increase Post-Release Survival Rates of Manta and Devil Rays in Tuna Purse Seine Fisheries. Biol. Conserv. 2024, 299, 110794. [Google Scholar] [CrossRef]
- Palacios, M.D.; Weiand, L.; Laglbauer, B.J.L.; Cronin, M.R.; Fowler, S.; Jabado, R.W.; Ko Gyi, T.; Fernando, D.; De Bruyne, G.; Shea, S.K.H.; et al. Global Assessment of Manta and Devil Ray Gill Plate and Meat Trade: Conservation Implications and Opportunities. Environ. Biol. Fishes 2025, 108, 611–638. [Google Scholar] [CrossRef]
- Venables, S.; Winstanley, G.; Bowles, L.; Marshall, A.D. A Giant Opportunity: The Economic Impact of Manta Rays on the Mozambican Tourism Industry—An Incentive for Increased Management and Protection. Tour. Mar. Environ. 2016, 12, 51–68. [Google Scholar] [CrossRef]
- Stewart, J.D.; Stevens, G.M.W.; Marshall, G.J.; Abernathy, K. Are Mantas Self Aware or Simply Social? A Response to Ari and D’Agostino 2016. J. Ethol. 2017, 35, 145–147. [Google Scholar] [CrossRef]
- Perryman, R.J.Y.; Carpenter, M.; Lie, E.; Sofronov, G.; Marshall, A.D.; Brown, C. Reef Manta Ray Cephalic Lobe Movements Are Modulated during Social Interactions. Behav. Ecol. Sociobiol. 2021, 75, 51. [Google Scholar] [CrossRef]
- Gómez-García, M.D.J.; Blázquez-Moreno, M.D.C.; Stewart, J.D.; Leos-Barajas, V.; Fonseca-Ponce, I.A.; Zavala-Jiménez, A.A.; Fuentes, K.; Ketchum, J.T. Quantifying the Effects of Diver Interactions on Manta Ray Behavior at Their Aggregation Sites. Front. Mar. Sci. 2021, 8, 639772. [Google Scholar] [CrossRef]
- Fong, V.; Hoffmann, S.; Pate, J. Using Drones to Assess Volitional Swimming Kinematics of Manta Ray Behaviors in the Wild. Drones 2022, 6, 111. [Google Scholar] [CrossRef]
- Pate, J.; Marshall, A. Urban Manta Rays: Potential Manta Ray Nursery Habitat along a Highly Developed Florida Coastline. Endanger. Species Res. 2020, 43, 51–64. [Google Scholar] [CrossRef]
- Bucair, N.; Hinojosa-Alvarez, S.; Marshall, A.D.; Pate, J.; Francini, C.L.B.; Garrido, A.G.; Capel, K.C.C.; Loboda, T.S.; Monteiro, J.S.; Bruno, C.E.M.; et al. An Integrative Taxonomy Investigation Unravels a Cryptic Species of Mobula Rafinesque, 1810 (Mobulidae, Myliobatiformes), from the Atlantic Ocean. Environ. Biol. Fishes 2025, 108, 1801–1835. [Google Scholar] [CrossRef]
- Pate, J.H. Unpublished Data From Stakeholder Engagement; Marine Megafauna Foundation: West Palm Beach, FL, USA, 2025. [Google Scholar]
- Pate, J.H. Personal Observations During Field Surveying; Marine Megafauna Foundation: West Palm Beach, FL, USA, 2025. [Google Scholar]
- Bourke, E.; Raoult, V.; Williamson, J.E.; Gaston, T.F. Estuary Stingray (Dasyatis Fluviorum) Behaviour Does Not Change in Response to Drone Altitude. Drones 2023, 7, 164. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://www.r-project.org/ (accessed on 1 September 2025).
- Peters, K.J.; Parra, G.J.; Skuza, P.P.; Möller, L.M. First Insights into the Effects of Swim-with-dolphin Tourism on the Behavior, Response, and Group Structure of Southern Australian Bottlenose Dolphins. Mar. Mammal Sci. 2013, 29, E484–E497. [Google Scholar] [CrossRef]
- Carpenter, B.; Gelman, A.; Hoffman, M.D.; Lee, D.; Goodrich, B.; Betancourt, M.; Brubaker, M.; Guo, J.; Li, P.; Riddell, A. Stan: A Probabilistic Programming Language. J. Stat. Softw. 2017, 76, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Zuur, A.F.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; ISBN 978-0-387-87457-9. [Google Scholar]
- Tennessen, J.B.; Holt, M.M.; Ward, E.J.; Hanson, M.B.; Emmons, C.K.; Giles, D.A.; Hogan, J.T. Hidden Markov Models Reveal Temporal Patterns and Sex Differences in Killer Whale Behavior. Sci. Rep. 2019, 9, 14951. [Google Scholar] [CrossRef] [PubMed]
- McElreath, R. Statistical Rethinking: A Bayesian Course with Examples in R and Stan; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2016; ISBN 978-1-4822-5344-3. [Google Scholar]
- Frixione, M.G.; Gómez García, M.D.J.; Gauger, M.F.W. Drone Imaging of Elasmobranchs: Whale Sharks and Golden Cownose Rays Co-Occurrence in a Zooplankton Hot-Spot in Southwestern Sea of Cortez. Food Webs 2020, 24, e00155. [Google Scholar] [CrossRef]
- Butcher, P.; Colefax, A.; Gorkin, R.; Kajiura, S.; López, N.; Mourier, J.; Purcell, C.; Skomal, G.; Tucker, J.; Walsh, A.; et al. The Drone Revolution of Shark Science: A Review. Drones 2021, 5, 8. [Google Scholar] [CrossRef]
- Oleksyn, S.; Tosetto, L.; Raoult, V.; Joyce, K.E.; Williamson, J.E. Going Batty: The Challenges and Opportunities of Using Drones to Monitor the Behaviour and Habitat Use of Rays. Drones 2021, 5, 12. [Google Scholar] [CrossRef]
- Finger, J.S.; Dhellemmes, F.; Guttridge, T.L.; Kurvers, R.H.J.M.; Gruber, S.H.; Krause, J. Rate of Movement of Juvenile Lemon Sharks in a Novel Open Field, Are We Measuring Activity or Reaction to Novelty? Anim. Behav. 2016, 116, 75–82. [Google Scholar] [CrossRef]
- Finger, J.S.; Guttridge, T.L.; Wilson, A.D.M.; Gruber, S.H.; Krause, J. Are Some Sharks More Social than Others? Short- and Long-Term Consistencies in the Social Behavior of Juvenile Lemon Sharks. Behav. Ecol. Sociobiol. 2018, 72, 17. [Google Scholar] [CrossRef]
- Byrnes, E.E.; Brown, C. Individual Personality Differences in P Ort J Ackson Sharks H Eterodontus Portusjacksoni. J. Fish. Biol. 2016, 89, 1142–1157. [Google Scholar] [CrossRef]
- Hernández-Navarro, C.; Elorriaga-Verplancken, F.R.; Galván-Magaña, F.; Valdivia-Anda, G.; Peña, R.; Ketchum, J.T.; Hoyos-Padilla, E.M. Stress Biomarkers in the Giant Manta (Mobula birostris) Associated to Tourism in the Revillagigedo National Park, Mexico. Open J. Vet. Med. 2023, 13, 136–146. [Google Scholar] [CrossRef]
- How to Swim with Manta Rays 2018. Available online: https://swimwithmantas.org/ (accessed on 1 August 2025).
- Pate, J. Evidence of Reproductive and Feeding Habitat for Manta Rays off Florida’s Atlantic Coast. Environ. Biol. Fishes 2025, 108, 691–698. [Google Scholar] [CrossRef]
- Williams, R.; Lusseau, D.; Hammond, P.S. Estimating Relative Energetic Costs of Human Disturbance to Killer Whales (Orcinus orca). Biol. Conserv. 2006, 133, 301–311. [Google Scholar] [CrossRef]
- Domínguez-Sánchez, P.S.; Širović, A.; Fonseca-Ponce, I.A.; Zavala-Jiménez, A.A.; Rubin, R.D.; Kumli, K.R.; Ketchum, J.T.; Galván-Magaña, F.; Wells, R.J.D.; Stewart, J.D. Occupancy of Acoustically Tagged Oceanic Manta Rays, Mobula birostris, in Bahia de Banderas, Mexico. Mar. Biol. 2023, 170, 128. [Google Scholar] [CrossRef]
- Pate, J.H. Unpublished Data from Field Surveys; Marine Megafauna Foundation: West Palm Beach, FL, USA, 2025. [Google Scholar]
- Scacco, U.; Gennari, E.; Di Crescenzo, S.; Fanelli, E. Looking into the Prevalence of Bycatch Juveniles of Critically Endangered Elasmobranchs: A Case Study from Pelagic Longline and Trammel Net Fisheries of the Asinara Gulf (Western Mediterranean). Front. Mar. Sci. 2023, 10, 1303961. [Google Scholar] [CrossRef]
- Stepanuk, J.; Heywood, E.; Lopez, J.; DiGiovanni Jr, R.; Thorne, L. Age-Specific Behavior and Habitat Use in Humpback Whales: Implications for Vessel Strike. Mar. Ecol. Prog. Ser. 2021, 663, 209–222. [Google Scholar] [CrossRef]
- Osada, K. Relationship of Zooplankton Emergence, Manta Ray Abundance and SCUBA Diver Usage in Kona Hawai’i. Master’s Thesis, University of Hawai’i at Hilo, Hilo, HI, USA, 2010. [Google Scholar]
- Meyer, L.; Apps, K.; Bryars, S.; Clarke, T.; Hayden, B.; Pelton, G.; Simes, B.; Vaughan, L.M.; Whitmarsh, S.K.; Huveneers, C. A Multidisciplinary Framework to Assess the Sustainability and Acceptability of Wildlife Tourism Operations. Conserv. Lett. 2021, 14, e12788. [Google Scholar] [CrossRef]

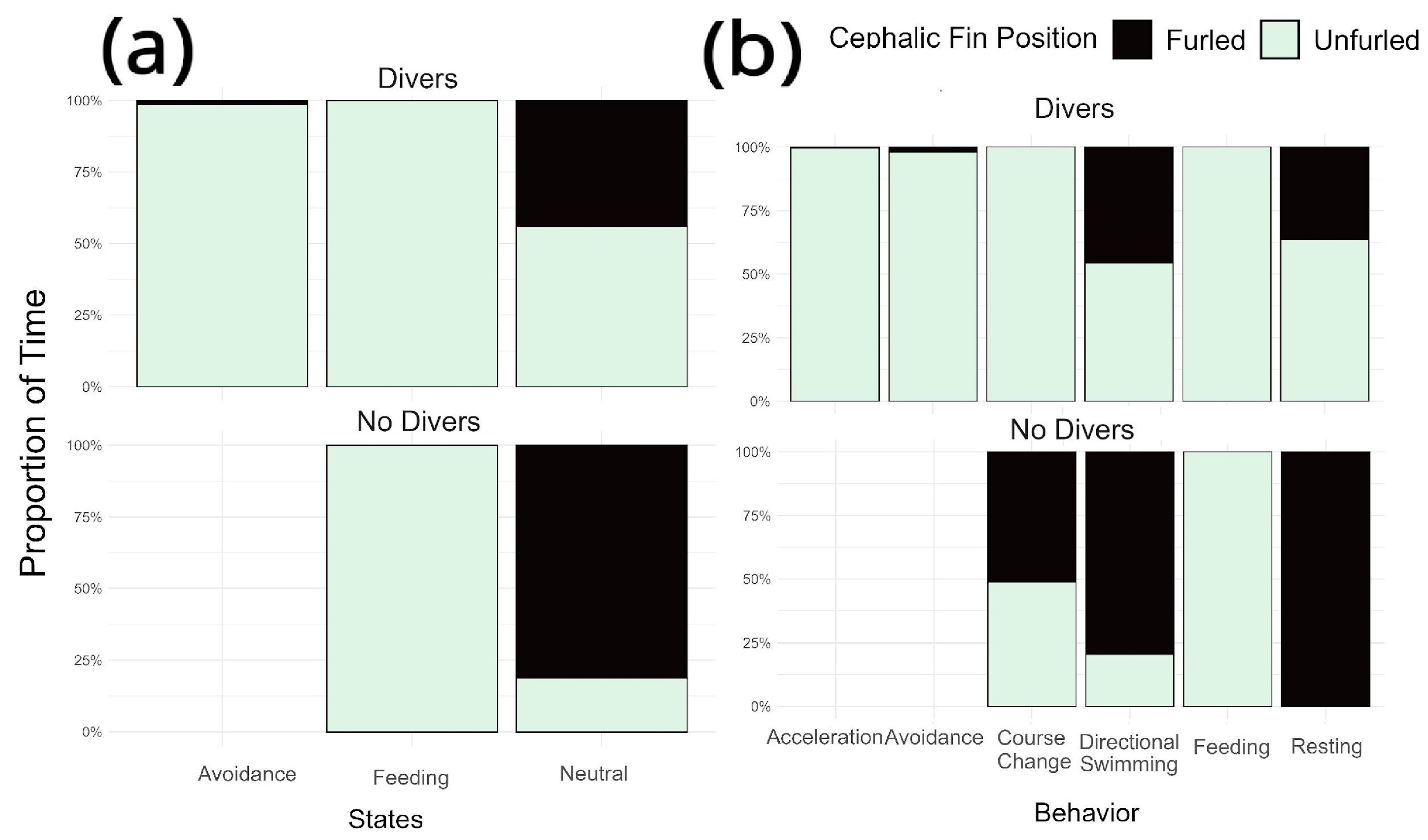
| Behavior | Behavioral State | Description |
|---|---|---|
| 1. Acceleration | Avoidance | The focal manta ray suddenly increases its speed. Speed increase is accompanied by noticeably wider and quicker wingbeats. Or the manta ray performs a single wing beat, leaving its fins curved upwards in an “Omega-like” shape (ω) |
| 2. Defensive/Avoidance movements | Focal manta ray changes direction suddenly in response to a source of stimuli (A diver, fish, another manta ray, a boat, etc.). Sometimes the manta ray can perform a downwards somersault, “avoidance somersault”, exposing its ventral side towards the stimuli and swimming away. | |
| 3. Course Changes | Neutral | Focal manta ray gradually changes its course either in a wide or sharp turn without significantly increasing its current speed or performing sudden movements |
| 4. Directional swimming/traveling | Focal manta ray remains swimming in a straight or semi-straight line. Also called “transiting” or “cruising”. | |
| 5. Resting | Focal manta ray appears to be immobile, typically facing into a current. Pectoral fins are moving slightly. Wingbeat frequency varies. Sometimes the manta ray sinks noticeably due to the lack of horizontal movement. | |
| 6. Feeding | Feeding | Focal manta ray appears to be feeding. Cephalic fins must be unfurled. Manta ray can feed while swimming in a straight line or in circles with frequent turns to remain in the same area or performing backwards somersaults. |
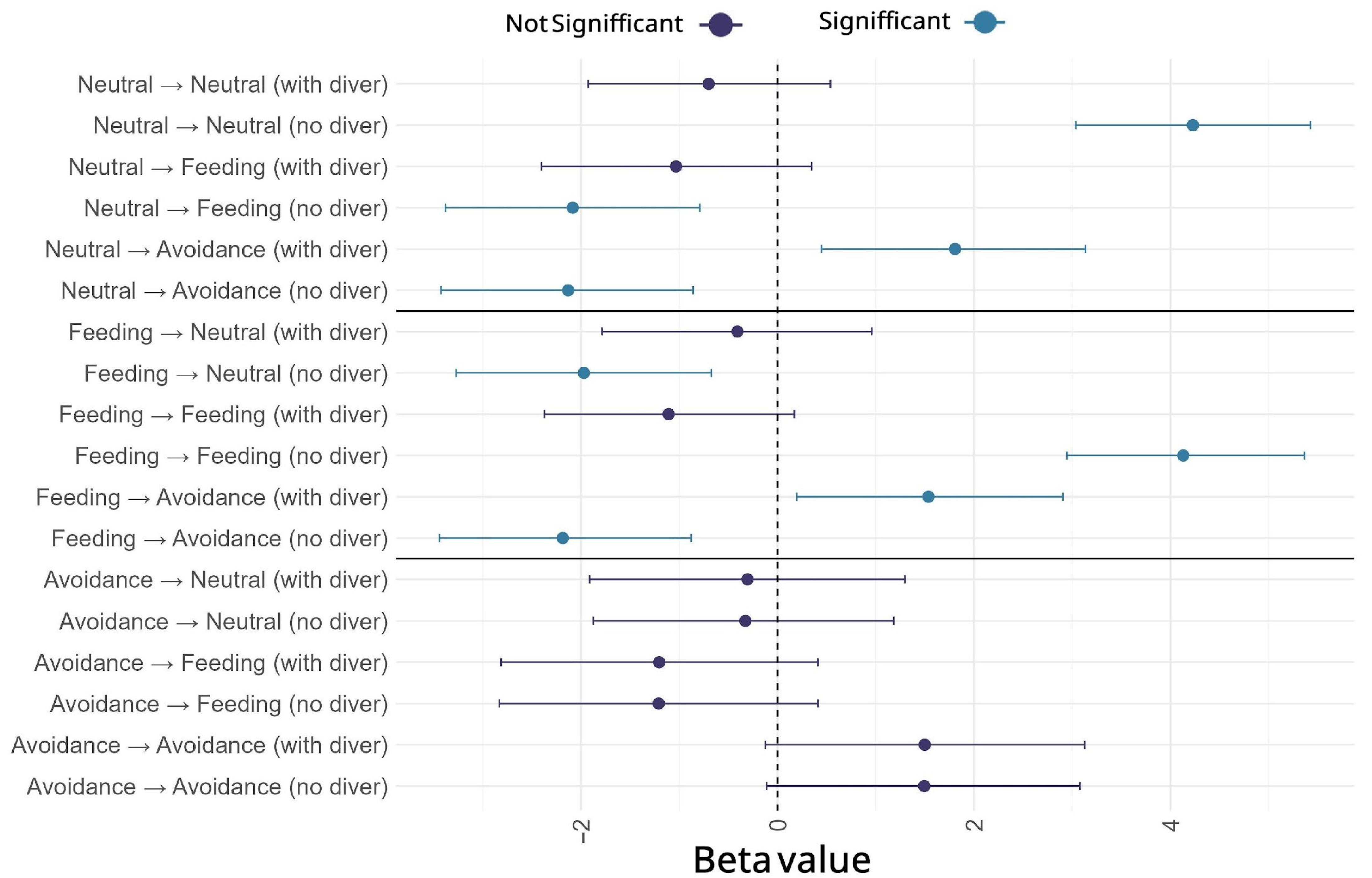
| Response | Predictor | Divers | p |
|---|---|---|---|
| % time | Behavior | No * | <0.01 |
| Yes * | <0.01 | ||
| % time | States | No | 0.62 |
| Yes * | <0.01 | ||
| % time | Lobes | No * | 0.016 |
| Yes * | <0.01 | ||
| % time | Lobes (excluding feeding) | No * Yes * | <0.01 <0.01 |
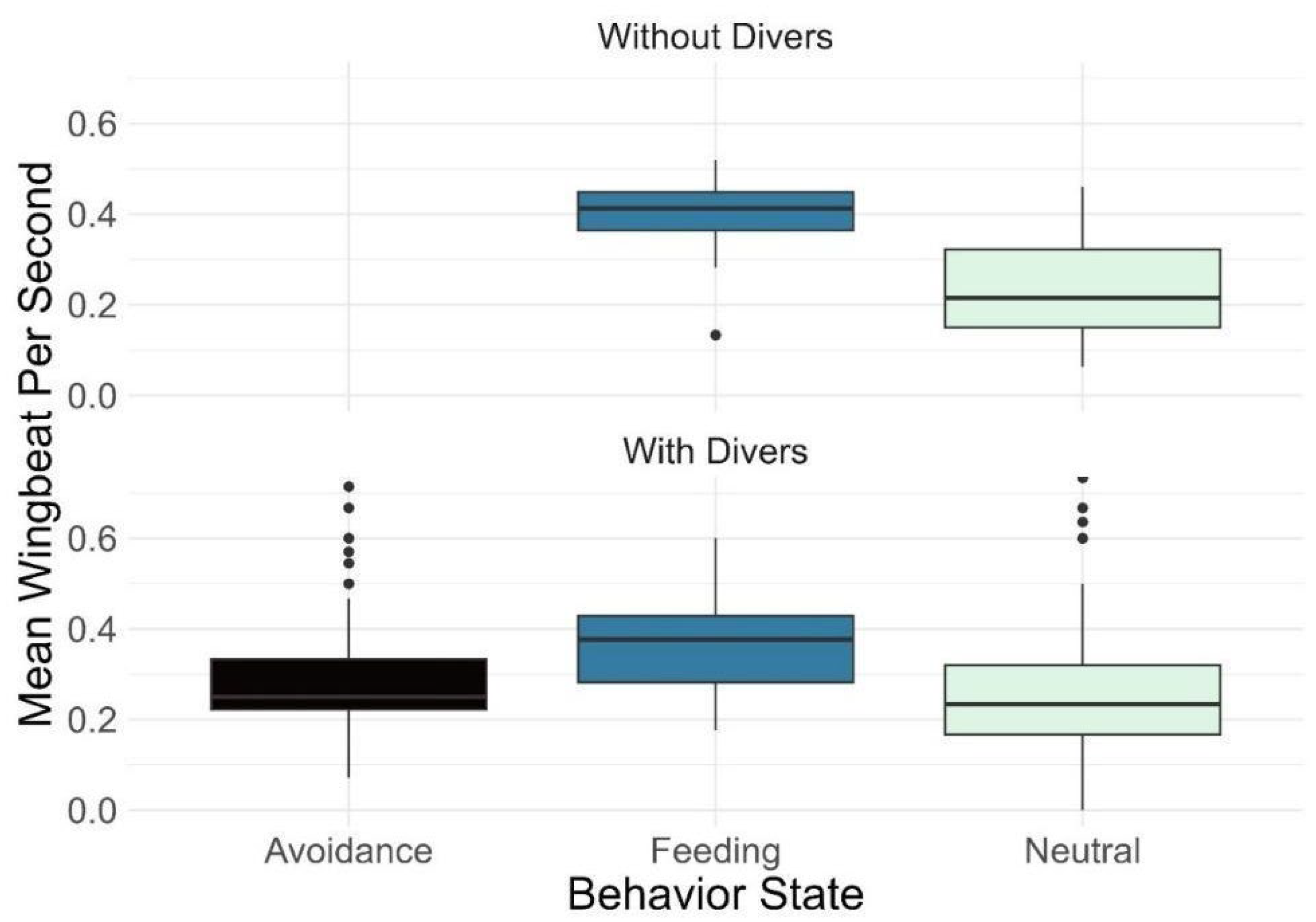
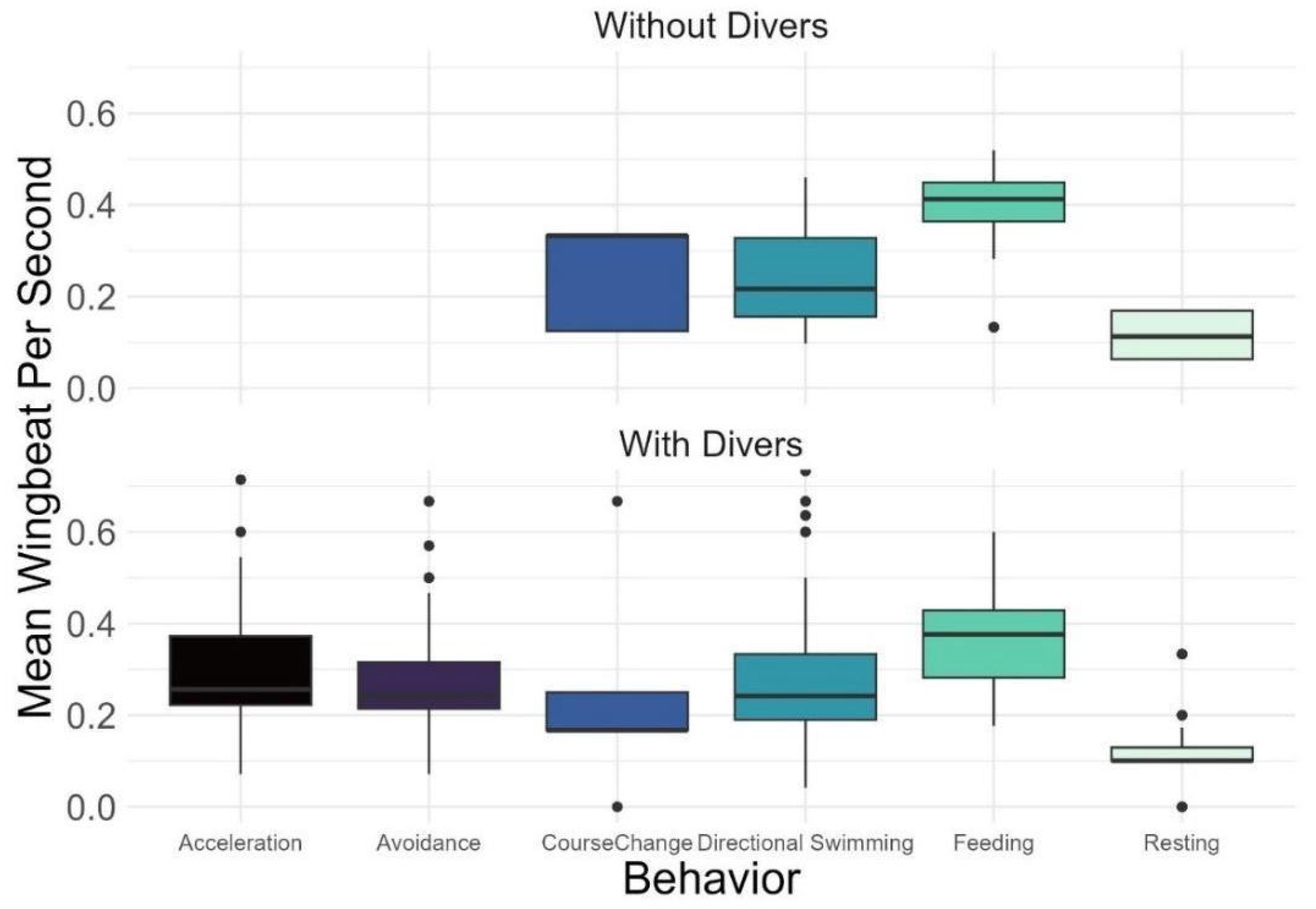
| Wingbeat | States | No * Yes * | <0.01 <0.01 |
| Paired Comparison States | Divers | p |
|---|---|---|
| Neutral-Feeding | No | 0.91 |
| Feeding-Avoidance | Yes * | <0.01 |
| Neutral-Avoidance | Yes | 0.09 |
| Neutral-Feeding | Yes * | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-García, M.d.J.; O’Brien, A.L.; Pate, J.H. Using Drone Footage to Analyze the Effect of Diver Presence on Juvenile Manta Ray Behavior. Drones 2025, 9, 781. https://doi.org/10.3390/drones9110781
Gómez-García MdJ, O’Brien AL, Pate JH. Using Drone Footage to Analyze the Effect of Diver Presence on Juvenile Manta Ray Behavior. Drones. 2025; 9(11):781. https://doi.org/10.3390/drones9110781
Chicago/Turabian StyleGómez-García, Miguel de Jesús, Amanda L. O’Brien, and Jessica H. Pate. 2025. "Using Drone Footage to Analyze the Effect of Diver Presence on Juvenile Manta Ray Behavior" Drones 9, no. 11: 781. https://doi.org/10.3390/drones9110781
APA StyleGómez-García, M. d. J., O’Brien, A. L., & Pate, J. H. (2025). Using Drone Footage to Analyze the Effect of Diver Presence on Juvenile Manta Ray Behavior. Drones, 9(11), 781. https://doi.org/10.3390/drones9110781






