Abstract
The controlled contact between two micro-sized objects, such as beads and cells, and the assessment of their adhesion status is demonstrated in this research. The controlled contact is carried out in a microfluidic channel under flow conditions and makes use of a combination of hydrodynamic traps, flow drag force and dielectrophoretic (DEP) force to maintain the two objects in contact for the desired duration in a first step. Then, the pair objects are separated in the second step in order to explore their adhesion status. Adhesion events are mediated by the bond formed between a receptor and its ligand, and their binding kinetic parameters can be extracted from the measurements using the proposed device.
1. Introduction
The binding between a membrane-bound receptor and its ligand mediates cell–cell adhesion, communication, and cell adaptation to its environment. Failure of the receptors to properly function threatens the homeostasis of the organism, and our understanding of the resulting pathologies relies on our capability to study the associated binding kinetics. Current methods for the controlled contact between two objects, such as atomic force microscopy, optical tweezers, or a dual pipette assay, are typically cumbersome, slow, and leave no hope for scalability [1].
2. Discussion
We thus propose a microfluidic device for the controlled contact between two objects. The operation of the device is illustrated in Figure 1 and starts with the hydrodynamic trapping of the first type of object in custom vertical traps (a) made using an innovative fabrication process [2]. This type of trap is totally transparent and has no effect on other particles once filled with a cell. The second type of object is directed to and trapped in contact with the first object using the DEP force created by the electrodes patterned in their vicinity (b,c) [3]. The forced contact is released by stopping the voltage applied to the electrodes and letting the flow drag the second type of object (d). A receptor–ligand binding event is assessed by observing the adhesion state after the forced contact is released. A scanning electron microscope (SEM) image of the device is shown in Figure 1e.
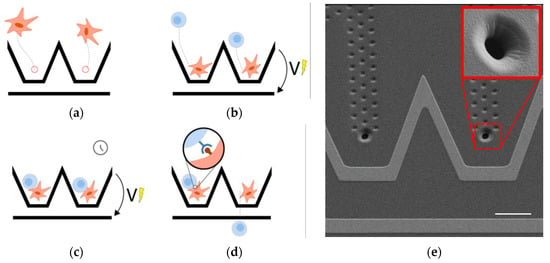
Figure 1.
(a–d) Process for the controlled contact between two cells and the exploration of their adhesion status after a defined, forced contact time. (e) SEM image of the proposed device with the hydrodynamic trap highlighted in the inset and the electrodes used for the DEP manipulation patterned in their vicinity.
The concept is demonstrated by measuring the lifetime of pairs formed by cancer cells and T-cell clones that are TCR-specific to cancer cell peptides, as illustrated in Figure 2.
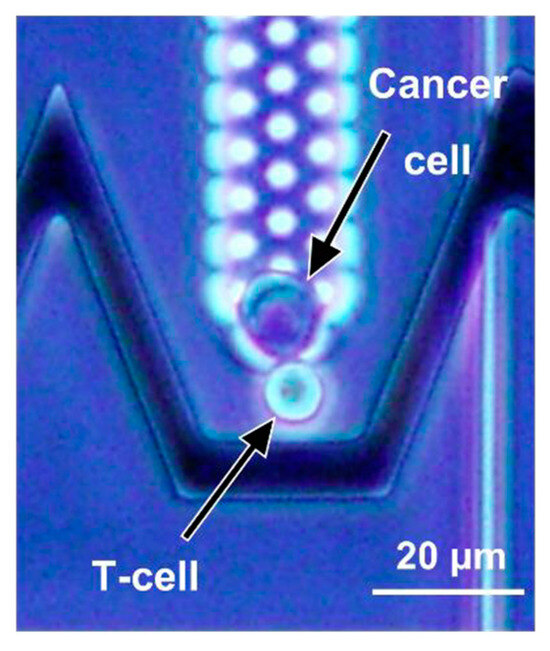
Figure 2.
This microscopy picture represents the controlled contact between a cancer cell (in the hydrodynamic trap) and a T-cell (manipulated by DEP) using the proposed device.
Author Contributions
Conceptualization: C.L., L.K., A.B. (Arnaud Bertsch), A.B. (Aude Bolopion), R.L., C.B. and P.R.; Investigation: C.L., L.K., L.S. and R.L.; Data Curation: C.L. and L.K.; Writing original draft: C.L., L.K. and L.S.; Editing: C.L., L.K., A.B. (Arnaud Bertsch), M.G., A.B. (Aude Bolopion), L.S., R.L., C.B. and P.R.; Supervision: A.B. (Arnaud Bertsch), M.G., A.B. (Aude Bolopion), R.L., C.B. and P.R. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the EIPHI Graduate School under Contract ANR-17-EURE-0002, by the French Agence Nationale de la Recherche and the Swiss National Science Foundation through the CoDiCell project (contract “ANR-17-CE33-0009” and “FNS 00021E_175,592/1”, respectively), and by the French ROBOTEX network and its Micro and Nanorobotics center under Grant ANR-10-EQPX-44-01.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, B.; Chen, W.; Zhu, C. Molecular force spectroscopy on cells. Annu. Rev. Phys. Chem. 2015, 66, 427–451. [Google Scholar] [CrossRef]
- Lipp, C.; Uning, K.; Cottet, J.; Migliozzi, D.; Bertsch, A.; Renaud, P. Planar hydrodynamic traps and buried channels for bead and cell trapping and releasing. Lab Chip 2021, 21, 3686–3694. [Google Scholar] [CrossRef]
- Lipp, C.; Koebel, L.; Bertsch, A.; Gauthier, M.; Bolopion, A.; Renaud, P. Dielectrophoretic Traps for Efficient Bead and Cell Trapping and Formation of Aggregates of Controlled Size and Composition. Front. Bioeng. Biotechnol. 2022, 10, 910578. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).