Multicomponent reactions have attracted significant attention since they are performed without the need to isolate any intermediate during their process; this diminishes the time and saves both energy and raw materials [1,2]. Derivatives of 1-Amidoalkyl-2-naphthol are of importance, as they can be easily converted to biologically active compounds through amide hydrolysis reaction [3]. Recently, various catalysts have been used to prepare these compounds through a multicomponent reaction. In this research, metal–organic frameworks (MOFs) were utilized as heterogeneous catalysts. They are often crystalline solids consisting of metal ions or clusters coordinated to mostly rigid organic linkers such as aromatic polycarboxylates or polyamines to form one-, two-, or three-dimensional porous structures [4]. We report a green and convenient method for the synthesis of 1-amidoalkyl-2-naphthols from the reaction between β-naphthol, aromatic aldehydes, and amide derivatives under microwave irradiation in the presence of Cu2(NH2-BDC)2(DABCO)-Sal-Co(II) as a modified catalyst through a post-synthesis method. (Scheme 1).
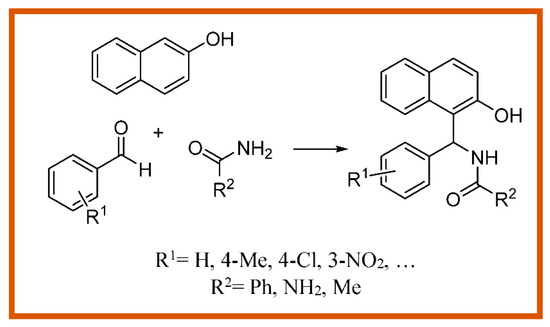
Scheme 1.
Synthesis of 1-Amidoalkyl-2-naphthol derivatives catalyzed by metal–organic frameworks (MOFs).
Cu2(NH2-BDC)2(DABCO) was synthesized and then modified with salicylaldehyde and cobalt acetate (II) salt by the ball-milling technique.
References
- Devi, I.; Bhuyan, P.J. Sodium bromide catalysed one-pot synthesis of tetrahydrobenzo[b]pyrans via a three-component cyclocondensation under microwave irradiation and solvent free conditions. Tetrahedron Lett. 2004, 45, 8625. [Google Scholar] [CrossRef]
- Mashraqui, S.H.; Patil, M.B.; Mistry, H.D. A Three-component Reaction of Phenol, Aldehyde, and Active Methylene Substrate under Lewis acid Catalysis: Successful Trapping of o-Quinone Methide to Afford Benzopyran Systems. Ghadigaonkar, S.; Meetsma, A. Chem. Lett. 2004, 33, 1058. [Google Scholar] [CrossRef]
- Shen, A.Y.; Chen, C.L.; Lin, C.C. Electrophysiological basis for the bradycardic effects of 1-(1-pyrrolidinylmethyl)-2-naphthol in rodents. J. Physiol. 1992, 35, 45. [Google Scholar]
- Cook, T.R.; Zheng, Y.R.; Stang, P.J. Metal–Organic Frameworks and Self-Assembled Supramolecular Coordination Complexes: Comparing and Contrasting the Design, Synthesis, and Functionality of Metal–Organic Materials. Chem. Rev. 2013, 113, 734–777. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).