Carbonated hydroxyapatite (CHAp) is an inorganic compound with various applications that presents better homogeneity and consolidating effect than HAp [1]. The insertion of metallic ions into the structure can improve the physicochemical properties of apatite. In our previous research [2], the inclusion of different dopants (Ag, Sr, Ba, K, Zn) into the apatite structure by replacing the calcium ions was evidenced by a complete characterization of synthesized CHAp substituted with these metallic ions. This study aimed to obtain carbonated hydroxyapatite substituted with magnesium (Mg-CHAp) and to evaluate its consolidation capacity on artificial stone samples.
Mg-CHAp was synthesized by the nanoemulsion method at room temperature, and it was characterized by Fourier transform infrared spectroscopy, X-ray diffraction, and optical microscopy. The effectiveness of Mg-CHAp as a consolidation treatment for artificial stone was assessed in terms of mechanical strength, water absorption, humidity, and chromatic parameters.
The XRD spectrum for carbonated hydroxyapatite substituted with magnesium, presented in Figure 1, highlights the specific apatite structure and Mg presence. The efficiency of the consolidation treatment was influenced by the application method of the consolidating agent, the solution concentration, and the amounts of the absorbed consolidator on the stone surface.
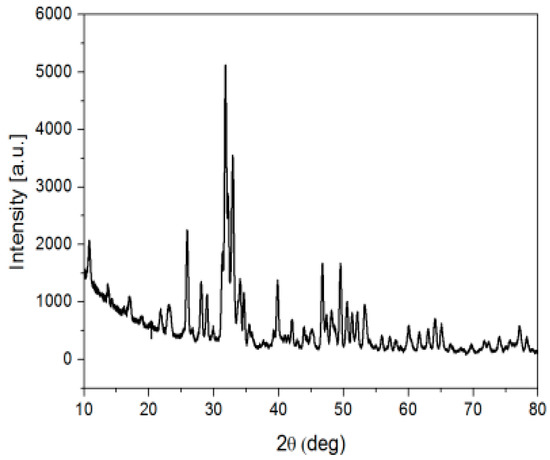
Figure 1.
XRD diffractogram for Mg-CHAp.
Mg-CHAp was successfully synthesized by the nanoemulsion technique. The treatment of the model samples with Mg-CHAp did not influence the chromatic parameters of the artificial stones. The highest values of compressive strength were obtained on the stone samples treated by brushing with a 0.25 g/L concentration of the consolidator.
Acknowledgments
This work was supported by a grant of the Romanian Ministry of Research and Innovation, CCCDI–UEFISCDI, project number PN-III-P1-1.2-PCCDI-2017-0476/51PCCDI/2018, within PNCDI III and PN.19.23.03.01 contract No. 23N/2019 within the Nucleu Program.
References
- Wong, W.; Noor, A.-F.M. Synthesis and sintering-wet carbonation of nano-sized carbonated hydroxyapatite. Procedia Chem. 2016, 19, 98–105. [Google Scholar] [CrossRef][Green Version]
- Ion, R.-M.; Iancu, L.; Vasilievici, G.; Grigore, M.E.; Andrei, E.R.; Radu, G.I.; Grigorescu, R.M.; Teodorescu, S.; Bucurica, I.A.; Ion, M.L. Ion-substituted carbonated hydroxyapatite coatings for model stone samples. Coatings 2019, 9, 231. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).