Nucleosides with a norbornane fragment as sugar moiety (Figure 1) were found to have antiviral and anticancer activity [1]. Previously we obtained new carbocyclic nucleosides with an optically pure bicyclo[2.2.1]heptane fragment with antiviral activity against Influenza viruses and coxsackivirus B4 [2] similar to that of the most active norbornane nucleosides in this class of compounds. Now we present the synthesis of new 1’homocarbanucleosides, molecular docking, antiviral activity and correlation between the docking score and experimental found herpes simplex type-1 virus activity. Synthesis of the compounds started from an optically active intermediate in a sequence of reactions which conducted to a key 6-chloropurine intermediate.
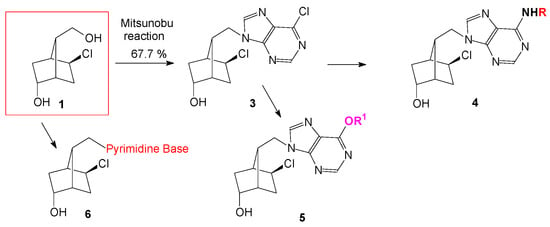
Figure 1.
New carbocyclic nucleosides with a constrained norbornane scaffold as sugar moiety.
Molecular docking was performed on a professional soft CLC Drug Discovery Workbench Software. The protein-ligand complex was realized based on the X-ray structure of herpes simplex type-1 thymidine kinase (TK) in complex with acyclovir (AC2), which was downloaded from the Protein Data Bank (PDB ID: 2KI5). Antiviral activity against adeno-, herpes- and influenza viruses was done at Pasteur Institute of Epidemiology and Microbiology, Department of Virology, St. Petersburg, Russia and against enterovirus 71 (EV71), yellow fever and Chikungunya viruses, at Rega Institute, Laboratory of Virology and Chemotherapy, Leuven-Belgium, using published procedures. A total of 18 compounds were synthesized, fully characterized, and tested for their antiviral activity. Seven other synthesized compounds were not yet tested. A molecular docking study and the correlation between the experimental and predicted data were realized. Two compounds (6j and 6d) had lower IC50 (15 ± 2 and 21 ± 4 µM) and one compound had IC50 similar to that of acyclovir (28 ± 4 µM) in experimental activity against herpes simplex type-1 virus [3] .
Acknowledgments
The funds were provided by PN 19-41 01 03.
References
- Sala, M.; Palma, A.; Hrebabecky, H.; Dejmek, M.; Dracinsky, M.; Leysen, P.; Neyts, J.; Mertlikova-Kaiserova, H.; Nencka, R. SAR studies of 9-norbornylpurines as Coxsackievirus B3 inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 4271–4275. [Google Scholar] [CrossRef] [PubMed]
- Tănase, C.; Drăghici, C.; Cojocaru, A.; Galochkina, A.V.; Orshanskaya, J.R.; Zarubaev, V.V.; Shova, S.; Enache, C.; Maganu, M. New carbocyclic N6-substituted adenine and pyrimidine nucleoside analogues with a bicyclo[2.2.1]heptane fragment as sugar moiety; synthesis, antiviral, anticancer activity and X-ray crystallography. Bioorg. Med. Chem. 2015, 23, 6246–6254. [Google Scholar] [CrossRef] [PubMed]
- Tănase, C.; Drăghici, C.; Hanganu, A.; Pintilie, L.; Maganu, M.; Volobueva, A.; Sinegubova, E.; Zarubaev, V.V.; Neyts, J.; Jochmans, D.; et al. New HSV-1 Anti-Viral 1′-Homocarbocyclic Nucleoside Analogs with an Optically Active Substituted Bicyclo[2.2.1]Heptane Fragment as a Glycoside Moiety. Molecules 2019, 24, 2446. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).