The aim of this article is the study of the fragmentation reactions of octaethoxycyclotetrasiloxane (CTET) initiated by electronic impact in the ionization chamber of a double focusing mass spectrometer. Octaethoxycyclotetrasiloxane as tetraethoxysilane (TEOS) tetramer, with structural formula (C2H5O)8Si4O4 and molecular weight M = 536, is obtained in sol-gel process by hydrolysis–condensation reactions. The stereochemistry-optimized formula of CTET by MOPAC 7 program (computer program used in computational chemistry) is shown in Figure 1.
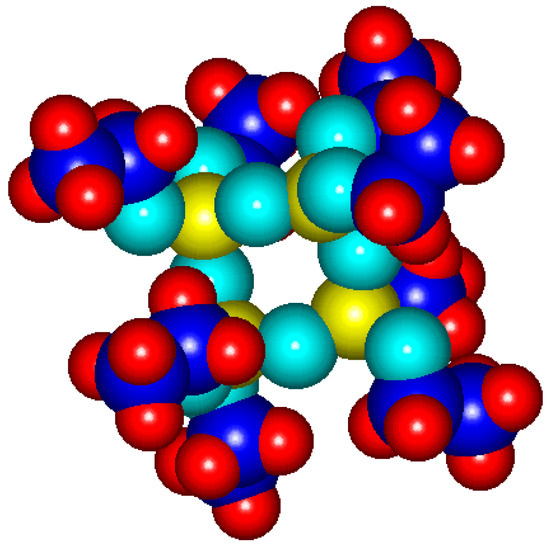
Figure 1.
Legend: Si: yellow, O: cyan, C: blue, and H: red.
Mass spectrum of an organic substance such as octaethoxycyclotetrasiloxane obtained in sol-gel process is the result of a series of unimolecular consecutive and competitive chemical reactions, which constitutes a pattern of fragmentation (Figure 2a,b; integral spectrum and 250–540 amu, respectively).
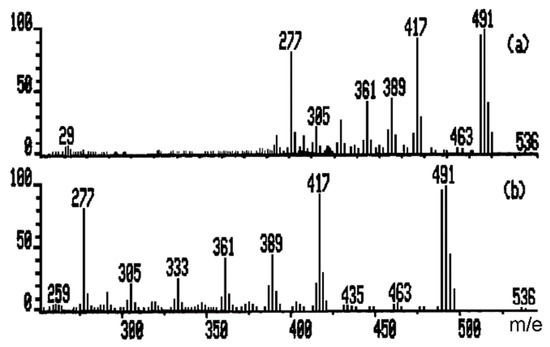
Figure 2.
Mass spectrum of octaethoxycyclotetrasiloxane (integral spectrum and 250–540 amu).
The experimental data for this paper were obtained on a GC-MS tandem produced by VG-Analytical, England. The working conditions for 70-SE, VG Analytical double focusing mass spectrometer are as follows. B/E linked scan: This method of scanning allows for obtaining daughter ions m2+ from a preset precursor ion m1+; (B/E)(1-E)1/2 linked scan: It is used to obtain ions which lose small molecules with a preset mass (e.g., ethanol, ethylene, water, etc.).
The TEOS tetramer fraction, separated and identified by GC-MS, is at the center of the oligomer distribution of the reaction mixture TEOS:H2O:EtOH in acid catalysis; these molecular species have high stability and are very important for the characterization of the sol-gel process in the transition from solution to gel.
The reaction pathway from m/e 536 to m/e 277 in the mass spectrum of the CTET can be highlighted by linked scans. Figure 3 shows the linked scan B/E(1-E)1/2 for the elimination of ethylene (Figure 3a) and ethanol (Figure 3b) and the linked scan B/E for the daughter ions of the ions with m/e 536 (Figure 3c), m/e 463 (Figure 3d), m/e 435 (Figure 3e), m/e 417 (Figure 3f), and m/e 333 (Figure 3g) for successive eliminations of ethylene. Based on experimental data mentioned above, we can write the reactions for obtaining the fragmentation ion m/e 277 according to equations presented in Figure 4.
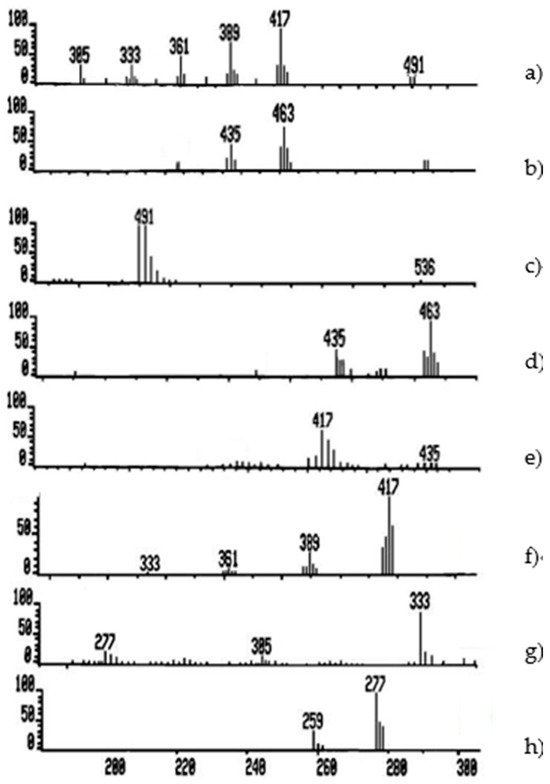
Figure 3.
The linked scan B/E(1-E)1/2 for the elimination of ethylene (a) and ethanol (b) and the linked scan B/E for the daughter ions (c–h).
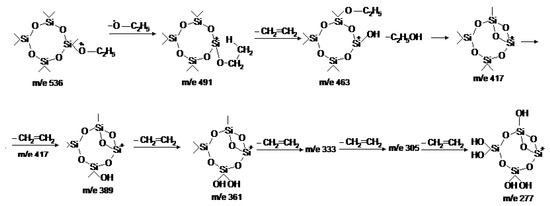
Figure 4.
The reactions for obtaining the fragmentation ion m/e 277 from molecular ion at m/e 536.
The m/e ion 277 with the ionic formula H5O10Si4 is characteristic ion for bicyclic, cyclic, branched, cyclic, branched, and linear tetramers and can be used to identify and measure the TEOS tetramer fraction. The existence of this ion is argued by the M+1 and M+2 isotopic effects for the molecular species of tetramers of TEOS [1,2,3]. There is a good agreement between the experimental values for the ion with m/e 277 and intensity 76.0% (14,3% and 11.8%) in the mass spectrum of CTET and those calculated theoretically by MS Interpreter software (15.5% and 12.6%) [4].
The reaction path from m/e 536 to m/e 277 in the mass spectrum of the CTET is obtained experimentally by the B/E and B/E(1-E)1/2 linked scans. Thus, there can be written the fragmentation pathways for the primary event (ethoxy group removal) and successive eliminations of six ethylene and ethanol. The existence of this ion is confirmed by the M+1 and M+2 isotopic effects.
Acknowledgments
This work was supported by a NUCLEU Program, conducted with MCI support, project number P.N.19.23.01.03.
References
- Badescu, V. Contributions to the interpretation of mass spectrum of hexaethoxydisiloxane. Linked scans and M+1, M+2 isotopic effects. Rev. Chim. (Bucharest), accepted.
- Badescu, V. Contributions to the interpretation of mass spectrum of tetraethoxysilane (TEOS). Part II: Charge induced reactions. Eliminations of neutral fragments. Rev. Roum. Chim. 2015, 60, 1107–1115. [Google Scholar]
- Badescu, V. Contributions to the interpretation of mass spectrum of tetraethoxysilane (TEOS). Part I: The molecular ion. Primary events. Rev. Roum. Chim. 2014, 59, 875–882. [Google Scholar]
- Badescu, V. Reactivita Teaalcoxizilor Studiata Prinreactiiionice in Fazagazoasa. Ph.D. Thesis, Roumanian Academy, Bucharest, Romania, 1998. [Google Scholar]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).