Entropy in Multiple Equilibria, Systems with Two Different Sites †
Abstract
1. Introduction
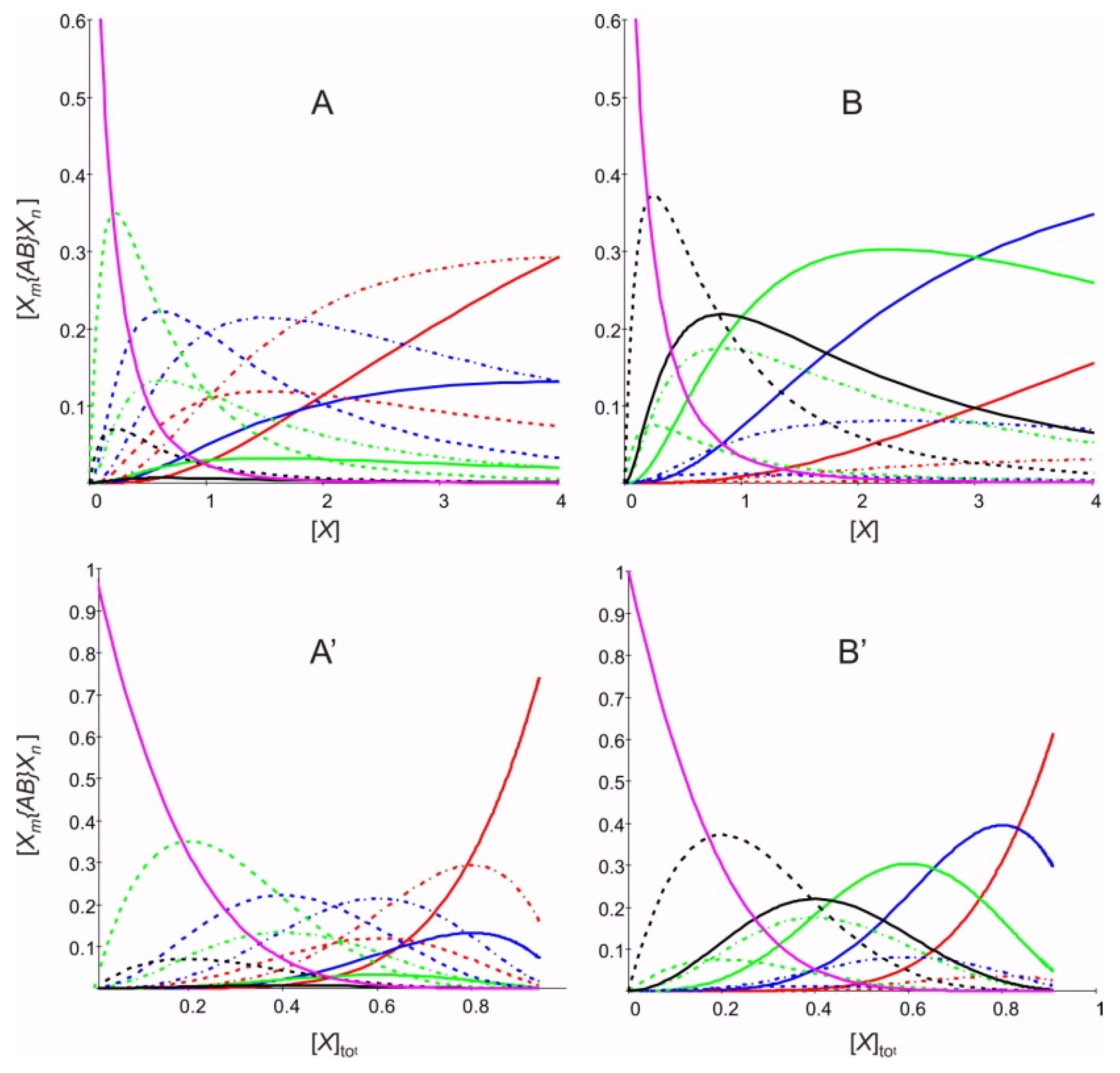
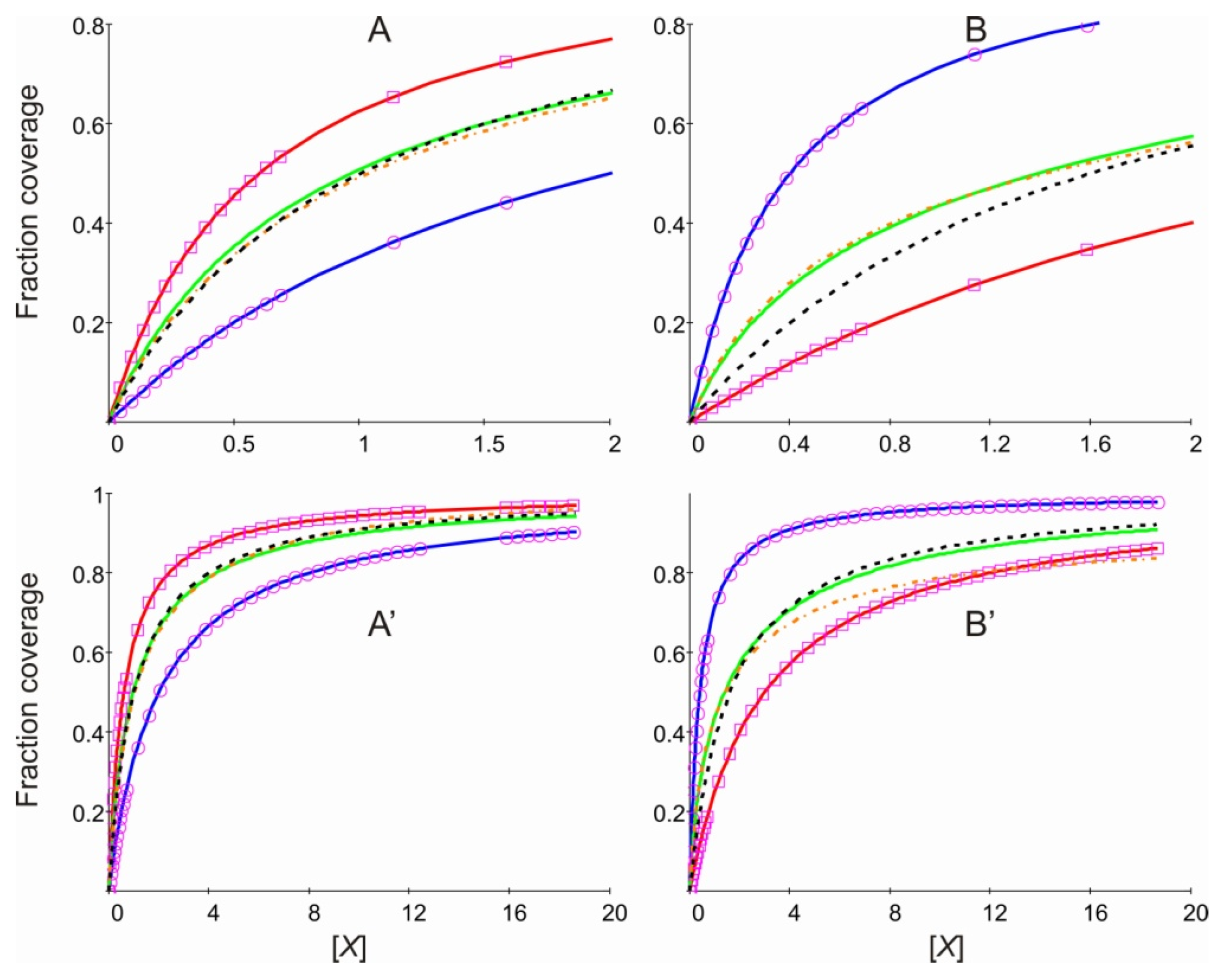
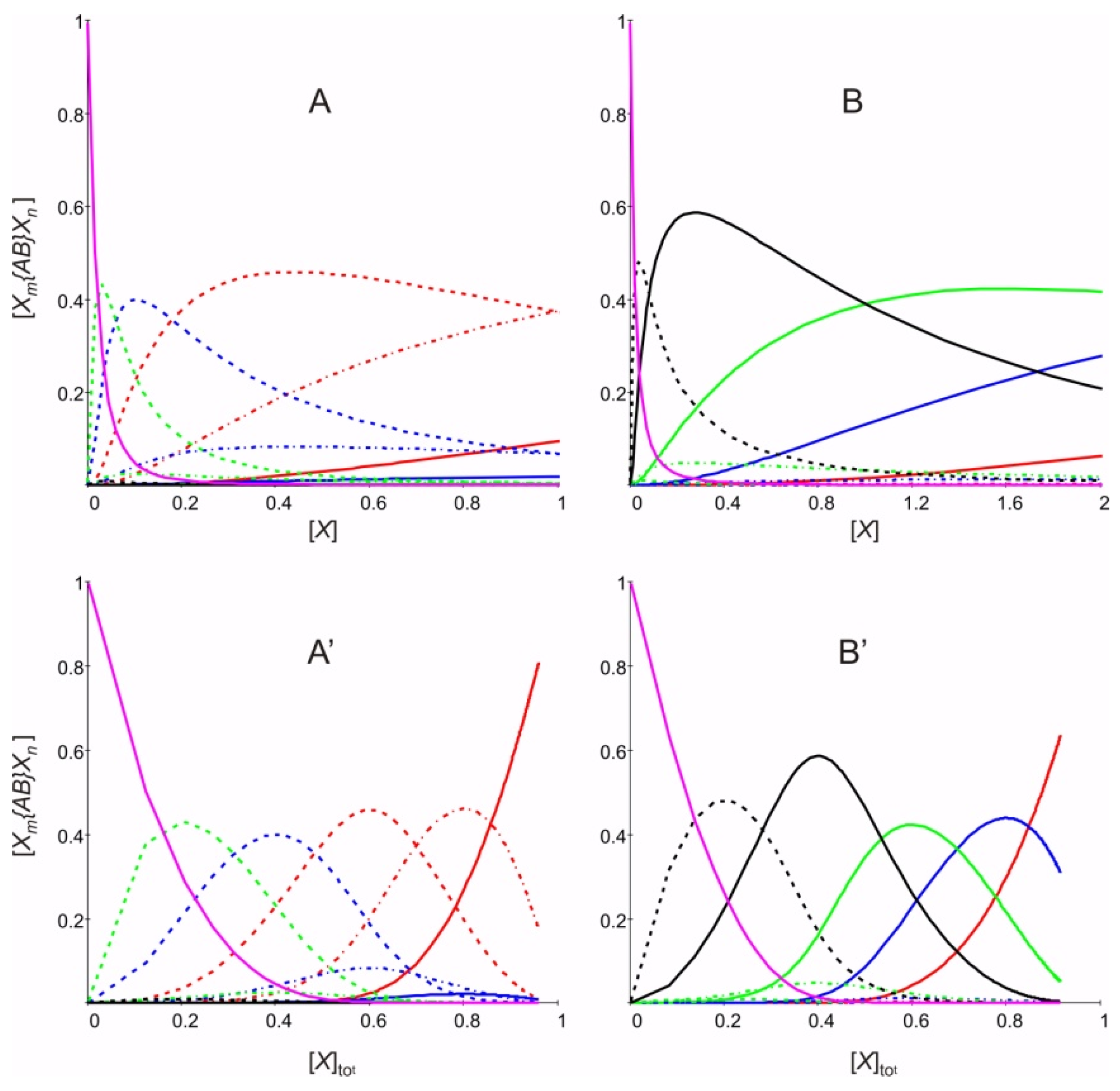
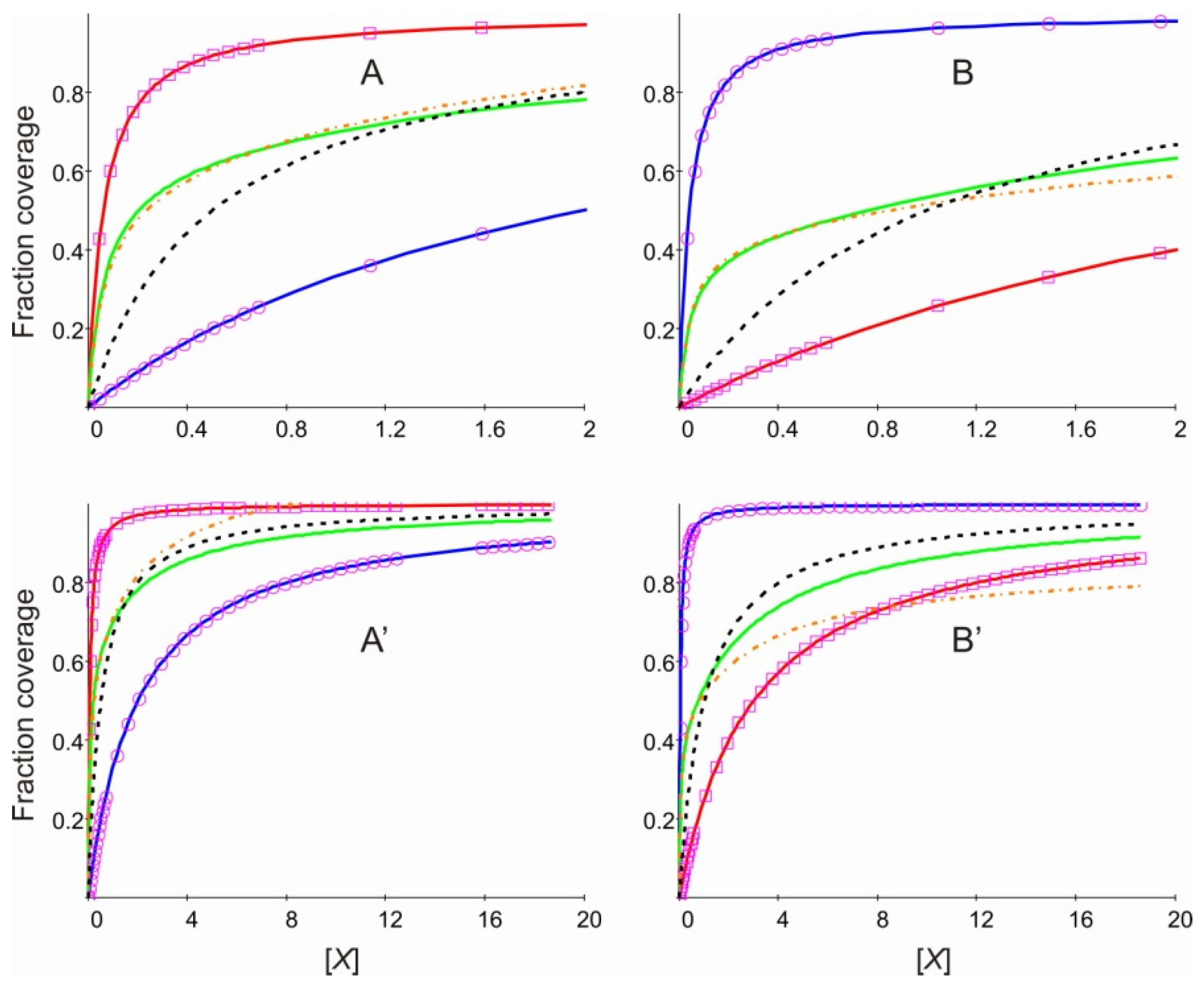
2. Results and Discussion
Conflicts of Interest
Appendix A
| C1 = | = | 5 K1 K2 K3 K10 K11 | |
| C2 = | = | 4 K1 K2 K10 K11 | |
| C3 = | = | 3 K1 K10 K11 | |
| C4 = | = | 4 K4 K5 K6 K10 | |
| C5 = | = | 3 K4 K5 K10 | |
| C6 = | = | 2 K4 K10 | |
| C7 = | = | 3 K7 K8 K9 | |
| C8 = | = | 2 K7 K8 | |
| C9 = | = | K7 | |
| C10 = | = | 2 K10 K11 | |
| C11 = | = | K10 |
References
- Calzaferri, G. Entropy in multiple equilibria, theory and applications. Phys. Chem. Chem. Phys. 2017, 19, 10611–10621. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Denbigh, K. The Principles of Chemical Equilibrium, 2rd ed.; Cambridge University Press: Cambridge, UK, 1966. [Google Scholar]
- Tabacchi, G. Supramolecular organization and confined nanospaces. Chem. Phys. Chem. 2018, in press. [Google Scholar] [CrossRef]
- Barrer, R.M. Zeolites and Clay Minerals as Sorbents and Molecular Sieves; Academic: London, UK, 1978; ISBN 0-12-079350-4. [Google Scholar]
- Nagaj, J.; Stokowa-Sołtys, K.; Kurowska, E.; Frączyk, T.; Jeżowska-Bojczuk, M.; Bal, W. Revised coordination model and stability constants of Cu (II) complexes of tris buffer. Inorg. Chem. 2013, 52, 13927–13933. [Google Scholar] [CrossRef]
- David, C.; Companys, E.; Galceran, J.; Garcés, J.L.; Mas, F.; Rey-Castro, C.; Salvador, J.; Puy, J. Competitive Cd2+/H+ complexation to polyacrylic acid described by the stepwise and intrinsic stability constants. J. Phys. Chem. 2008, 112, 10092–10100. [Google Scholar] [CrossRef]
- Prigogine, I. Thermodynamics of Irreversible Processes, 3rd ed.; Wiley & Sons: Hoboken, NJ, USA, 1967. [Google Scholar]
- Dubler, T.; Maissen, C.; Calzaferri, G. Diskussion Chemischer Gleichgewichte. Z. Naturforsch. 1976, 31, 569–579. [Google Scholar] [CrossRef]
- Breck, D.W. Zeolite Molecular Sieves; John Wiley: New York, NY, USA, 1974; ISBN 0-471-09985-6. [Google Scholar]
- Schoonheydt, R.A.; Weckhuysen, B.M. Editorial Highlight: Molecules in confined spaces. Phys. Chem. Chem. Phys. 2009, 11, 2794–2798. [Google Scholar] [CrossRef]
- Schulz-Ekloff, G.; Wöhrle, D.; van Duffel, B.; Schoonheydt, R.A. Chromophores in porous silicas and minerals: Preparation and optical properties. Microporous Mesoporous Mater. 2002, 51, 91–138. [Google Scholar] [CrossRef]
- Craciun, G.; Feinberg, M. Multiple Equilibria in Complex Chemical Reaction Networks: Semiopen Mass Action Systems. SIAM J. Appl. Math. 2010, 70, 1859–1877. [Google Scholar] [CrossRef]
- Roduner, E.; Radhakrishnan, S.G. In command of non-equilibrium. Chem. Soc. Rev. 2016, 45, 2768–2784. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Leiggener, C.; Calzaferri, G. Particle distribution in a microporous material: Experiments with zeolite A. Chem. Phys. Chem. 2005, 6, 1071–1080. [Google Scholar] [CrossRef]
- Calzaferri, G.; Huber, S.; Maas, H.; Minkowski, C. Host-guest antenna materials. Angew. Chem. Int. Ed. 2003, 42, 3732–3758. [Google Scholar] [CrossRef] [PubMed]
- Valtchev, V.; Mintova, S.; Tsapatsis, M. (Eds.) Ordered Porous Solids, 1st ed.; Elsevier: Oxford, UK, 2009; ISBN 978-0-444-53189-6. [Google Scholar]
- Sola-Llano, R.; Martínez-Martínez, V.; Fujita, Y.; Hortigüela, L.G.; Alfayate, A.; Uji-I, H.; Fron, E.; Pérez-Pariente, J.; López-Arbeloa, I. Formation of a nonlinear optical host-guest hybrid material by tight confinement of LDS 722 into aluminophosphate 1D nanochannels. Chem. Eur. J. 2016, 22, 15700–15711. [Google Scholar] [CrossRef] [PubMed]
- Al-Aqar, R.; Atahan, A.; Benniston, A.C.; Perks, T.; Waddell, P.G.; Harriman, A. Exciton Migration and Surface Trapping for a Photonic Crystal Displaying Charge-Recombination Fluorescence. Chem. Eur. J. 2016, 22, 15420–15429. [Google Scholar] [CrossRef]
- Ramamurthy, V.; Inoue, Y. (Eds.) Supramolecular Photochemistry: Controlling Photochemical Processes; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 978-0-470-23053-4. [Google Scholar]
- Tabacchi, G.; Fois, E.; Calzaferri, G. Structure of Nanochannel Entrances in Stopcock-Functionalized Zeolite L Composites. Angew. Chem. Int. Ed. 2015, 54, 11112–11116. [Google Scholar] [CrossRef]
- Tabacchi, G.; Calzaferri, G.; Fois, E. One-dimensional self-assembly of perylene-diimide dyes by unidirectional transit of zeolite channel openings. Chem. Commun. 2016, 52, 11195–11198. [Google Scholar] [CrossRef]
- Cejka, J.; van Bekkum, H.; Corma, A.; Schüth, F. (Eds.) Studies in Surface Science and Catalysis, Vol. 168: Introduction to Zeolite Science and Practice, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 978-0-444-53063-9. [Google Scholar]
- Gigli, L.; Arletti, R.; Vitillo, J.G.; Alberto, G.; Martra, G.; Devaux, A.; Vezzalini, G. Thionine dye confined in zeolite L: synthesis location and optical properties. J. Phys. Chem. C 2015, 119, 16156–16165. [Google Scholar] [CrossRef][Green Version]
- Getman, R.B.; Bae, J.S.; Wilmer, C.E.; Snurr, R.Q. Review and analysis of molecular simulations of methane, hydrogen, and acetylene storage in metal–organic frameworks. Chem. Rev. 2012, 112, 703–723. [Google Scholar] [CrossRef]
- Lencione, D.; Gehlen, M.H.; Trujillo, L.N.; Leitao, R.C.F.; Albuquerque, R.Q. The spatial distribution of the photostability of thionine in zeolite L nanochannels investigated by Photobleaching Lifetime Imaging Microscopy. Photochem. Photobiol. Sci. 2016, 15, 398–404. [Google Scholar] [CrossRef]
- Bussemer, B.; Dreiling, I.; Grummt, U.-W.; Mohr, G.J. Spectroscopic and quantum chemical study of the Brønsted acid sites in zeolite L channels with acidochromic cyanine dyes. J. Photochem. Photobiol. A Chem. 2009, 204, 90–96. [Google Scholar] [CrossRef]
- Woodtli, P.; Giger, S.; Müller, P.; Sägesser, L.; Zucchetto, N.; Reber, M.J.; Ecker, A.; Brühwiler, D. Indigo in the nanochannels of zeolite L: Towards a new type of colorant. Dyes Pigment. 2017. [Google Scholar] [CrossRef]
- Kunzmann, A.; Seifert, R.; Calzaferri, G. Particle distribution in a microporous material. J. Phys. Chem B 1999, 103, 18–26. [Google Scholar] [CrossRef]
- Van Speybroeck, V.; Hemelsoet, K.; Joos, L.; Waroquier, M.; Bell, R.G.; Catlow, C.R.A. Advances in theory and their application within the field of zeolite chemistry. Chem. Soc. Rev. 2015, 44, 7044–7111. [Google Scholar] [CrossRef]
- Gigli1, L.; Arletti, R.; Tabacchi, G.; Fabbiani, M.; Vitillo, J.G.; Gianmario, M.; Devaux, A.; Miletto, I.; Quartieri, S.; Calzaferri, G. Structure and Host-Guest Interactions of Perylene-Bisimide Dyes in Zeolite L Nanochannels. J. Phys. Chem. C in press. 2018. [Google Scholar] [CrossRef]
| K3 | |
| K2 | |
| K1 | |
| K6 | |
| K5 | |
| K4 | |
| K9 | |
| K8 | |
| K7 | |
| K11 | |
| K10 |
| C1 = | = | 5 K1 K2 K3 K10 K11 | |
| C2 = | = | 4 K1 K2 K10 K11 | |
| C3 = | = | 3 K1 K10 K11 | |
| C4 = | = | 4 K4 K5 K6 K10 | |
| C5 = | = | 3 K4 K5 K10 | |
| C6 = | = | 2 K4 K10 | |
| C7 = | = | 3 K7 K8 K9 | |
| C8 = | = | 2 K7 K8 | |
| C9 = | = | K7 | |
| C10 = | = | 2 K10 K11 | |
| C11 = | = | K10 | |
| C12 = | = |
| K1 | K2 | K3 | K4 | K5 | K6 | K7 | K8 | K9 | K10 | K11 |
|---|---|---|---|---|---|---|---|---|---|---|
| K5 | K6 | K1 | K2 | K3 | K1 | K2 | K3 | |||
| K8 | K9 | |||||||||
| f(3,2)K1 | f(3,1)f(3,2)K1 | f(2,1)K10 | ||||||||
| K1 | K1 | K1 | K1 | K1 | K1 | K1 | K1 | K10 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calzaferri, G. Entropy in Multiple Equilibria, Systems with Two Different Sites. Proceedings 2018, 2, 168. https://doi.org/10.3390/ecea-4-05019
Calzaferri G. Entropy in Multiple Equilibria, Systems with Two Different Sites. Proceedings. 2018; 2(4):168. https://doi.org/10.3390/ecea-4-05019
Chicago/Turabian StyleCalzaferri, Gion. 2018. "Entropy in Multiple Equilibria, Systems with Two Different Sites" Proceedings 2, no. 4: 168. https://doi.org/10.3390/ecea-4-05019
APA StyleCalzaferri, G. (2018). Entropy in Multiple Equilibria, Systems with Two Different Sites. Proceedings, 2(4), 168. https://doi.org/10.3390/ecea-4-05019





