Abstract
Increased neopterin levels reflect the activation of the cellular immune system which is of importance in the pathogenesis and progression of various diseases such as cancer. However, the effects of neopterin on the biological activity of Hepatocellular carcinoma cells are not yet illuminated. In this study we aim to find the effects of neopterin on viability/cytotxicity and motility of five different HCC cell lines with MTT, SRB and wound healing assays. According to our results, HCC cell lines can be grouped into two categories based on their proliferative and motility response to neopterin, as sensitive and resistant. This data provides us with variable results concerning the proliferation and motility of the cell lines under the effect of neopterin. The underlying molecular mechanisms will be examined in further studies, with a focus on the effect of neopterin on the signalling pathways. Overall, neopterin might have an important role in the hepatocarcinogenesis.
1. Introduction
Neopterin (6-d-erythrotrihydroxypropyl-pterin) is a low molecular mass metabolite of guanosine triphosphate, produced and secreted from human monocyte-derived macrophages and dendritic cells upon stimulation [1]. Neopterin (NP) is a well-established marker for cellular immune activation, and its concentration in plasma closely correlates with the amount of oxidative stress originated from the immune system in some disease like HIV infection, autoimmune diseases and malignancies [2]. Increased NP levels reflect the activation of the cellular immune system which is of importance in the pathogenesis and progression of Hepatocellular Carcinoma (HCC) [3,4]. HCC, known as one of the most common and aggressive cancers among humans, is the fifth most common cancer and the third leading cause of cancer deaths worldwide. Thus, a significant focus must be dedicated to study this disease with the purpose of developing more efficient and effective treatments. Some studies showed that the concentrations of NP are very closely linked with the tumor size in patients with HCC [4,5]. However, the effects of NP on the biological activity of HCC cells are not yet illuminated. In this study our aim is to understand the effects of NP on specific biological activities such as the proliferation and motility of five different HCC cell lines.
2. Materials and Methods
2.1. Cell Lines and Cell Culture
HCC cell lines, (HuH-7, PLC/PRF/5, Hep-3B, SNU449, PLC/PRF/5 and SK-HEP-1) were kindly provided by Prof. Dr. Mehmet Öztürk (Izmir Biomedicine and Genome Centre). All cell lines were maintained in regular DMEM-10, at 5% CO2 at 37 °C. NP treatment was carried out along 48 h for both viability and motility experiments.
2.2. MTT and SRB Assay
Cells were seeded in a 48 well plate, after O/N incubation, cells were treated with increasing concentrations of NP containing medium. For MTT; following 48-h treatment of NP, 0.5 mg/mL MTT (Thiazolyl Blue Tetrazolium Bromide, M2128, Sigma, Saint Louis, MO, USA) was added to the medium. After the formation of formazan crystals, medium was removed and formazan crystals were dissolved with 100 µL DMSO. Absorption of solution was measured at 570 nm. For SRB; following 48-h treatment of NP, 25 µL cold TCA 50% was added on each well and plates were kept at cold. After fixation, cell were stained with 0.04 % SRB. Absorption was measured at 570 nm. The cell viability was calculated as viable cell percentage.
2.3. Wound Healing (2 D Motility) Assay
After reaching 90% of confluency in 6 well plates, wound created by a sterile tip than washed and NP containing medium was added on cells. Following 48 incubation cells were fixed with methanol and stained with 0.5% crystal violet. Photos taken from wound area was measured by ImageJ software.
2.4. Statistical Analysis
Experiments have been carried out as triplicates independently. In viability experiment, the results were reported as mean ± SE for six repetitive analysis. The effect on motility was analyzed by semi-quantitative methods. One way ANOVA test was used for data analysis. p < 0.05 was considered as statistically significant. All the analyses were performed using GraphPad Prism 5 software.
3. Results
3.1. Effects of NP on HuH-7 Cells
NP drastically increase the motility of HuH-7 cell lines and NP concentrations up to 500 µM have no statistically significant effect on the proliferation of HuH-7 cells. p > 0.05 as shown Figure 1.
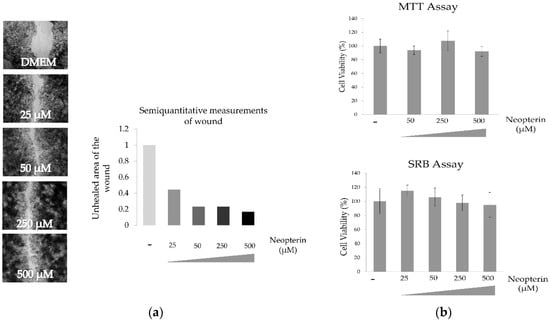
Figure 1.
Effect of neopterin on wound healing (a) and cytotoxicity (b) of HuH-7 cell line.
3.2. Effects of NP on PLC/PRF/5 Cells
At lower concentrations (25–50 µM), NP slightly increased the motility and up to 500 µM have no statistically significant effect on the viability of PLC/PRF/5 cells as shown Figure 2.
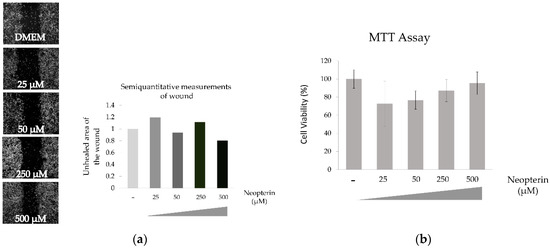
Figure 2.
Effect of neopterin on wound healing (a) and cytotoxicity (b) of PLC/PRF/5 cell line.
3.3. Effects of NP on Hep-3B Cells
NP shows little or no effect of the motility or viability of Hep-3B cells as shown Figure 3.
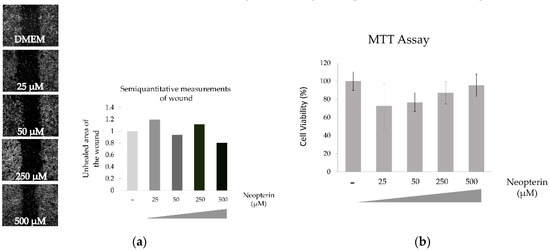
Figure 3.
Effect of neopterin on wound healing (a) and cytotoxicity (b) of Hep-3B cell line.
3.4. Effects of NP on SNU449 Cells
NP have no statistically significant effect on the viability of SNU449 cells even in highest examined concentration as shown Figure 4.
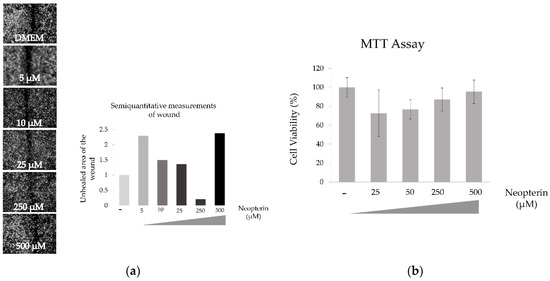
Figure 4.
Effect of neopterin on wound healing (a) and cytotoxicity (b) of SNU449 cell line.
3.5. Effects of NP on SK-HEP-1 Cells
NP has a significant effect on the motility of the SK-HEP-1 cells. Cells were treated up to 100 µM, because it is toxic over 250 µM. Wound healing capacity is best reflected by in the 10 µM treatment of NP. In the other hand cytotoxicity effect of NP on SK-HEP-1 cell line significantly increased in a concentration-dependent manner as shown Figure 5. *** p < 0.001, **** p < 0.0001.
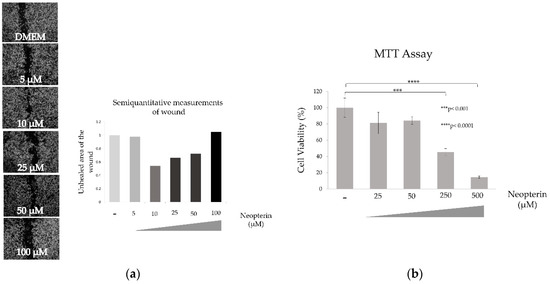
Figure 5.
Effect of neopterin on wound healing (a) and cytotoxicity (b) of SK-HEP-1 cell line.
4. Discussion
This is the only study analyzing the induced effect of NP on HCC biological activity. Our results showed that the HCC cell lines can be grouped into two categories according to their proliferative response to NP, as sensitive and resistant. HuH-7, PLC/PRF/5, Hep-3B, SNU449 cells were resistant even under a treatment with high concentrations of NP. SK-Hep1 showed a statistically significant decrease in the proliferation. Our data also showed an increase in the motility of HuH-7, SK-Hep1, and SNU449 cells under the treatment of neopterin but not in PLC/PRF/5 and Hep-3B cells. For this reason, neopterin can be a good focus to develop new treatment strategies against this chief liver malignancy. In further studies, we are planning to analyze the underlying molecular mechanism of neopterin effectiveness on signalling pathways.
Acknowledgments
This research received no external funding.
References
- Werner, E.R.; Werner-Felmayer, G.; Fuchs, D.; Hausen, A.; Reibnegger, G.; Wachter, H. Parallel induction of tetrahydrobiopterin biosynthesis and indoleamine 2,3-dioxygenase activity in human cells and cell lines by interferon-gamma. Biochem. J. 1989, 262, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Huber, C.; Batchelor, J.R.; Fuchs, D.; Hausen, A.; Lang, A.; Niederwieser, D.; Reibnegger, G.; Swetly, P.; Troppmair, J.; Wachter, H. Immune response-associated production of neopterin—Release from macrophages primarily under control of interferon-gamma. J. Exp. Med. 1984, 160, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Capece, D.; Fischietti, M.; Verzella, D.; Gaggiano, A.; Cicciarelli, G.; Tessitore, A.; Zazzeroni, F.; Alesse, E. The inflammatory microenvironment in hepatocellular carcinoma: A pivotal role for tumor-associated macrophages. BioMed Res. Int. 2013, 2013, 187204. [Google Scholar] [CrossRef] [PubMed]
- Wilmer, A.; Nölchen, B.; Tilg, H.; Herold, M.; Pechlaner, C.; Judmaier, G.; Dietze, O.; Vogel, W. Serum neopterin concentrations in chronic liver disease. Gut 1995, 37, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Lahdou, I.; Oweira, H.; Daniel, V.; Terness, P.; Schmidt, J.; Weiss, K.H.; Longerich, T.; Schemmer, P.; Opelz, G.; et al. Serum levels of chemokines CCL4 and CCL5 in cirrhotic patients indicate the presence of hepatocellular carcinoma. Br. J. Cancer 2015, 113, 756–762. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).