Abstract
The objective of this study is to evaluate the trabecular structure in hypodivergent individuals using fractal analysis, with a particular focus on specific mandibular regions. This study aims to assess the impact of hypodivergent growth patterns on bone microarchitecture. This research involved a methodological approach using panoramic radiographs to assess trabecular structure at specific regions of the mandible using fractal analyses. The dimensions of the fractals were calculated with the use of the box-counting technique by the software Image J (v1.53c; Bethesda, MD, USA, National Institutes of Health), while the statistical evaluations were carried out with the Jamovi Software (The Jamovi Project, version 2.3.21.0). The study found significant differences in fractal dimension values between hypodivergent individuals and the control group, particularly in the condyle and angulus regions, indicating a less complex trabecular structure in hypodivergent individuals. This study concludes that individuals with a hypodivergent growth pattern exhibit alterations in trabecular bone structure within the mandibular condyle and angulus regions, characterized by reduced complexity. These findings suggest that increased occlusal forces and mechanical stress associated with this growth pattern may contribute to changes in trabecular architecture. Understanding these variations is essential for orthodontic and maxillofacial diagnosis, treatment planning, and biomechanical considerations, particularly in cases requiring vertical dimension management or anchorage control.
1. Introduction
The hypodivergent growth pattern is a vertical direction abnormality that is frequently observed in the craniofacial region. The hypodivergent growth pattern is a craniofacial characteristic defined by a reduced lower facial height and a relatively flat mandibular plane angle.
In individuals with hypodivergent facial morphology, the mandible tends to exhibit an upward and forward growth direction, leading to a more horizontal facial profile. Orthodontically, hypodivergent individuals often present with a deep bite and reduced lower anterior facial height. The reduced vertical growth may also influence dental compensation mechanisms, occlusal relationships, and treatment planning strategies. Since vertical dimension control is a key consideration in orthodontic interventions, understanding the characteristics of hypodivergent growth is essential for optimizing treatment outcomes. This growth pattern can arise during the growth period due to a range of etiological factors, including differential growth in the condylar, sutural, and alveolar regions, as well as functional factors and masticatory muscles. The hypodivergent growth model is characterized by the vertical positioning of the masticatory muscles, which corresponds to the upward and forward growth of the condyle [,,]. Individuals characterized by a low-angle facial pattern demonstrate increased bite force and masticatory muscle activity, which significantly influence dental and skeletal anomalies. Increased masticatory muscle activity, particularly in the temporal and masseter muscles, affects mandibular development and trabecular bone density [,].
Trabecular bone density is a crucial parameter for orthodontic treatments, and density measurements are commonly used to evaluate it. Recent studies suggest that new methods of analyzing trabecular microstructure could enhance the assessment of trabecular bone density []. Fractal geometry, with its principles of “self-similarity” and “a lack of well-defined scale,” offers a valuable tool for the modeling of complex structures, such as trabecular bone. This modeling capability provides deeper quantitative insights into the intricate branching structural architecture of trabecular bone [].
Fractal analysis (FA) is a technique applied in the medical and dental fields to identify bone trabeculation [,]. This method enables a quantitative analysis of the intricate structure of bone tissue and its alterations at the microscopic level, thereby elucidating the effects of diverse pathological conditions or therapeutic interventions on bone density [,]. In the domain of orthodontics, this technique has been employed to evaluate the trabecular structure of bone in relation to various malocclusions or therapeutic interventions [,]. In the domain of orthodontics, this technique has been employed to evaluate the trabecular structure of bone in relation to various malocclusions or therapeutic interventions. The existing literature has investigated the effects of increased occlusal forces on the density of trabecular bone structure in patients with bruxism using the FA technique. The results indicate a decrease in bone trabeculation in specific regions of the mandible [,]. The current literature provides a limited number of studies that have analyzed the structure of bone trabeculation using the FA technique in individuals with hypodivergent vertical abnormalities characterized by an increase in bite force and masticatory muscle activity.
This study’s objective is to identify variations in the trabecular bone structure of individuals whose growth patterns are hypodivergent compared to those with normal vertical dimensions, focusing on distal regions near the mental foramen, the angulus region below the mandibular canal, and the mandibular condyle centre. To achieve this, the structure of the trabecular bone will be assessed with the use of the FA technique on panoramic radiographs.
2. Materials and Methods
The flow chart for the study is presented in Figure 1.
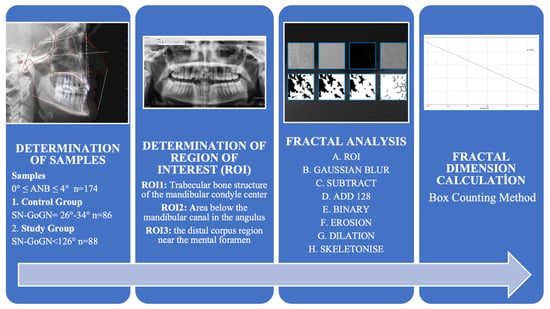
Figure 1.
Flowchart of Study.
2.1. Ethical Consideration
This study was approved by the Ethics Committee for Non-Invasive Clinical Practices of University of Health Sciences, Turkey (Approval No: 2022/227, 5 June 2021). All procedures adhered to the ethical standards of the responsible committee and the Helsinki Declaration (2008 revision). Informed consent was obtained from all participants.
2.2. Sample Size Determination
To assess the number of samples required for the study, power analysis was conducted using the software G*Power version 3.1.2 (University of Kiel, Kiel, Germany). The analysis showed that for a significance level of α = 0.05 and an effect size of 0.25, a minimum of 86 samples in each of the groups was required to achieve 90% power. In this study, each participant provided measurements from three different regions of the mandible, resulting in a total of 264 measurements for the study group (n = 88) and 258 measurements for the control group (n = 86).
2.3. Radiological Evaluation
The study utilised panoramic radiographs obtained with a 2D digital panoramic X-ray unit (ProMax, Planmeca, Helsinki, Finland). These radiographs were captured using settings of 5 mA, 70 kVp, and an exposure time of 15 s [].
2.4. Sample Identification
Sample identification involved selecting participants based on specific criteria. Individuals were required to be in growth stage S5 or S6 according to Lamparski’s vertebral assessment method []. Additionally, participants needed to have skeletal and dental class I (0° ≤ ANB ≤ 4°), and exhibit either a normal (26–34°) or hypodivergent (<26°) growth pattern according to the SN-GoGN angle.
Exclusion criteria included any history of trauma in the jaw and face area, abnormalities in the mandibular condyle region, temporomandibular joint (TMJ) dysfunction, or maxillofacial deformity. These criteria ensured the selection of suitable participants for the study. However, patients with any known systemic diseases, metabolic disorders, or regular medication usage that could potentially affect bone metabolism were excluded based on their medical history recorded during routine anamnesis.
2.5. Examination and Standardisation of Radiographic Images
Tagged Image File Format (TIFF) was used to store the panoramic images. To ensure uniformity across the radiographs, the size of each image was standardised to 2944 × 1436 pixels with the use of Adobe Photoshop Express (v25.25.0; San Jose, CA, USA: Adobe Inc.) The images were scaled to ensure consistency and to provide an appropriate basis for FA. A computer with an Intel Iris Plus Graphics 655 graphics card (1536 MB), 2.3 GHz quad-core Intel Core i5 processor (2.3 GHz quad-core) (Santa Clara, CA, USA), and 2133 MHz LPDDR3 RAM (8 GB) was used to analyse the radiographs.
2.6. Determination of Regions of Interest (ROI)
The chosen ROIs are in line with established anatomical landmarks used in prior fractal analysis studies. Ensuring consistency and comparability across research [,,,,,]. To ensure consistency and reliability in this study, a single researcher consistently cropped the defined ROI from the right side for each patient. The selected ROI was obtained by cropping a 64 × 64 pixel frame over the raw image using ImageJ software, with trabecular bone selected from the mandibular condyle’s geometric center (ROI1), the angulus region below the mandibular canal (ROI2), and the distal regions near the mental foramen (ROI3). To minimize variability introduced by different observers, each ROI was measured twice by the same researcher with a two-week interval. Intraobserver reliability was assessed using the Intraclass Correlation Coefficient (ICC), confirming the consistency of the measurements (Figure 2) [,]. (e.g., grammar, spelling, punctuation, and formatting) does not need to be declared.
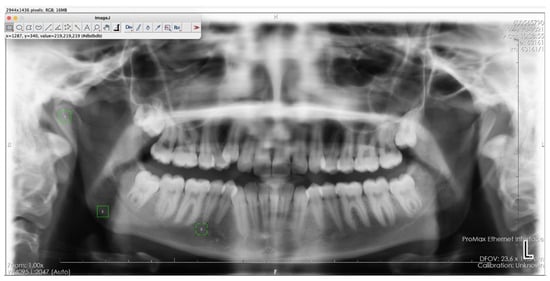
Figure 2.
ROI1, ROI2, and ROI3 areas selected on a panoramic radiograph using ImageJ software. 1: The mandibular condyle’s geometric center (ROI1), 2: The angulus region below the mandibular canal (ROI2), 3: The distal regions near the mental foramen (ROI3).
2.7. Image Analysis
The Mac OS X-compatible ImageJ v1.52 software was chosen for the FA. This platform, developed in Java, is a variant of the ImageJ Software created by the National Institutes of Health (NIH) and offers an extensive array of tools for image processing and analysis. The FA was carried out in accordance with the method as described by the authors White and Rudolph []. The FA process involves a series of image processing steps [,] (Table 1) (Figure 3).

Table 1.
Image processing steps of the FA process.
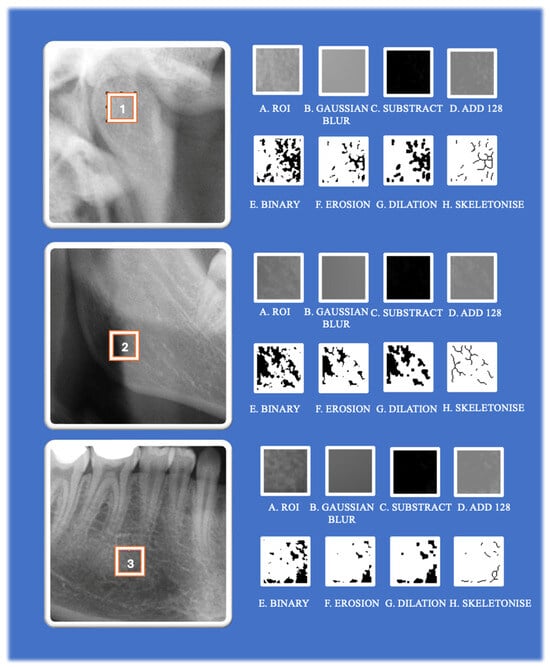
Figure 3.
The image processing steps involved in fractal analysis using ImageJ software. 1: ROI1, 2: ROI 2, 3: ROI3.
2.8. Calculation of Fractal Dimension (FD)
In this study, the FD was calculated using the “box counting method,” which is widely recognised in dental radiology literature as a reliable and reproducible technique for assessing trabecular bone complexity on 2D radiographs. This method is particularly effective in assessing the fractality of structures, especially in images featuring complex and irregular patterns such as trabecular bone. In this study, to calculate the FD, the images were segmented into squares of various sizes—specifically, 2, 3, 4, 6, 8, 12, 16, 32, and 64 pixels—utilising the ‘fractal box counter’ tool (Figure 4) []. For every pixel size, the study calculated both the count of squares encompassing trabeculae and the overall quantity of squares present in the image. These figures were then plotted on a logarithmic scale. FD was determined by the slope of the curve that most accurately fits the points plotted on this logarithmic graph. The relationship between the size of the boxes and the total number was calculated from the log-log graph using the formula (Figure 4) [,,,].
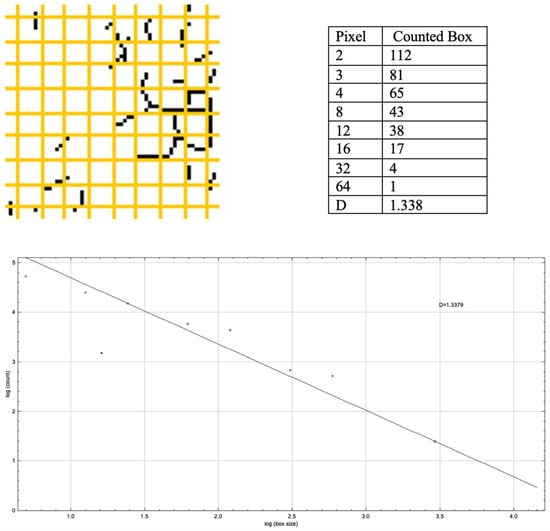
Figure 4.
Fractal box counter and the log-log plot graph from ImageJ Software, from which the FD is obtained. Yellow grid cells denote the box-counting grid, and black dots mark the intersections where the structure occupies a box.
2.9. Statistical Analysis
In this study, the intraclass correlation coefficient (ICC) was employed to evaluate intra-observer reliability. The ICC values for the measurements ranged between 0.89 and 0.95, indicating a high degree of reliability. The average ICC value was 0.91 (p < 0.001). All measurements were repeated by the same, single-blinded investigator with a two-week interval.
The parameters obtained from the FA were evaluated using the open-source Jamovi software (The Jamovi Project, www.jamovi.org, version 2.3.21.0). To test the normality of the sample distribution, the Shapiro–Wilk test was applied. Since the data were not normally distributed for between-group comparisons, the Kruskal–Wallis test was applied, followed by Dwass–Steel–Critchlow–Fligner (DSCF) pairwise comparisons. The DSCF method is a non-parametric post-hoc test that inherently accounts for multiple comparisons, thus eliminating the need for additional correction procedures such as Bonferroni.
3. Results
A statistically significant difference was observed between the two groups in the SN-GoGN angle, with the study group showing an angle of 24.8 ± 2.41° and the control group displaying 31.1 ± 1.19° (p < 0.0005). However, there was no statistically significant difference in the ANB angle between the two groups (Supplementary Table S1).
All participants were selected based on cervical vertebral maturation stages S5 or S6 according to Lamparski’s method, ensuring skeletal maturity and comparability in developmental status. The mean age of participants in the study group was 22.54 ± 3.60 years (22.30 ± 3.95 for females, 22.74 ± 3.31 for males), while the control group had a mean age of 23.96 ± 4.30 years (24.09 ± 4.38 for females, 23.71 ± 4.40 for males). No statistically significant differences were found in age between males and females within either group (p = 0.755 and p = 0.633), nor between the female participants of both groups (p = 0.103) or between male participants (p = 0.452). These findings indicate that age and sex distribution were balanced across the groups, minimizing potential confounding effects related to these variables (Supplementary Table S2).
Analysis of the relationship between gender and FDs showed statistically significant differences between FD values in ROI1, ROI2 and ROI3 regions (respectively 1.16 ± 0.111, 1.24 ± 0.138, p = 0.033, 1.25 ± 0.123, 1.47 ± 0.170, p < 0.001; 1.25 ± 0.166, 1.35 ± 0.149, p = 0.019) in the study group and ROI1 and ROI2 regions (respectively 1.35 ± 0.155, 1.44 ± 0.109, p = 0.023; 1.42 ± 0.159, 1.57 ± 0.112, p < 0.001) in the control group (Table 2). In these regions, FD values are lower in females than in males.

Table 2.
Comparison of FD values by gender.
A significant difference was found between the control group and the study group in FA results of individuals in the condyle and angulus regions (respectively 1.34 ± 0.144, 1.20 ± 0.131, p < 0.001;1.43 ± 0.157, 1.37 ± 0.186, p = 0.037), whereas no statistically significant difference was found between FA values in the corpus region (Table 3). In these regions, the FA values of hypodivergent individuals were lower than those of individuals with normal vertical ratios.

Table 3.
Descriptive statistics and between-within group comparisons.
Table 3 shows the results of the within-group comparisons of the control group and the study group. According to the results of this table, no statistically significant difference was found between ROI1 and ROI3 in the control group. However, a significant difference was found between ROI1 and ROI2 and between ROI2 and ROI3 (respectively ROI1 = 1.34 ± 0.144, ROI2 = 1.43 ± 0.157, ROI3 = 1.32 ± 0.123, p = 0.017, p < 0.001). For the study group, no statistically significant difference was found between ROI2 and ROI3. However, a significant difference was found between ROI1 and ROI2, and between ROI1 and ROI3 (respectively ROI1 = 1.20 ± 0.131, ROI2 = 1.37 ± 0.186, ROI3 = 1.30 ± 0.149, p < 0.001, p = 0.003) (Supplementary Table S3).
4. Discussion
This study makes a contribution to existing knowledge in the field of orthodontics by analysing the mandibular trabecular bone structure of individuals with hypodivergent growth patterns using the FA technique. The available literature offers only a limited number of studies that have examined the impact of this specific growth pattern on the structure of the mandible. The findings of this study suggest that the trabecular bone structures in the angulus and condyle regions of individuals with hypodivergent growth patterns are less complex compared to those in individuals with normal vertical dimensions.
FA has the ability to reveal micro-level features and scale-independent patterns of complex biological structures, particularly bone tissue. This method has been reported to quantitatively assess the complexity and branching characteristics of trabecular bone structure, revealing details that cannot be detected by conventional methods []. Fractal analysis (FA) is a valuable tool for assessing trabecular bone microarchitecture, offering insights into complex biological structures at a micro-level. The literature, including both recent and well-established studies, supports the use of panoramic radiographs for FA, as they consistently demonstrate trabecular patterns and allow for the evaluation of key anatomical regions, including the gonial, condylar, and dentoalveolar areas [,,,,,,,,]. While Cone Beam Computed Tomography (CBCT) provides high-resolution 3D imaging and may offer advantages for detailed structural assessment, its role in FA remains uncertain. Some studies suggest that FA results from CBCT may not be fully compatible with histological findings, raising concerns about its reliability in quantitative trabecular analysis [,]. A recent study comparing CBCT, panoramic, and periapical radiographs for FA concluded that CBCT-derived FDs were inconsistent and did not correlate with those obtained from 2D. This discrepancy is largely attributed to CBCT’s larger voxel size, which can limit its ability to capture finer trabecular structures. In light of these findings, the authors of the study recommended the use of periapical and panoramic radiographs for fractal analysis of trabecular patterns in clinical settings []. From an ethical standpoint, CBCT is not routinely taken for general orthodontic assessment, and it would not be justifiable to expose patients to additional radiation solely for research purposes. Since panoramic radiographs are already a standard part of orthodontic diagnostics, their use in this study aligns with both clinical practice and ethical guidelines. Selecting only patients with existing CBCT scans would compromise sample standardization, as CBCT imaging is typically obtained for specific clinical indications rather than as part of routine assessments. This could introduce selection bias and limit the generalizability of the findings. Given the literature support, routine availability, and validated use of panoramic radiographs in FA, this approach provides a reliable and ethical methodology for evaluating mandibular trabecular structure in hypodivergent individuals. Additionally, panoramic radiographs are frequently utilized in routine dental examinations, and the anatomical regions examined in this study can be evaluated simultaneously on a single radiograph. In our study, panoramic radiographs were used instead of CBCT primarily due to ethical considerations, as CBCT imaging is not routinely justified in healthy individuals solely for research purposes due to its higher radiation exposure. While CBCT provides three-dimensional data, recent literature has also highlighted concerns regarding the inconsistency between FD values derived from CBCT and histological findings. Therefore, panoramic radiographs, which are commonly used in clinical practice and supported by several fractal analysis studies, were considered an ethically and methodologically appropriate imaging modality for this investigation.
Previous research has demonstrated that the values calculated from digital radiographs for FD are not significantly affected by X-ray exposure or beam orientation. However, ROI selection prior to FD evaluation can influence the final results []. The mandibular condyle region (ROI1) is located at the centre of the TMJ movements. The ROI2, which is situated in the posterior part of the mandible, refers to the angulus region. This region, which is the inferior attachment point of the masseter muscle, is an important area for load-bearing and stress distribution during TMJ movements. The trabecular bone structure of the distal corpus of the mental foramen (ROI3) can provide information about the mechanical properties and the load-bearing capacity of the anterior region of the mandible [,,].
The literature has investigated the impact of gender on FD and has reported that male FD values are generally higher than female FD values [,,]. Our study also showed higher FD values in males compared to females, similar to the literature. The difference in FD values between the genders might be associated with denser and more complex trabecular structures in males, whereas a more porous trabecular structure and a reduced number of trabeculae were observed in females.
In this study, a notable decrease in FD values was observed in the angulus and condyle regions of hypodivergent individuals compared to the control group. The observed decrease in FD values is indicative of reduced trabecular complexity, which may be associated with resorptive changes [,,,]. These differences may be associated with increased activity of the masseter and temporal muscles in hypodivergent individuals, which is typically associated with increased bite forces and masticatory muscle activity. The predominance of fast-twitch type II fibers in the masseter muscles of hypodivergent individuals has been linked to increased occlusal forces, which may contribute to trabecular bone changes [,]. Güleç et al. [] investigated the relationship between bruxism and the mandibular trabecular bone structure using digital panoramic radiographs. They selected ROIs from the mandibular condyle and found that individuals with bruxism exhibited lower FD values in this region. This finding aligns with the results of the present study, as the condyle region in the present study also showed significantly lower FD values, suggesting that similar mechanisms may be at play. These results support the hypothesis that increased muscle activity may influence trabecular bone structure through mechanical stress and adaptation. According to Wolff’s law, bone remodels in response to mechanical stress; however, excessive or non-physiological forces may disrupt the architecture and reduce trabecular complexity []. Guleç et al.’s findings in bruxism patients and animal studies further suggest that while physiological loads can enhance trabecular organization, excessive repetitive loads may instead lead to architectural degradation, contributing to reduced FD values [,,,]. A recent 3D finite element analysis (FEA) using CBCT- and MRI-derived mandibular models demonstrated that variations in mandibular growth patterns significantly affect stress distribution in the condylar region. Specifically, hypodivergent morphologies were associated with increased mechanical loading on the condylar zone []. Such biomechanical findings support our hypothesis that increased occlusal forces and masticatory muscle activity in hypodivergent individuals may contribute to the observed reduction in trabecular complexity. Although FEA was not employed in the present research, the consistency of our FD-based results with previous biomechanical models underscores the relevance of mechanical stress in trabecular bone adaptation. Moreover, future investigations incorporating electromyographic (EMG) assessments and bite force measurements—alongside FEA modelling—could provide more direct and robust evidence linking muscle function to trabecular microarchitecture. These complementary approaches would offer valuable functional and structural perspectives, enabling a more precise evaluation of causality. Notably, no significant difference was found in ROI3 (corpus mandibulae region) between the groups. This result may stem from the anatomical and biomechanical nature of the corpus region, which is less subject to concentrated occlusal forces or masseteric muscle activity compared to the condyle and angulus. Moreover, due to the two-dimensional nature of panoramic radiography, this region may be more affected by structural superimposition and limited resolution, potentially masking subtle trabecular changes. Previous studies using similar imaging modalities and ROI selection also reported non-significant findings in this region [,,], suggesting that both regional biomechanics and imaging constraints could contribute to this pattern. These observations support the view that trabecular adaptation is region-specific and that methodological limitations must be considered when interpreting site-based differences in FD values.
The clinical relevance of these findings lies in their potential impact on orthodontic treatment planning, risk assessment, and bone adaptation mechanisms. The observed changes in trabecular bone structure align with previous studies on bruxism, where increased muscle forces were associated with altered FD values. Since hypodivergent individuals exhibit higher masticatory activity, their bone response may influence tooth movement, anchorage control, and susceptibility to mechanical stress during orthodontic treatment. Although FD is not a direct measure of bone density or strength, its ability to reflect trabecular complexity may offer supplementary insights into individual biomechanical profiles. Recognizing such patterns could serve as a supportive indicator for tailoring force applications or considering enhanced anchorage strategies, especially in individuals with potentially compromised trabecular architecture. Furthermore, since panoramic radiographs are routinely obtained in orthodontic assessments, the integration of FA into clinical workflows might provide additional microstructural information without subjecting patients to extra procedures or radiation. While these interpretations remain secondary and should be validated by further studies, they may contribute to more informed, patient-specific decision-making in orthodontics. Additionally, these findings may hold value for restorative dentistry and implantology, where bone microarchitecture plays a key role in treatment planning and success.
In this study, FA was used to assess the complexity and micro-level characteristics of the mandibular trabecular structure in individuals with hypodivergent growth patterns. Our findings highlight the different structural variations in the mandibular condyle, angulus, and corpus regions of these individuals. However, the exclusive focus on a Turkish Caucasian population constitutes a limitation that may affect the generalizability of the findings to broader or more ethnically diverse populations. While this homogeneity helped control for potential confounding genetic and morphological variables in the current study, future research should aim to include multi-centre samples with more diverse ethnic backgrounds to validate and expand upon these results. Although strict inclusion and exclusion criteria were applied to minimise systemic confounding, certain influencing factors—such as nutritional status, metabolic bone conditions, and environmental exposures—were not available for retrospective analysis due to the limitations of standard orthodontic anamnesis records. These factors, along with age- and gender-related variability, could have subtle effects on trabecular structure. Therefore, future prospective studies should incorporate broader clinical datasets and apply multivariate analysis techniques (e.g., regression modelling) to better isolate the independent effects of growth patterns and control for such variables. Additionally, while previous literature supports the use of panoramic radiographs as a reliable method for fractal analysis, it is important to acknowledge the inherent limitations of 2D imaging, such as magnification errors, geometric distortion, and anatomical superimposition. These factors may affect the standardisation and accuracy of FD measurements, particularly in posterior mandibular regions. While our study focused on a standard orthodontic patient population, in whom routine CBCT imaging is not ethically justified, this limitation restricts the depth of microarchitectural assessment. Future studies incorporating advanced three-dimensional imaging modalities or histological validation are warranted to enhance anatomical accuracy and provide a more comprehensive evaluation of trabecular complexity.
5. Conclusions
Within the limits of this study, FA values indicate that trabecular bone structure in the mandibular condyle and angulus is influenced by vertical growth patterns. Individuals with a hypodivergent growth pattern exhibit reduced FD values in these regions, suggesting that increased occlusal forces and mechanical stress may alter trabecular architecture. These findings have clinical relevance for orthodontic treatment planning and risk assessment. Understanding trabecular variations in hypodivergent patients may help optimize force application, anchorage strategies, and biomechanical considerations. Additionally, these alterations may impact maxillofacial surgery, restorative, and prosthetic treatments. Future studies with longitudinal data and advanced imaging could further clarify these relationships.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fractalfract9080517/s1.
Author Contributions
G.B.D.: Data curation: methodology, investigation, formal analysis, resources, visualization, and writing—original draft. R.D.: Data curation: formal analysis, methodology, investigation, visualization, and resources. K.G.T.: Conceptualization, methodology, formal analysis, and writing—review and editing. G.S.D.: Methodology: writing—review and editing, supervision. S.G.: Conceptualization: writing—review and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data that support the findings of this research article are available from the corresponding author, upon reasonable request.
Acknowledgments
During the preparation of this work, the authors utilised ChatGPT-4 to check the grammar, spelling, and translation of the text. Following the use of this tool, the authors reviewed and amended the content as necessary and take full responsibility for the publication’s content.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| FA | Fractal analyses |
| TIFF | Tagged Image File Format |
| ROI | Regions of Interest |
| FD | Fractal Dimension |
| CBCT | Cone Beam Computed Tomography |
| TMJ | Temporomandibular joint |
References
- Buschang, P.H.; Jacob, H.; Carrillo, R. The Morphological Characteristics, Growth, and Etiology of the Hyperdivergent Phenotype. Semin. Orthod. 2013, 19, 212–226. [Google Scholar] [CrossRef]
- Vianna-Lara, M.S.; Caria, P.H.F.; De Tosello, D.O.; Lara, F.; Amorim, M.M. Electromyographic Activity of Masseter and Temporal Muscles with Different Facial Types. Angle Orthod. 2009, 79, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.M.; Miyamoto, K.; Saifuddin, M.D.; Ishizuka, Y.; Tanne, K. Masticatory muscle activity in children and adults with different facial types. Am. J. Orthod. Dentofac. Orthop. 2000, 118, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Franciotti, R.; Moharrami, M.; Quaranta, A.; Bizzoca, M.E.; Piattelli, A.; Aprile, G.; Perrotti, V. Use of fractal analysis in dental images for osteoporosis detection: A systematic review and meta-analysis. Osteoporos. Int. 2021, 32, 1041–1052. [Google Scholar] [CrossRef]
- Geraets, W.G.M.; Van der Stelt, P.F. Fractal properties of bone. Dentomaxillofac. Radiol. 2000, 29, 144–153. [Google Scholar] [CrossRef]
- Southard, T.E.; Southard, K.A.; Jakobsen, J.R.; Hillis, S.L.; Najim, C.A. Fractal dimension in radiographic analysis of alveolar process bone. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1996, 82, 569–576. [Google Scholar] [CrossRef]
- Korkmaz, Y.N.; Arslan, S. Evaluation of the trabecular structure of the mandibular condyles by fractal analysis in patients with different dentofacial skeletal patterns. Australas. Orthod. J. 2021, 37, 93–99. [Google Scholar] [CrossRef]
- Akbulut, S.; Bayrak, S.; Korkmaz, Y.N. Prediction of rapid palatal expansion success via fractal analysis in hand-wrist radiographs. Am. J. Orthod. Dentofac. Orthop. 2020, 158, 192–198. [Google Scholar] [CrossRef]
- Gulec, M.; Tassoker, M.; Ozcan, S.; Orhan, K. Evaluation of the mandibular trabecular bone in patients with bruxism using fractal analysis. Oral Radiol. 2021, 37, 36–45. [Google Scholar] [CrossRef]
- Kolcakoglu, K.; Amuk, M.; Sarıbal, G.S. Evaluation of mandibular trabecular bone by fractal analysis on panoramic radiograph in paediatric patients with sleep bruxism. Int. J. Paediatr. Dent. 2022, 32, 776–784. [Google Scholar] [CrossRef]
- Lamparski, D. Skeletal age assessment utilizing cervical vertebrae. Am. J. Orthod. 1975, 67, 458–459. [Google Scholar] [CrossRef]
- Kato, C.N.A.O.; Barra, S.G.; Tavares, N.P.K.; Amaral, T.M.P.; Brasileiro, C.B.; Mesquita, R.A.; Abreu, L.G. Use of fractal analysis in dental images: A systematic review. Dentomaxillofac. Radiol. 2020, 49, 20180457. [Google Scholar] [CrossRef] [PubMed]
- Eninanç, İ.; Yeler, D.Y.; Çınar, Z. Investigation of mandibular fractal dimension on digital panoramic radiographs in bruxist individuals. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 131, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Tepe, R.D.; Toraman, K.O.; Kayhan, K.B.; Ozcan, I.; Karabas, H.C. Fractal Analysis of Mandible in Panoramic Radiographs of Patients Received Radiotherapy for Nasopharyngeal Carcinoma. J. Clin. Densitom. 2025, 28, 101531. [Google Scholar] [CrossRef]
- Arsan, B.; Köse, T.E.; Çene, E.; Özcan, İ. Assessment of the trabecular structure of mandibular condyles in patients with temporomandibular disorders using fractal analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 123, 382–391. [Google Scholar] [CrossRef]
- White, S.C.; Rudolph, D.J. Alterations of the trabecular pattern of the jaws in patients with osteoporosis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1999, 88, 628–635. [Google Scholar] [CrossRef]
- Shrout, M.K.; Potter, B.J.; Hildebolt, C.F. The effect of image variations on fractal dimension calculations. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1997, 84, 96–100. [Google Scholar] [CrossRef]
- GV, V.; Tripathi, T. Fractal analysis of mandibular trabecular pattern following protraction facemask therapy: A pilot study. J. Orofac. Orthop. 2024, 1–9. [Google Scholar] [CrossRef]
- Dedeoğlu, N.; Eşer, G.; Özen, D.Ç.; Altun, O. Five-year change of panoramic radiomorphometric indices and fractal dimension values in type 2 diabetes patients. Oral Radiol. 2024, 40, 49–57. [Google Scholar] [CrossRef]
- Balkan, E.P.; Paksoy, C.S.; Bağış, N. Fractal analysis of the effects on mandibular bone of botulinum toxin therapy of the masseter muscle in patients with bruxism. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2024, 137, 83–88. [Google Scholar] [CrossRef]
- Esmaeili, F.; Bayat, N.; Tolouei, A.E.; Azimzadeh, M.; Nateghi, M.; Rahimipour, K. Evaluation of the changes in trabecular bone density of angle and condyle regions of the mandible before and after COVID-19 contraction using fractal analysis. J. Oral Maxillofac. Surg. Med. Pathol. 2024, 37, 175–179. [Google Scholar] [CrossRef]
- Akay, G.; Pamukcu, U.; Atas, O.K. Evaluation of the long-term structural change caused by root canal treatment in the bone around the tooth by fractal analysis. Oral Radiol. 2025, 1–9. [Google Scholar] [CrossRef]
- Corpas, L.D.S.; Jacobs, R.; Quirynen, M.; Huang, Y.; Naert, I.; Duyck, J. Peri-implant bone tissue assessment by comparing the outcome of intra-oral radiograph and cone beam computed tomography analyses to the histological standard. Clin. Oral Implant. Res. 2011, 22, 492–499. [Google Scholar] [CrossRef]
- Saribal, S.; Ersu, G.; Yilmaz, N. The effects of technical factors on the fractal dimension in different dental radiographic images. Eur. Oral Res. 2023, 57, 68–74. [Google Scholar] [CrossRef]
- Yavuz, E.; Yardimci, S. Comparison of periapical radiography, panoramic radiography, and CBCT in the evaluation of trabecular bone structure using fractal analysis. Oral Radiol. 2024, 40, 394–400. [Google Scholar] [CrossRef]
- Alman, A.C.; Johnson, L.R.; Calverley, D.C.; Grunwald, G.K.; Lezotte, D.C.; Hokanson, J.E. Diagnostic capabilities of fractal dimension and mandibular cortical width to identify men and women with decreased bone mineral density. Osteoporos. Int. 2012, 23, 1631–1636. [Google Scholar] [CrossRef]
- Frost, H.M. Wolff’s Law and bone’s structural adaptations to mechanical usage: An overview for clinicians. Angle Orthod. 1994, 64, 175–188. [Google Scholar]
- Su, K.; Yuan, L.; Yang, J.; Du, J. Numerical Simulation of Mandible Bone Remodeling under Tooth Loading: A Parametric Study. Sci. Rep. 2019, 9, 14887. [Google Scholar] [CrossRef]
- Ju, Y.-I.; Sone, T. Effects of Different Types of Mechanical Loading on Trabecular Bone Microarchitecture in Rats. J. Bone Metab. 2021, 28, 253. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Zhu, J.; Zheng, F.; Zhu, Y.; Sui, S.; Liu, Y.; Yin, D. Associations between condylar height relative to occlusal plane and condylar osseous condition and TMJ loading based on 3D measurements and finite element analysis. Sci. Rep. 2024, 14, 28919. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).