Abstract
Apart from some information on dengue virus (DENV), there is limited data on the circulation of arboviruses in Burkina Faso. The aim of this study was to investigate antibody prevalence against six arboviruses in four regions of the country to document previous virus exposure. Serum samples collected between August 2018 and December 2022 from people infected with viral hepatitis B and C in Bobo-Dioulasso were used to detect IgG antibodies against DENV, Chikungunya virus (CHIKV), Zika virus (ZIKV), Yellow fever virus (YFV), Rift Valley fever virus (RVFV) and Crimean-Congo hemorrhagic fever virus (CCHFV) using commercial ELISA kits. A total of 1808 serum samples, accompanied by basic epidemiologic data (sex, age and residency) were included in this study. We observed an IgG antibodies seroprevalence of 75.4% for DENV, 30.8% for CHIKV, 2.9% for ZIKV, 1.2% for RVFV, 1.1% for CCHFV and 1.1% for YFV. Age, sex, and place of residence were significantly associated with seropositivity for DENV and age and sex with CHIKV seropositivity. The results suggested widespread circulation of DENV and CHIKV and possible circulation of CCHFV and RVFV in humans in Burkina Faso. The importance of strengthening arbovirus surveillance by including additional arboviruses in the diagnostic panel is emphasized.
1. Introduction
Arboviruses are transmitted by arthropod vectors including mosquitoes, ticks, and sandflies [1]. They are responsible for arboviral infections, which are asymptomatic in about 75% of cases. When symptomatic, arboviral infections typically present as an influenza-like syndrome with nonspecific signs and symptoms [2].
Arbovirus infections are more common in tropical countries, where climatic conditions are conducive to the emergence and re-emergence of vectors. However, in recent years, many countries have experienced outbreaks of arboviral diseases due to advanced deforestation, globalization and climate change [3,4]. Currently, arbovirus infections are considered a global public health concern [4,5].
The arboviruses with the greatest impact on humans are dengue (DENV) and Zika viruses (ZIKV) of the family Flaviviridae and chikungunya virus (CHIKV) of the family Togaviridae [6,7]. DENV, one of the most extensively studied arboviruses, has been estimated to cause between 50 and 100 million cases of dengue fever and 500,000 cases of severe dengue requiring hospitalization each year worldwide [8]. It is present in at least 128 countries and approximately 4 billion people are at risk of infection each year [4,5]. In 2013, the emergence of CHIKV in the Caribbean and its rapid spread to 45 countries and territories in the Americas highlighted the high epidemic potential of this virus [5]. CHIKV is now widespread globally [9]. Despite the low mortality rates associated with CHIKV, infection with this virus has a significant impact on the quality of life and results in significant economic losses [10]. In 2024, as of April 30, approximately 240,000 cases of CHIKV disease and more than 90 deaths have been reported worldwide [11]. First identified in Uganda in 1947, ZIKV has spread rapidly around the world. The 2015 epidemic recorded approximately 1,500,000 suspected cases in the Americas, Caribbean and Pacific [12]. ZIKV infection is usually mild. However, it can cause neurological disorders such as congenital microcephaly, Guillain-Barré syndrome, myelitis, and meningoencephalitis [13]. As for yellow fever (YF), despite the existence of an effective vaccine, it is considered a re-emerging arboviral disease. Indeed, in recent years, YF cases have been continuously reported in tropical countries, especially in Africa, where 16 YF outbreaks have been reported in 13 countries [14]. Clinically, the majority of human infections with yellow fever virus (YFV) are asymptomatic or mild. However, severe YF is estimated to occur in 12% of infected individuals, with a case fatality rate of approximately 47% [15,16]. Rift Valley Fever Virus (RVFV) was discovered in Kenya in 1930 and primarily infects domestic animals (sheep, goats, cattle) and humans [17]. Human infection ranges from self-limiting febrile illness to life-threatening hemorrhagic episodes [18]. Since its discovery, RVFV has caused numerous epidemics in Africa and the Arabian Peninsula [17,19,20]. Crimean-Congo hemorrhagic fever (CCHF) is a zoonosis caused by the Crimean-Congo hemorrhagic fever virus (CCHFV). It belongs to the Nairoviridae family and is transmitted to humans mainly by the bite of a tick of the genus Hyalomma [21]. Symptomatic patients initially present with nonspecific illness, which can progress to a potentially fatal outcome. Epidemics have been reported, and mortality rates varied from region to region, ranging from about 5% to 80% [22,23].
Although vector-borne diseases pose a serious threat to the health of the world’s population [24], epidemiologic data are limited in Africa, due to limited surveillance, a nonexistent or poor screening system, and low awareness, despite the historical presence and recent epidemics on the continent [25].
Burkina Faso, a tropical country in West Africa, has faced recurrent dengue epidemics since 2013 [26,27,28]. ZIKV and CHIKV circulation in Burkina Faso has recently been demonstrated [29,30] and YFV infections were reported between 2003 and 2008 through the national febrile jaundice surveillance system [31]. To our knowledge, RVFV and CCHFV circulation in the human population has not yet been confirmed in the country. Although Burkina Faso has a surveillance system for arboviruses, this surveillance is mainly focused on DENV, resulting in a knowledge gap regarding other arboviruses. In addition, the few existing data on the circulation of these viruses are old, geographically limited, and involve a small population. We conducted a seroprevalence study for antibodies against DENV, CHIKV, ZIKV, YFV, CCHFV and RVFV in four regions of Burkina Faso to gain an overview of the burden of these infections as a basis for prevention and control strategies.
2. Materials and Methods
2.1. Study Design
This was a cross-sectional study based on serum samples collected at the Assaut-Hépatites Center, as part of follow-up of patients infected with hepatitis B or C viruses in Bobo-Dioulasso, Burkina Faso. Assaut-Hépatites is a nongovernmental organization (NGO) that operates a referral center for the treatment of viral hepatitis, receiving infected patients from various regions of Burkina Faso daily. Approximately 8 mL of whole blood was collected from each patient by venipuncture. The blood samples were centrifuged at 4000 rpm for five minutes at 25 °C and the resulting serum was aliquoted into two screw-cap tubes. One aliquot was used for molecular analysis of hepatitis viruses and the second was stored at −80 °C for subsequent analyses.
2.2. Sample Size Calculation
The target sample size was estimated at 954 using the Schwartz formula: n = , where
n is the required sample size,
z is the z-score corresponding to the desired confidence level (1.96 for 95% CI),
p is the expected prevalence (66.3% for anti-DENV IgG antibodies [32]) and the desired precision (0.03).
2.3. Sample Selection and Data Collection
For this study, 1808 samples were randomly selected using STATA version 17.0 (StataCorp LLC, College Station, TX, USA) software. Selection criteria included sufficient serum volume, proper labeling, and adequate storage at −80 °C. Sociodemographic data, including sex, age, and place of residence, were extracted from the database. The samples included in the study were collected between August 2018 and December 2022 and were from participants residing in the Hauts Bassins, Boucle du Mouhoun, Sud-Ouest and Cascades regions located in the Western part of Burkina Faso (Figure 1).
Figure 1.
Map showing the study regions.
2.4. Serological Detection
Serum samples were tested for the presence of immunoglobulin G (IgG) class antibodies against DENV, CHIKV, ZIKV, YFV, RVFV and CCHFV using commercial ELISA kits (Table 1). Cut-off values were calculated according to the manufacturers’ instructions. Results were interpreted as negative, equivocal, or positive. Equivocal results were retested and in case they were equivocal again, they were considered as negative for further analyses. Positive and negative controls supplied with the commercial kits were tested on each plate to ensure assay validity.

Table 1.
Characteristics of the ELISA tests.
2.5. Statistical Analysis
Data were entered using Microsoft Excel and analyzed using STATA version 17.0 (StataCorp LLC, College Station, TX, USA). Categorical variables were expressed as percentages with 95% CIs; comparisons used chi-square or Fisher’s exact test as appropriate. Quantitative variables (age) were expressed as mean ± SD. Multiple logistic regression analyses were performed to assess associations between participant characteristics (age, sex, and region of residence) and seropositivity to each arbovirus. Crude and adjusted odds ratios (cORs, aORs) with 95% CIs were calculated. A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Study Population
Men accounted for 60.7% of the 1808 hepatitis patients (1097/1808, Table 2). The mean age was 35.7 ± 11.5 years (range 3–83), with the 30–39-year age group most represented (697 participants, 38.0%). Most participants resided in the Hauts Bassins region (n = 1244; 68.8%).

Table 2.
Sociodemographic characteristics of study participants.
3.2. Seroprevalence of Antibodies Against Arboviruses
IgG antibodies against at least one arbovirus were detected in 1472 samples (81.4%; 95% CI: 79.5–83.1). The IgG antibodies seroprevalence was 75.4% (1363/1808; 95% CI: 73.3–77.4) for DENV, 30.7% (556/1808; 95% CI: 28.2–32.5) for CHIKV, 2.9% (53/1808; 95% CI: 2.1–3.7) for ZIKV, 1.2% (21/1808; 95% CI: 0.7–1.7) for RVFV, 1.1% (20/1808; 95% CI: 0.7–1.6) for CCHFV and 1.1% (19/1808; 95% CI: 0.6–1.6) for YFV. A significant increase in seroprevalence with age was observed for DENV from 27.3% among <20-year-olds to 90.5% among ≥50-year-olds (p < 0.001) and for CHIKV from 11.4% among <20-year-olds to 38.2% among ≥50-year-olds (p < 0.001, Table 3). Furthermore, the prevalence of IgG antibodies for DENV was significantly higher in males (77.2%) than in females (72.6%, p = 0.029), whereas the reverse was true for CHIKV (males: 28.8% versus females: 33.8%; p = 0.026). Significant differences between regions were observed only for anti-DENV IgG, with prevalence rates ranging from 73.6% in the Boucle du Mouhoun region to 85.3% in the Sud-Ouest region (p = 0.002, Table 3).

Table 3.
Seroprevalence of IgG antibodies against arboviruses by sociodemographic characteristics.
Seropositivity for IgG antibodies against two or more arboviruses was detected in 724 (724/1471; 49.2%) of the positive samples. Double positivity was observed in 665 (665/1471; 45.2%) and triple positivity in 59 samples (59/1471; 4.0%) (Table 4). Anti-DENV IgG/anti-CHIKV IgG (455/1471; 30.9%) and anti-DENV IgG/anti-ZIKV IgG (50/1471; 3.4%) were the most frequently detected combinations. Among triple positives, anti-DENV IgG/anti-CHIKV IgG/anti-ZIKV IgG (21/1471; 1.4%) and anti-CCHFV IgG/anti-RVFV IgG/anti-YFV IgG (16/1471; 1.1%) were the most common (Figure 2).

Table 4.
Factors associated with seropositivity against arboviruses.
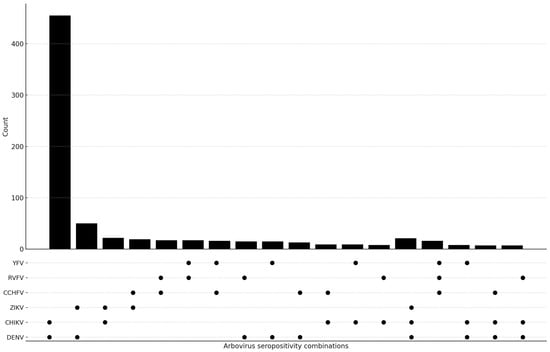
Figure 2.
The UpSet plot shows how often dual and triple arbovirus IgG seropositivity patterns appear together, and how much they overlap. The upper bar chart shows the number of participants with each serological combination, while the dot matrix indicates the viruses included in each pattern. The filled circles on the graph show the IgG results. The figure shows how the different viruses (DENV, CHIKV, ZIKV, CCHFV, RVFV and YFV) are exposed to each other.
3.3. Associations Between Participant Characteristics and Seropositivity
Multiple logistic regression analysis was performed to assess the association between certain variables and arbovirus seropositivity. Age, sex and place of residence were significantly associated with DENV seropositivity, while age and sex were associated with CHIKV seropositivity (Table 4). Participants aged 20–29 years were five times more likely to be positive for anti-DENV (aOR: 5.4; 95% CI: 3.2–9.2; p = 0.001) and nearly three times more likely for anti-CHIKV (aOR: 2.6; 95% CI: 1.3–5.3; p = 0.005) compared with those under 20 years. Seropositivity increased among participants ≥50 years, who were 27 times more likely to be DENV seropositive (aOR: 27.3; 95% CI: 14.5–54.0; p = 0.001) and approximately five times more likely to be CHIKV seropositive (aOR: 4.8; 95% CI: 2.3–9.8; p = 0.001) than participants under 20 years. Male sex was also positively associated with DENV seropositivity (aOR: 1.2; 95% CI: 1.0–1.6; p = 0.01) and negatively associated with CHIKV seropositivity (aOR: 0.8; 95% CI: 0.6–1.0; p = 0.03). Regarding place of residence, only living in the Sud-Ouest region was significantly associated with DENV seropositivity (aOR: 2.3; 95% CI: 1.3–4.0; p = 0.003) (Table 4).
The following factors were found to be associated with seropositivity to DENV and CHIKV in the study population (N = 724). The study examined a range of variables, including age, gender, and geographical location. Both cOR and aOR with 95% CI are shown to assess associations with arbovirus IgG seropositivity.
4. Discussion
The results of our study showed seroprevalences ranging from 1.1% for anti-YFV IgG to 75.4% for anti-DENV IgG. The high seroprevalence of anti-DENV IgG confirms the endemic circulation of DENV in Burkina Faso and supports data reported in previous studies [27,28,32,33]. Our study did not differentiate between serotypes, but previous studies have documented the presence of all 4 of them in the country [8,24,34,35]. The seroprevalence of 30.7% for anti-CHIKV IgG, which is similar to that reported by Lim et al. (29.1%) [29] in their 2015 population based study, also demonstrates wide circulation of CHIKV. Since CHIKV was also detected in vectors from Burkina Faso [36], this virus might be considered as possible cause of febrile infections and be included in the diagnostic panel of local health facilities. In contrast to the recently reported anti-ZIKV IgG seroprevalence of 22.6% in blood donors [30], we found a prevalence of only 2.8%, which may be attributable to differences in study populations (blood donors vs. HBV/HCV patients) and analytical methods (ELISA vs. Luminex – Microneutralization). We found a seroprevalence of 1.1% for anti-YFV IgG. Although the test used could not determine whether it was a result of previous contact or vaccination, national surveillance data confirmed the presence of the virus in Burkina Faso [31].
For the first time, our study provides serological indications of possible exposure to CCHFV (1.1%) and RVFV (1.2%) in the human population in Burkina Faso. Although seroprevalence levels were low, these findings suggest that these viruses may be present in the country and underline the relevance of including them in arbovirus surveillance efforts. Previous evidence of CCHFV exposure in sheep in Burkina Faso has been reported [37]. Additionally, studies from neighboring West African countries (Ghana, Senegal, Mauritania, Nigeria) [24,38,39,40] and other African regions (Cameroon, Tunisia) [41,42] support the potential for circulation of these viruses in both human and animal populations.
Multivariate analysis showed that IgG antibodies seroprevalence for DENV increased significantly with age, possibly because of continuous exposure risk and recurrent DENV epidemics since 2013 [35]. This result is in agreement with other studies conducted in the country [8,32], showing the association between age and seropositivity. The same association was found for anti-CHIKV IgG, suggesting endemic circulation of CHIKV in Burkina Faso and potentially also under detection during epidemics [29].
The seroprevalence was higher in males (77.2%) than in females (72.6%) for anti-DENV IgG and inverse for anti-CHIKV IgG (males: 28.8% versus females: 33.8%), which is consistent with other studies showing a higher risk of arbovirus infection in men (CHIKV) [43,44], (DENV) [45] or in women (DENV) [46]. This difference in exposure according to sex is difficult to explain since both viruses have the same vectors, but may be related to the sociocultural characteristics of each country [47]. In Burkina Faso, women spend most of their time in households that are often close to breeding sites of mosquitoes, which would explain at least the higher exposure to CHIKV, while men might be more exposed to sylvatic DENV.
Th IgG antibodies seroprevalence for DENV was very high in all four regions, ranging from 73% in the Boucle du Mouhoun region to 85% in the Sud-Ouest region. The positive association between residence in the Sud-Ouest region and exposure to dengue virus might be related to the high rainfall in this region, which favors the development and maintenance of vectors.
Although seroprevalence studies are important to assess arbovirus disease burden, the correct interpretation of the results poses a challenge especially for flaviviruses, which share several conserved epitopes, potentially leading to cross-reactions [48,49]. However, the magnitude of these cross-reactions varies depending on the type of flavivirus, the type of test used, and the target protein [50] and has been reported to range between 15.4% and 84% for DENV and other flaviviruses (YFV, West Nile Virus and Japanese encephalitis virus) [50,51], while another study reported no cross-reactivity of antibodies produced by yellow fever vaccine and a ZIKV ELISA and only 3.9% for one of three DENV ELISA kits tested [52]. Cross-reactivity of antibodies against CHIKV and DENV or other flaviviruses has been reported to be low (7%) [53], which is important for our study, where most of the double and triple positives involved DENV and CHIKV. Although the ELISA tests used in our study seemed to have high specificity (Table 1), not all cross-reactions relevant for this study were assessed by the manufacturers. Thus, we cannot exclude a certain overestimation of some antibody prevalence rates, but retain the main information, which is the confirmation of the circulation of the studied arboviruses in Burkina. This is especially relevant for RVFV and CCHFV, for which to the best of our knowledge, no cross-reactions with flaviviruses or togaviruses have been reported and for which we found two single positive samples each. In addition to the potential overestimation of seroprevalence rates, the retrospective nature of the study did not allow to include certain relevant variables (e.g., occupation, travel, living with pets, etc.) to assess associations with arbovirus exposure risk.
It should be noted that the study has some limitations. We were unable to perform confirmatory neutralization tests that would have distinguished actual infections from potentially cross-reactive IgG responses, particularly among flaviviruses. In addition, while the ELISA kits used report high diagnostic performance, their accuracy may vary in the West African context where multiple arboviruses co-circulate. A certain degree of cross-reactivity with related nairoviruses or phleboviruses cannot be excluded, since the ELISA test detects binding antibodies rather than functional antibodies. Finally, the use of samples from patients with viral hepatitis and the lack of information on occupation, travel or contact with animals may limit the generalizability of our findings.
5. Conclusions
Our results indicate a high seroprevalence of DENV and CHIKV, suggesting widespread circulation of these viruses in Burkina Faso. IgG antibodies against CCHFV and RVFV were detected, suggesting possible human exposure for the first time in the country; however, confirmatory testing using virus neutralization assays is needed. Strengthening arbovirus surveillance, including additional relevant viruses in diagnostic panels and using sensitive and specific diagnostic tools, will provide a clearer understanding of disease burden and inform prevention and control strategies.
Author Contributions
A.M.S., A.S.N. and J.M.H. designed the study. A.M.S., A.B.S.B. and M.N.G.O. selected the samples and extracted information from the database. A.S. coordinated and summarized the laboratory work and J.D. performed the statistical analyses. A.M.S. and J.M.H. interpreted the data and wrote the manuscript. I.B. and H.G.O. reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Luxembourg government through the Ministry of Foreign and European Affairs, Defence, Development Cooperation and Foreign Trade and the Luxembourg Institute of Health (“Microbiology for Development” project) as well as the Institut de Recherche en Sciences de la Santé (IRSS) in Burkina Faso.
Institutional Review Board Statement
This study was approved by the Institutional Ethics Board for Health Research on 19 January 2024 (IRB approval no.002-24/CEIRES). The blood samples used in this study had been collected as part of laboratory diagnosis. Patients were informed during enrolment that an aliquot would be stored at −80 °C for future research purposes. The samples were pseudonymized, and the database was accessible only to the investigators. The results were reported to health authorities.
Informed Consent Statement
Informed consent was obtained from the participants in this study.
Data Availability Statement
The authors confirm that the relevant data generated during this study are included in the article. All data supporting these results are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank “Assaut-Hépatites” for agreeing to make the database and sample library available to the research team for this study. We would also like to thank the medical team responsible for collecting, processing and storing the samples. In addition, the authors are grateful to Maëlys Merel, Julie Vialle, Delphine Lentz, Thibault Albert and Agathe Durand for performing most of the ELISAs.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gutiérrez-Vera, E.; Patiño, L.; Castillo-Segovia, M.; Mora-Valencia, V.; Montesdeoca-Agurto, J.; Regato-Arrata, M. Seroprevalence of arboviruses in Ecuador: Implications for improved surveillance. Biomédica 2021, 41, 247–259. [Google Scholar] [CrossRef]
- Ribeiro, G.S.; Hamer, G.L.; Diallo, M.; Kitron, U.; Ko, A.I.; Weaver, S.C. Influence of herd immunity in the cyclical nature of arboviruses. Curr. Opin. Virol. 2020, 40, 1–10. [Google Scholar] [CrossRef]
- Salgado, B.B.; Maués, F.C.d.J.; Pereira, R.L.; Chiang, J.O.; Freitas, M.N.d.O.; Ferreira, M.S.; Martins, L.C.; Vasconcelos, P.F.d.C.; Ganoza, C.; Lalwani, P. Prevalence of arbovirus antibodies in young healthy adult population in Brazil. Parasit. Vectors 2021, 14, 403. [Google Scholar] [CrossRef]
- Ushijima, Y.; Abe, H.; Ondo, G.N.; Bikangui, R.; Loembé, M.M.; Zadeh, V.R.; Essimengane, J.G.E.; Mbouna, A.V.N.; Bache, E.B.; Agnandji, S.T.; et al. Surveillance of the major pathogenic arboviruses of public health concern in Gabon, Central Africa: Increased risk of West Nile virus and dengue virus infections. BMC Infect. Dis. 2021, 21, 265. [Google Scholar] [CrossRef] [PubMed]
- Fritzell, C.; Rousset, D.; Adde, A.; Kazanji, M.; Van Kerkhove, M.D.; Flamand, C. Current challenges and implications for dengue, chikungunya and Zika seroprevalence studies worldwide: A scoping review. PLoS Negl. Trop. Dis. 2018, 12, e0006533. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.; Pettersson, J.; Higgs, S.; Charrel, R.; de Lamballerie, X. Emerging arboviruses: Why today? One Health 2017, 4, 1–13. [Google Scholar] [CrossRef]
- Marbán-Castro, E.; Arrieta, G.J.; Martínez, M.J.; González, R.; Bardají, A.; Menéndez, C.; Mattar, S. High Seroprevalence of Antibodies against Arboviruses among Pregnant Women in Rural Caribbean Colombia in the Context of the Zika Virus Epidemic. Antibodies 2020, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.K.; Lim, J.K.; Seydou, Y.; Carabali, M.; Barro, A.; Dahourou, D.L.; Lee, K.S.; Nikiema, T.; Namkung, S.; Lee, J.-S.; et al. Clinical and epidemiologic characteristics associated with dengue during and outside the 2016 outbreak identified in health facility-based surveillance in Ouagadougou, Burkina Faso. PLoS Negl. Trop. Dis. 2019, 13, e0007882. [Google Scholar] [CrossRef]
- Patterson, J.; Sammon, M.; Garg, M. Dengue, Zika and Chikungunya: Emerging Arboviruses in the New World. West. J. Emerg. Med. 2016, 17, 671–679. [Google Scholar] [CrossRef]
- De Lima Cavalcanti, T.Y.V.; Pereira, M.R.; De Paula, S.O.; Franca, R.F.D.O. A Review on Chikungunya Virus Epidemiology, Pathogenesis and Current Vaccine Development. Viruses 2022, 14, 969. [Google Scholar] [CrossRef]
- ECDE. Chikungunya Virus Disease—Annual Epidemiological Report for 2022. Available online: https://www.ecdc.europa.eu/en/publications-data/chikungunya-virus-disease-annual-epidemiological-report-2022 (accessed on 25 June 2024).
- Karkhah, A.; Nouri, H.R.; Javanian, M.; Koppolu, V.; Masrour-Roudsari, J.; Kazemi, S.; Ebrahimpour, S. Zika virus: Epidemiology, clinical aspects, diagnosis, and control of infection. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 2035–2043. [Google Scholar] [CrossRef] [PubMed]
- Koppolu, V.; Shantha Raju, T. Zika virus outbreak: A review of neurological complications, diagnosis, and treatment options. J. Neurovirol. 2018, 24, 255–272. [Google Scholar] [CrossRef]
- WHO. Yellow Fever—African Region (AFRO). Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON510 (accessed on 25 June 2024).
- Johansson, M.A.; Vasconcelos, P.F.C.; Staples, J.E. The whole iceberg: Estimating the incidence of yellow fever virus infection from the number of severe cases. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 482–487. [Google Scholar] [CrossRef]
- Waggoner, J.J.; Rojas, A.; Pinsky, B.A. Yellow Fever Virus: Diagnostics for a Persistent Arboviral Threat. J. Clin. Microbiol. 2018, 56, e00827-18. [Google Scholar] [CrossRef]
- Wright, D.; Kortekaas, J.; Bowden, T.A.; Warimwe, G.M. Rift Valley fever: Biology and epidemiology. J. Gen. Virol. 2019, 100, 1187–1199. [Google Scholar] [CrossRef]
- Ikegami, T.; Makino, S. The Pathogenesis of Rift Valley Fever. Viruses 2011, 3, 493–519. [Google Scholar] [CrossRef]
- Madani, T.A.; Al-Mazrou, Y.Y.; Al-Jeffri, M.H.; Mishkhas, A.A.; Al-Rabeah, A.M.; Turkistani, A.M.; Al-Sayed, M.O.; Abodahish, A.A.; Khan, A.S.; Ksiazek, T.G.; et al. Rift Valley Fever Epidemic in Saudi Arabia: Epidemiological, Clinical, and Laboratory Characteristics. Clin. Infect. Dis. 2003, 37, 1084–1092. [Google Scholar] [CrossRef]
- Baba, M.; Masiga, D.K.; Sang, R.; Villinger, J. Has Rift Valley fever virus evolved with increasing severity in human populations in East Africa? Emerg. Microbes Infect. 2016, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Serretiell, E.; Astorri, R.; Chianese, A.; Stelitano, D.; Zannella, C.; Folliero, V.; Santella, B.; Galdiero, M.; Franci, G.; Galdiero, M. The emerging tick-borne Crimean-Congo haemorrhagic fever virus: A narrative review. Travel Med. Infect. Dis. 2020, 37, 101871. [Google Scholar] [CrossRef] [PubMed]
- Spengler, J.R.; Bente, D.A.; Bray, M.; Burt, F.; Hewson, R.; Korukluoglu, G.; Mirazimi, A.; Weber, F.; Papa, A. Second International Conference on Crimean-Congo Hemorrhagic Fever. Antivir. Res. 2018, 150, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Hawman, D.W.; Feldmann, H. Crimean–Congo haemorrhagic fever virus. Nat. Rev. Microbiol. 2023, 21, 463–477. [Google Scholar] [CrossRef]
- Nimo-Paintsi, S.C.; Mosore, M.; Addo, S.O.; Lura, T.; Tagoe, J.; Ladzekpo, D.; Addae, C.; Bentil, R.E.; Behene, E.; Dafeamekpor, C.; et al. Ticks and prevalence of tick-borne pathogens from domestic animals in Ghana. Parasit. Vectors 2022, 15, 86. [Google Scholar] [CrossRef] [PubMed]
- Gyasi, P.; Yakass, M.B.; Quaye, O. Analysis of dengue fever disease in West Africa. Exp. Biol. Med. 2023, 248, 1850–1863. [Google Scholar] [CrossRef]
- Tarnagda, Z.; Congo, M.; Sagna, T.; Ouédraogo, C.; Nikiéma, V.; Cissé, A.; Sanou, M.A.; Ouédraogo, A.; Ouédraogo, J.B.; Sangaré, L. Outbreak of dengue fever in Ouagadougou, Burkina Faso, 2013. Int. J. Microbiol. Immunol. Res. 2014, 2, 101–108. [Google Scholar]
- Tarnagda, Z.; Cissé, A.; Bicaba, B.W.; Diagbouga, S.; Sagna, T.; Ilboudo, A.K.; Tialla, D.; Lingani, M.; Sondo, K.A.; Yougbaré, I.; et al. Dengue Fever in Burkina Faso, 2016. Emerg. Infect. Dis. 2018, 24, 170–172. [Google Scholar] [CrossRef]
- Im, J.; Ridde, V.; Agnandji, S.T.; Lell, B.; Yaro, S.; Yang, J.S.; Hoinard, D.; Weaver, S.C.; Vanhomwegen, J.; Salje, H.; et al. The epidemiology of dengue outbreaks in 2016 and 2017 in Ouagadougou, Burkina Faso. Heliyon 2020, 6, e04389. [Google Scholar] [CrossRef]
- Lim, J.K.; Ridde, V.; Agnandji, S.T.; Lell, B.; Yaro, S.; Yang, J.S.; Hoinard, D.; Weaver, S.C.; Vanhomwegen, J.; Salje, H.; et al. Seroepidemiological Reconstruction of Long-term Chikungunya Virus Circulation in Burkina Faso and Gabon. J. Infect. Dis. 2022, 227, 261–267. [Google Scholar] [CrossRef]
- Tinto, B.; Kaboré, D.A.; Kania, D.; Kagoné, T.S.; Kiba-Koumaré, A.; Pinceloup, L.; Thaurignac, G.; de Perre, P.V.; Dabire, R.K.; Baldet, T.; et al. Serological Evidence of Zika Virus Circulation in Burkina Faso. Pathogens 2022, 11, 741. [Google Scholar] [CrossRef]
- Yaro, S.; Zango, A.; Rouamba, J.; Diabaté, A.; Dabiré, R.; Kambiré, C.; Tiendrebeogo, S.M.R.; Yonli, T.; Ouango, J.G.; Diagbouga, S.P. « Situation épidémiologique de la fièvre jaune au Burkina Faso de 2003 à 2008. Bull. Société Pathol. Exot. 2010, 103, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.K.; Carabali, M.; Edwards, T.; Barro, A.; Lee, J.-S.; Dahourou, D.; Lee, K.S.; Nikiema, T.; Shin, M.Y.; Bonnet, E.; et al. Estimating the Force of Infection for Dengue Virus Using Repeated Serosurveys, Ouagadougou, Burkina Faso. Emerg. Infect. Dis. 2021, 27, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Ouattara, C.A.; Traore, S.; Sangare, I.; Traore, T.I.; Meda, Z.C.; Savadogo, L.G.B. Spatiotemporal analysis of dengue fever in Burkina Faso from 2016 to 2019. BMC Public Health 2022, 22, 462. [Google Scholar] [CrossRef]
- Ridde, V.; Agier, I.; Bonnet, E.; Carabali, M.; Dabiré, K.R.; Fournet, F.; Ly, A.; Meda, I.B.; Parra, B. Presence of three dengue serotypes in Ouagadougou (Burkina Faso): Research and public health implications. Infect. Dis. Poverty 2016, 5, 23. [Google Scholar] [CrossRef]
- Tinto, B.; Kania, D.; Kagone, T.S.; Dicko, A.; Traore, I.; de Rekeneire, N.; Bicaba, B.W.; Hien, H.; Van de Perre, P.; Simonin, Y.; et al. Circulation du virus de la dengue en Afrique de l’Ouest: Une problématique émergente de santé publique. Médecine/Sciences 2022, 38, 152–158. [Google Scholar] [CrossRef]
- Hien, A.S.; Sangare, I.; Ouattara, E.L.P.; Sawadogo, S.P.; Soma, D.D.; Maiga, H.; Diabate, A.; Bonnet, E.; Ridde, V.; Fournet, F.; et al. Chikungunya (Togaviridae) and dengue 2 (Flaviviridae) viruses detected from Aedes aegypti mosquitoes in Burkina Faso by qRT-PCR technique: Preliminary results and perspective for molecular characterization of arbovirus circulation in vector populations. Front. Trop. Dis. 2022, 3, 920224. [Google Scholar] [CrossRef]
- Dahourou, L.D.; Akio, S.; Savadogo, M.; Yougbaré, B.; Ouoba, L.B.; Tapsoba, A.S.R.; Zerbo, L.H.; Ilboudo, A.K.; Abga, R.L.; Traoré, A.; et al. Serological evidence and factors associated with Crimean–Congo haemorrhagic fever in sheep in Burkina Faso. Vet. Med. Sci. 2024, 10, e1322. [Google Scholar] [CrossRef] [PubMed]
- Bukbuk, D.N.; Dowall, S.D.; Lewandowski, K.; Bosworth, A.; Baba, S.S.; Varghese, A.; Watson, R.J.; Bell, A.; Atkinson, B.; Hewson, R. Serological and Virological Evidence of Crimean-Congo Haemorrhagic Fever Virus Circulation in the Human Population of Borno State, Northeastern Nigeria. PLoS Negl. Trop. Dis. 2016, 10, e0005126. [Google Scholar] [CrossRef] [PubMed]
- El Ghassem, A.; Apolloni, A.; Vial, L.; Bouvier, R.; Bernard, C.; Khayar, M.S.; Ahmed, M.C.; Fausther-Bovendo, H.; Beyit, A.D.; Yahya, B.; et al. Risk factors associated with Crimean-Congo hemorrhagic fever virus circulation among human, livestock and ticks in Mauritania through a one health retrospective study. BMC Infect. Dis. 2023, 23, 764. [Google Scholar] [CrossRef] [PubMed]
- Sankhe, S.; Talla, C.; Thiam, M.S.; Faye, M.; Barry, M.A.; Diarra, M.; Dia, M.; Ndiaye, O.; Sembene, P.M.; Diop, B.; et al. Seroprevalence of Crimean-Congo Hemorrhagic Fever Virus and Rift Valley Fever Virus in human population in Senegal from October to November 2020. IJID Reg. 2023, 7, 216–221. [Google Scholar] [CrossRef]
- Bosworth, A.; Ghabbari, T.; Dowall, S.; Varghese, A.; Fares, W.; Hewson, R.; Zhioua, E.; Chakroun, M.; Tiouiri, H.; Jemaa, M.B. Serologic evidence of exposure to Rift Valley fever virus detected in Tunisia. New Microbes New Infect. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Sadeuh-Mba, S.A.; Wansi, G.M.Y.; Demanou, M.; Gessain, A.; Njouom, R. Serological evidence of rift valley fever Phlebovirus and Crimean-Congo hemorrhagic fever orthonairovirus infections among pygmies in the east region of Cameroon. Virol. J. 2018, 15, 63. [Google Scholar] [CrossRef]
- Périssé, A.R.S.; Souza-Santos, R.; Duarte, R.; Santos, F.; de Andrade, C.R.; Rodrigues, N.C.P.; Schramm, J.M.D.A.; da Silva, E.D.; Jacobson, L.D.S.V.; Lemos, M.C.F.; et al. Zika, dengue and chikungunya population prevalence in Rio de Janeiro city, Brazil, and the importance of seroprevalence studies to estimate the real number of infected individuals. PLoS ONE 2020, 15, e0243239. [Google Scholar] [CrossRef]
- Sissoko, D.; Moendandze, A.; Malvy, D.; Giry, C.; Ezzedine, K.; Solet, J.L.; Pierre, V. Seroprevalence and Risk Factors of Chikungunya Virus Infection in Mayotte, Indian Ocean, 2005-2006: A Population-Based Survey. PLoS ONE 2008, 3, e3066. [Google Scholar] [CrossRef]
- Muniz, P.T.; de Souza, V.A.F.; Pannuti, C.S.; Sperança, M.A.; Terzian, A.C.B.; Nogueira, M.L.; Yamamura, A.M.Y.; Freire, M.S.; da Silva, N.S.; Malafronte, R.S.; et al. Risk Factors for Dengue Virus Infection in Rural Amazonia: Population-based Cross-sectional Surveys. Am. J. Trop. Med. Hyg. 2008, 79, 485–494. [Google Scholar] [CrossRef]
- Soghaier, M.A.; Mahmood, S.F.; Pasha, O.; Azam, S.I.; Karsani, M.M.; Elmangory, M.M.; Elmagboul, B.A.; Okoued, S.I.; Shareef, S.M.; Khogali, H.S.; et al. Factors associated with dengue fever IgG sero-prevalence in South Kordofan State, Sudan, in 2012: Reporting prevalence ratios. J. Infect. Public Health 2014, 7, 54–61. [Google Scholar] [CrossRef]
- Salje, H.; Cauchemez, S.; Alera, M.T.; Rodriguez-Barraquer, I.; Thaisomboonsuk, B.; Srikiatkhachorn, A.; Lago, C.B.; Villa, D.; Klungthong, C.; Tac-An, I.A.; et al. Reconstruction of 60 Years of Chikungunya Epidemiology in the Philippines Demonstrates Episodic and Focal Transmission. J. Infect. Dis. 2016, 213, 604–610. [Google Scholar] [CrossRef]
- Chan, K.R.; Chan, K.R.; Ismail, A.A.; Thergarajan, G.; Raju, C.S.; Yam, H.C.; Rishya, M.; Sekaran, S.D. Serological cross-reactivity among common flaviviruses. Front. Cell. Infect. Microbiol. 2022, 12, 975398. [Google Scholar] [CrossRef]
- Da Silva, P.G.; Reis, J.A.S.D.; Rodrigues, M.N.; Da Silva Ardaya, Q.; Mesquita, J.R. Serological Cross-Reactivity in Zoonotic Flaviviral Infections of Medical Importance. Antibodies 2023, 12, 18. [Google Scholar] [CrossRef]
- Endale, A.; Medhin, G.; Darfiro, K.; Kebede, N.; Legesse, M. Magnitude of Antibody Cross-Reactivity in Medically Important Mosquito-Borne Flaviviruses: A Systematic Review. Infect. Drug Resist. 2021, 14, 4291–4299. [Google Scholar] [CrossRef] [PubMed]
- Koraka, P.; Zeller, H.; Niedrig, M.; Osterhaus, A.D.M.E.; Groen, J. Reactivity of serum samples from patients with a flavivirus infection measured by immunofluorescence assay and ELISA. Microbes Infect. 2002, 4, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Souza, N.C.S.E.; Félix, A.C.; De Paula, A.V.; Levi, J.E.; Pannuti, C.S.; Romano, C.M. Evaluation of serological cross-reactivity between yellow fever and other flaviviruses. Int. J. Infect. Dis. 2019, 81, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Kam, Y.-W.; Pok, K.-Y.; Eng, K.E.; Tan, L.-K.; Kaur, S.; Lee, W.W.L.; Leo, Y.-S.; Ng, L.-C.; Ng, L.F.P. Sero-Prevalence and Cross-Reactivity of Chikungunya Virus Specific Anti-E2EP3 Antibodies in Arbovirus-Infected Patients. PLoS Negl. Trop. Dis. 2015, 9, e3445. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).