Dietary β-1,3-Glucan Promotes Growth Performance and Enhances Non-Specific Immunity by Modulating Pattern Recognition Receptors in Juvenile Oriental River Prawn (Macrobrachium nipponense)
Abstract
1. Introduction
2. Materials and Methods
2.1. The Origin of Prawns and β-1,3-Glucan
2.2. Experimental Design and Dietary Composition
2.3. Experimental Animals and Feeding Trial
2.4. Analysis of Growth-Related Parameters
2.5. Aeromonas Hydrophila Challenge
2.6. Biochemical Parameters Analysis
2.7. 16S Illumina High-Throughput Sequencing Analysis Process
2.8. mRNA Expression Analysis
2.9. Data Analysis and Processing
3. Results
3.1. Growth Performance of Prawns
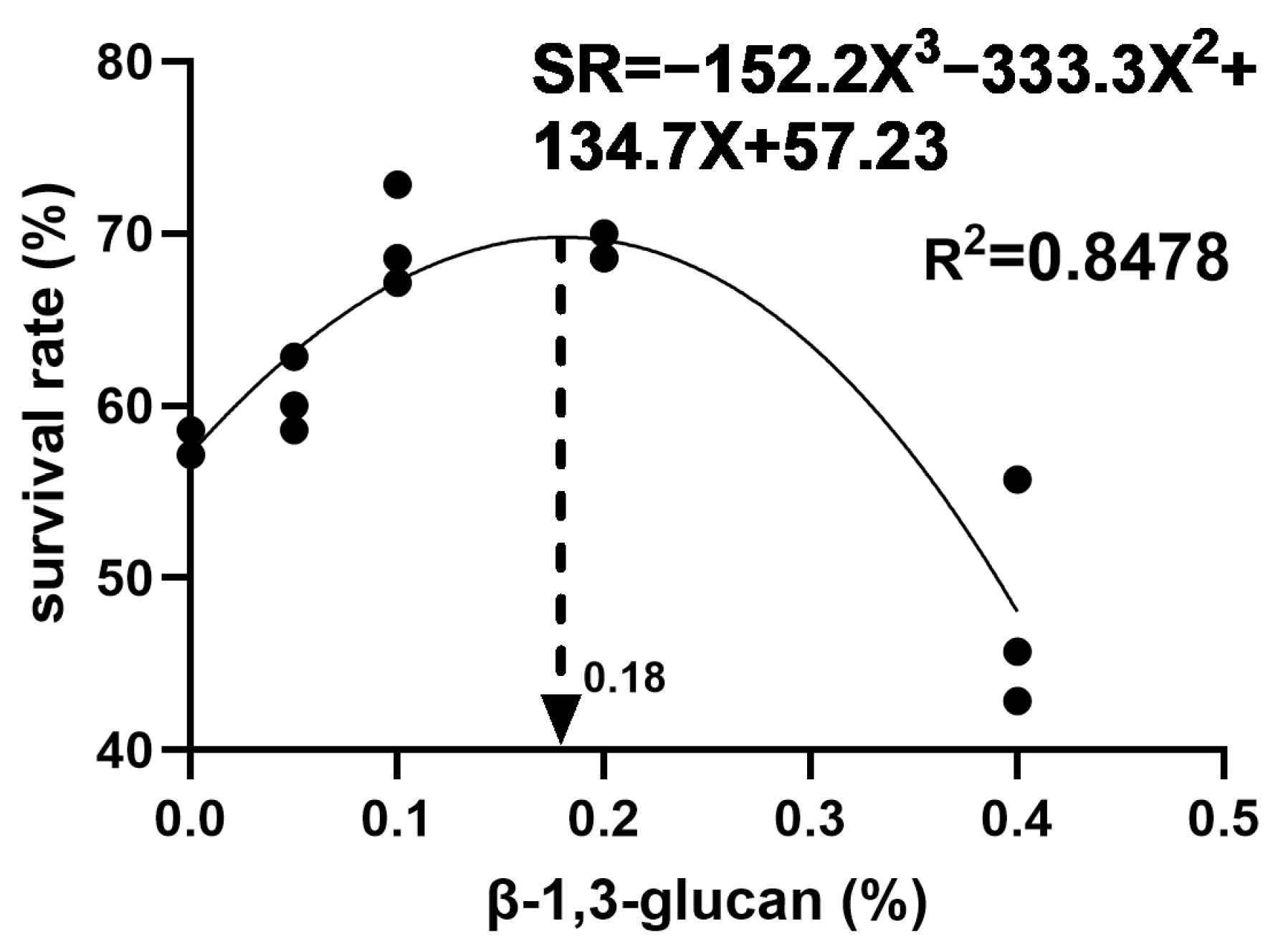
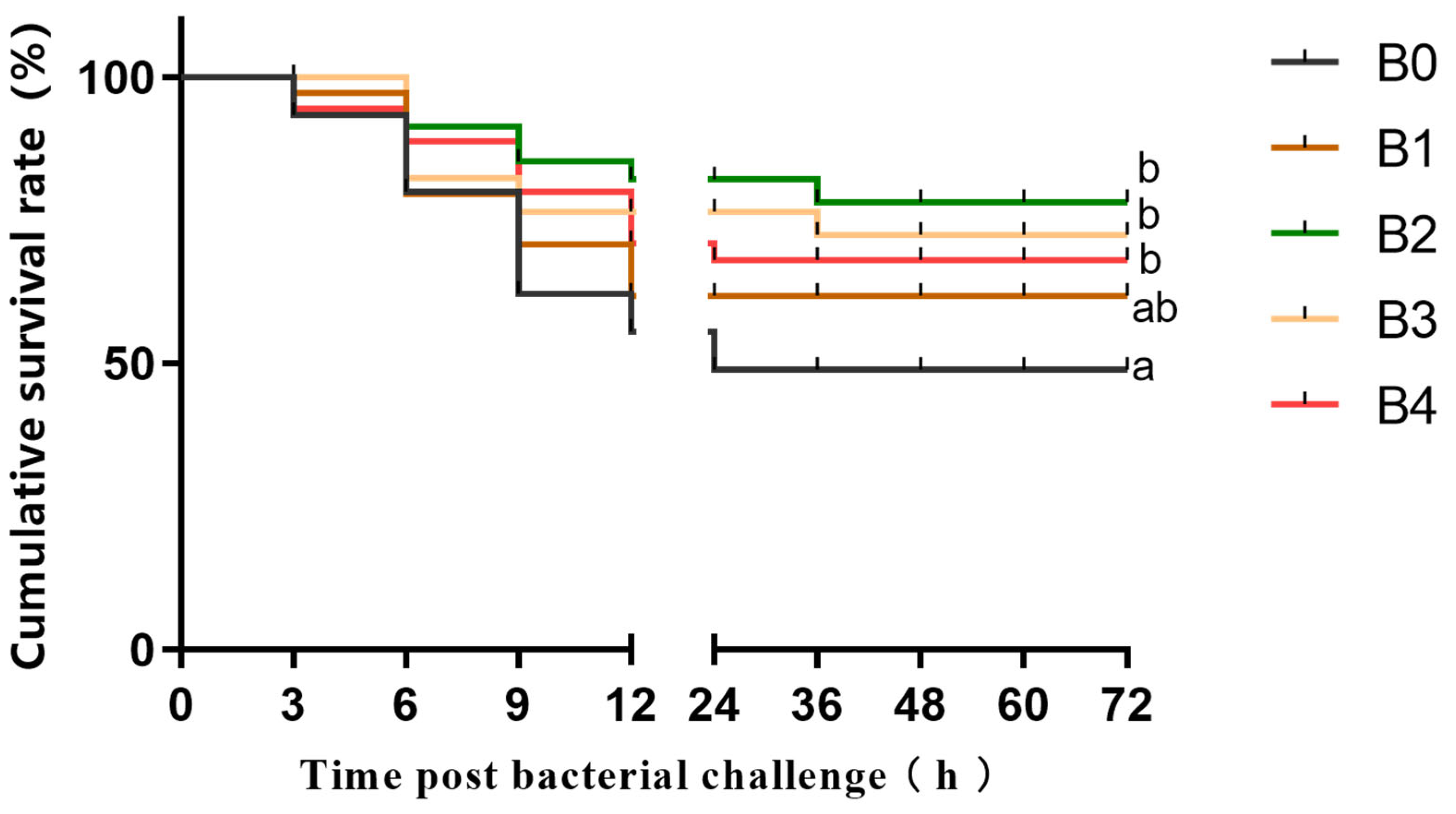
3.2. Survival Rate of the Prawns after A. hydrophila Challenge Test
3.3. Innate Immune Response and Antioxidant Enzyme Activity in the Hepatopancreas of Prawns
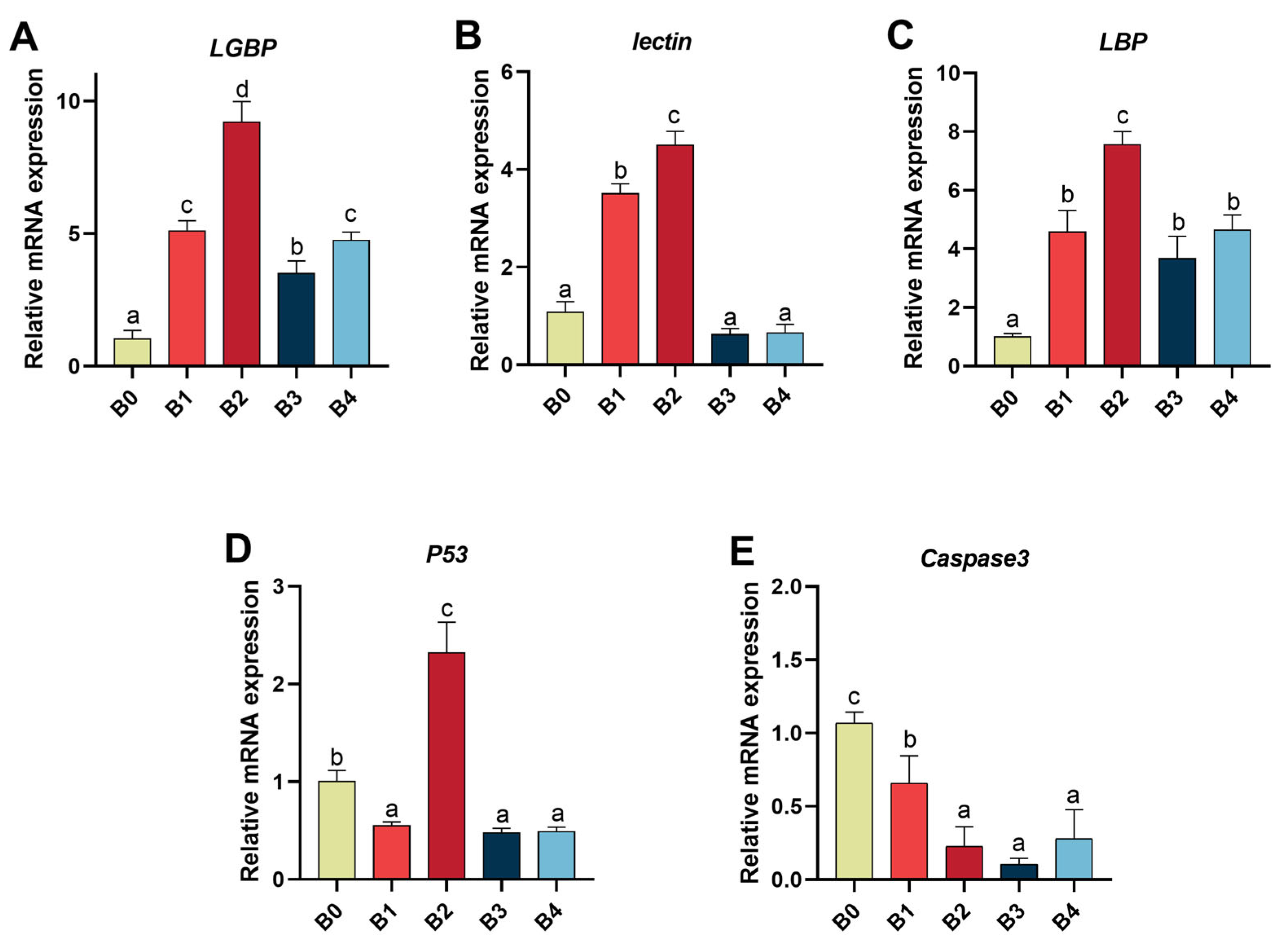
3.4. The mRNA Expression of Genes Related to PRRs and Apoptosis
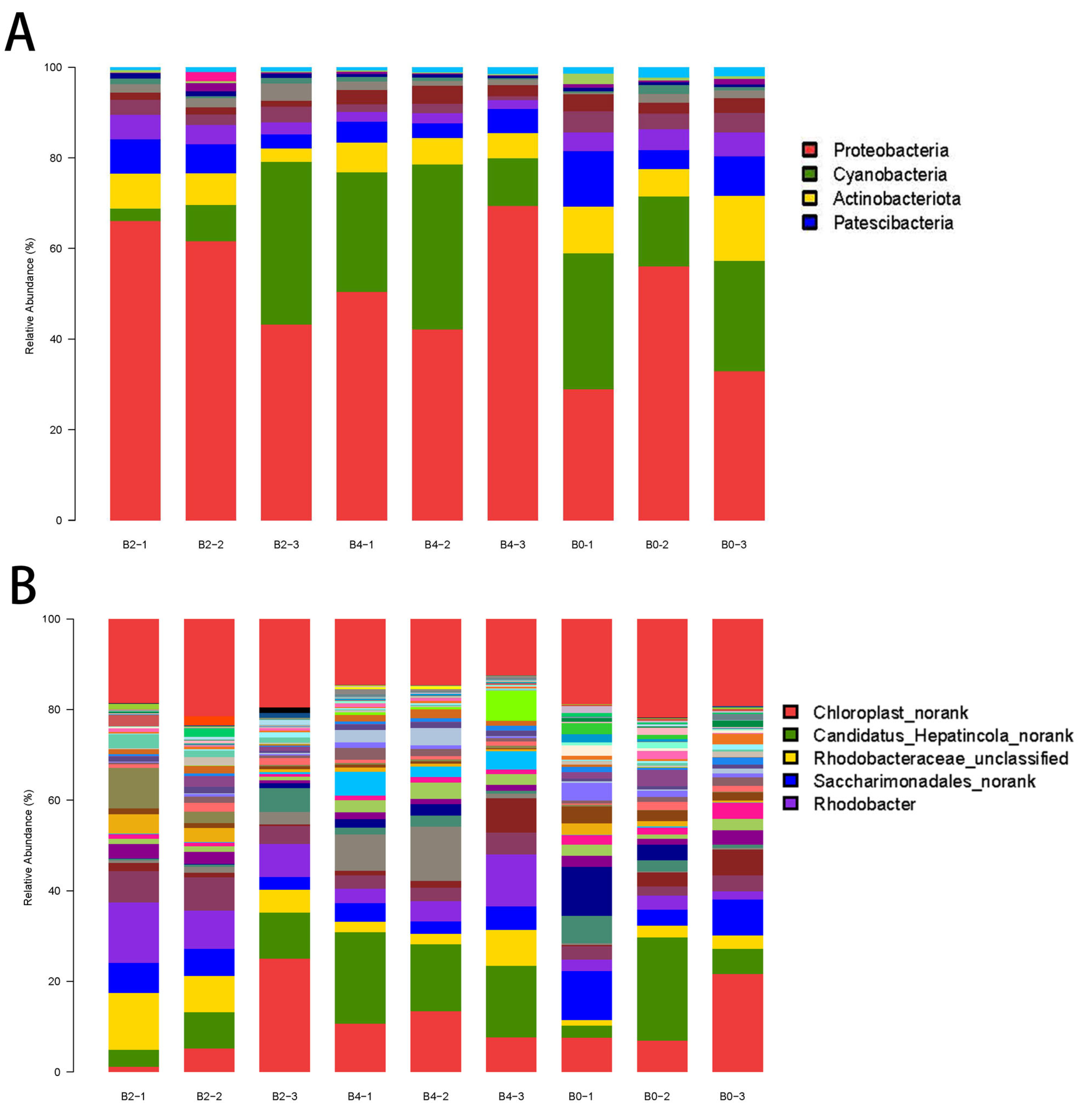
3.5. Gut Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bostock, J.; Mcandrew, B.; Richards, R.; Jauncey, K.; Telfer, T.; Lorenzen, K.; Little, D.; Ross, L.; Handisyde, N.; Gatward, I.; et al. Aquaculture: Global status and trends. Philos. Trans. R. Soc. Lond. B Biol. 2010, 365, 2897–2912. [Google Scholar] [CrossRef] [PubMed]
- Lafferty, K.D.; Harvell, C.D.; Conrad, J.M.; Friedman, C.S.; Kent, M.L.; Curis, A.M.; Powell, E.N.; Rondeau, D.; Saksida, S.M. Infectious diseases affect marine fisheries and aquaculture economics. Ann. Rev. Mar. Sci. 2015, 7, 471–496. [Google Scholar] [CrossRef] [PubMed]
- Defoirdt, T.; Sorgeloos, P.; Bossier, P. Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr. Opin. Microbiol. 2011, 14, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Chuah, L.O.; Effarizah, M.E.; Goni, A.M.; Rusul, G. Antibiotic application and emergence of multiple antibiotic resistance (MAR) in global catfish aquaculture. Curr. Environ. Health Rep. 2016, 3, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Limbu, S.M.; Chen, L.Q.; Zhang, M.L.; Du, Z.Y. A global analysis on the systemic effects of antibiotics in cultured fish and their potential human health risk: A review. Rev. Aquac. 2021, 13, 1015–1059. [Google Scholar] [CrossRef]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Khan, M.; Lively, J.A. Determination of sulfite and antimicrobial residue in imported shrimp to the USA. Aquacult. Rep. 2020, 18, 100529. [Google Scholar] [CrossRef]
- Wang, W.; Sun, J.; Liu, C.; Xue, Z. Application of immunostimulants in aquaculture: Current knowledge and future perspectives. Aquac. Res. 2017, 48, 1–23. [Google Scholar] [CrossRef]
- Bouallegui, Y. A comprehensive review on crustaceans’ immune system with a focus on freshwater crayfish in relation to crayfish plague disease. Front. Immunol. 2021, 12, 667787. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.T.; Kong, T.; Zhang, M.; Li, S. Pattern recognition receptors and their roles on the innate immune system of mud crab (Scylla paramamosain). Dev. Comp. Immunol. 2020, 102, 103469. [Google Scholar] [CrossRef]
- Hu, B.Q.; Wen, C.G.; Liu, Y. Identification and expression analysis on two forms bactericidal permeability-increasing protein (bpi)/lipopolysaccharide-binding protein (lbp) of triangle mussel, hyriopsis cumingii. Fish Shellfish Immunol. 2016, 53, 111–112. [Google Scholar] [CrossRef]
- Gao, X.; Ke, C.; Zhang, M.; Li, X.; Wu, F.; Liu, Y. Effects of the probiotic Bacillus amyloliquefaciens on the growth, immunity, and disease resistance of Haliotis discus hannai. Fish Shellfish Immunol. 2019, 94, 617–627. [Google Scholar] [CrossRef]
- Jin, P.; Zhou, L.; Song, X.; Qian, J.; Chen, L.; Ma, F. Particularity and universality of a putative Gram-negative bacteria-binding protein (GNBP) gene from amphioxus (Branchiostoma belcheri): Insights into the function and evolution of GNBP. Fish Shellfish Immunol. 2012, 33, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.G.; Aboobaker, A.A. Comparative genomic analysis of innate immunity reveals novel and conserved components in crustacean food crop species. BMC Genom. 2017, 18, 389. [Google Scholar] [CrossRef] [PubMed]
- Hoeger, U.; Schenk, S. Crustacean hemolymph lipoproteins. In Vertebrate and Invertebrate Respiratory Proteins, Lipoproteins and Other Body Fluid Proteins; Subcellular Biochemistry; Springer: Berlin/Heidelberg, Germany, 2020; pp. 35–62. [Google Scholar] [CrossRef]
- Hang, Y.; Ni, M.; Zhang, P.; Bai, Y.; Zhou, B.; Zheng, J.; Cui, Z. Identification and functional characterization of C-type lectins and crustins provide new insights into the immune response of Portunus trituberculatus. Fish Shellfish Immunol. 2022, 129, 170–181. [Google Scholar] [CrossRef]
- Wu, C.; Noonin, C.; Jiravanichpaisal, P.; Söderhäll, I.; Söderhäll, K. An insect TEP in a crustacean is specific for cuticular tissues and involved in intestinal defense. Insect Biochem. Mol. Biol. 2012, 42, 71–80. [Google Scholar] [CrossRef]
- Mateu, E.; Díaz, I. The challenge of PRRS immunology. Vet. J. 2008, 177, 345–351. [Google Scholar] [CrossRef]
- Chen, Q.; Bai, S.; Dong, C. A fibrinogen-related protein identified from hepatopancreas of crayfish is a potential pattern recognition receptor. Fish Shellfish Immunol. 2016, 56, 349–357. [Google Scholar] [CrossRef]
- Habib, Y.J.; Zhang, Z. The involvement of crustacean toll-like receptors in pathogen recognition. Fish Shellfish Immunol. 2020, 102, 169–176. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, S.; Li, Y.; Li, J.; Liu, H. Hemocyte-mediated phagocytosis in crustaceans. Front. Immunol. 2020, 11, 268. [Google Scholar] [CrossRef]
- Gallo, C.; Schiavon, F.; Ballarin, L. Insight on cellular and humoral components of innate immunity in Squilla mantis (Crustacea, Stomatopoda). Fish Shellfish Immunol. 2011, 31, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Paz, A.; Muhlia-Almazán, A. Uncovering and defragmenting the role of the Toll pathway in the innate immune responses of cultured crustaceans against viral pathogens. Rev. Aquac. 2020, 12, 1818–1835. [Google Scholar] [CrossRef]
- Bai, L.; Zhou, K.; Li, H.; Qin, Y.; Wang, Q.; Li, W. Bacteria-induced IMD-Relish-AMPs pathway activation in Chinese mitten crab. Fish Shellfish Immunol. 2020, 106, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, J. Pattern recognition receptors acting in innate immune system of shrimp against pathogen infections. Fish Shellfish Immunol. 2013, 34, 981–989. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, Q. Research progress in innate immunity of freshwater crustaceans. Dev. Comp. Immunol. 2020, 104, 103569. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Bu, X.; Xiao, S.; Lin, Z.; Wang, X.; Jia, Y.; Wang, X.; Qin, J.; Chen, L. Effect of single and combined immunostimulants on growth, anti-oxidation activity, non-specific immunity and resistance to Aeromonas hydrophila in Chinese mitten crab (Eriocheir sinensis). Fish Shellfish Immunol. 2019, 93, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Ojerio, V.; Corre, V.; Toledo, N.; Andrino-Felarca, K.; Nievales, L.; Traifalgar, F. Alginic acid as immunostimulant: Effects of dose and frequency on growth performance, immune responses, and white spot syndrome virus resistance in tiger shrimp Penaeus monodon (Fabricius, 1798). Aquac. Int. 2018, 26, 267–278. [Google Scholar] [CrossRef]
- Montero-Rocha, A.; McIntosh, D.; Sanchez-Merino, R.; Flores, I. Immunostimulation of white shrimp (Litopenaeus vannamei) following dietary administration of Ergosan. J. Invertebr. Pathol. 2006, 91, 188–194. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Sharifinia, M.; Ghaedi, G. β-glucan as a promising food additive and immunostimulant in aquaculture industry. Ann. N. Y. Acad. Sci. 2022, 22, 817–827. [Google Scholar] [CrossRef]
- Ibrahim, L.A.; ElSayed, E.E. The influence of water quality on fish tissues and blood profile in Arab al-Ulayqat Lakes, Egypt. Egypt. J. Aquat. Res. 2023, 49, 235–243. [Google Scholar] [CrossRef]
- Ding, Z.; Xiong, Y.; Zheng, J.; Zhou, D.; Kong, Y.; Qi, C.; Liu, Y.; Limbu, S.M. Modulation of growth, antioxidant status, hepatopancreas morphology, and carbohydrate metabolism mediated by alpha-lipoic acid in juvenile freshwater prawns Macrobrachium nipponense under two dietary carbohydrate levels. Aquaculture 2022, 546, 737314. [Google Scholar] [CrossRef]
- Shahbazi, S.; Bolhassani, A. Immunostimulants: Types and functions. Can. J. Infect. Dis. Med. Microbiol. 2016, 4, 45–51. [Google Scholar]
- Stier, H.; Ebbeskotte, V.; Gruenwald, J. Immune-modulatory effects of dietary Yeast Beta-1,3/1,6-D-glucan. Nutr. J. 2014, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Zhou, L.; Qu, Y.; Lu, K.; Han, F.; Li, E. Effects of different dietary β-glucan levels on antioxidant capacity and immunity, gut microbiota and transcriptome responses of white shrimp (Litopenaeus vannamei) under low salinity. Antioxidants 2022, 11, 2282. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhu, Y.; Yu, J.; Fang, L.; Li, Y.; Wang, M.; Liu, J.; Yan, P.; Xia, J.; Liu, G.; et al. Effects of sulfated β-glucan from Saccharomyces cerevisiae on growth performance, antioxidant ability, nonspecific immunity, and intestinal flora of the red swamp crayfish (Procambarus clarkii). Fish Shellfish Immunol. 2022, 127, 891–900. [Google Scholar] [CrossRef]
- Dawood, M.A.; Eweedah, N.M.; Moustafa, E.M.; Shahin, M.G. Synbiotic effects of Aspergillus oryzae and β-glucan on growth and oxidative and immune responses of Nile Tilapia, Oreochromis niloticus. Probiotics Antimicrob. Proteins 2020, 12, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Andrino, K.G.S.; Apines-Amar, M.J.S.; Janeo, R.L.; Corre, V.L., Jr. Effects of dietary mannan oligosaccharide (MOS) and β-glucan on growth, immune response and survival against white spot syndrome virus (WSSV) infection of juvenile tiger shrimp Penaeus monodon. Aquac. Aquar. Conserv. Legis. 2014, 7, 321–332. [Google Scholar]
- Tian, J.; Yang, Y.; Du, X.; Xu, W.; Zhu, B.; Huang, Y.; Ye, Y.; Zhao, Y.; Li, Y. Effects of dietary soluble β-1,3-glucan on the growth performance, antioxidant status, and immune response of the river prawn (Macrobrachium nipponense). Fish Shellfish Immunol. 2023, 138, 108848. [Google Scholar] [CrossRef]
- Tian, J.; Yang, Y.; Xu, W.; Du, X.; Ye, Y.; Zhu, B.; Huang, Y.; Zhao, Y.; Li, Y. Effects of β-1,3-glucan on growth, immune responses, and intestinal microflora of the river prawn (Macrobrachium nipponense) and its resistance against Vibrio parahaemolyticus. Fish Shellfish Immunol. 2023, 142, 109142. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.T.; Li, X.J.; Wang, S.C.; Li, D.; Li, W.W.; Wang, Q. Lipopolysaccharide and beta-1,3-glucan binding protein (LGBP) stimulates prophenoloxidase activating system in Chinese mitten crab (Eriocheir sinensis). Dev. Comp. Immunol. 2016, 61, 70–79. [Google Scholar] [CrossRef]
- Wongsasak, U.; Chaijamrus, S.; Kumkhong, S.; Boonanuntanasarn, S. Effects of dietary supplementation with β-glucan and synbiotics on immune gene expression and immune parameters under ammonia stress in Pacific white shrimp. Aquaculture 2015, 436, 179–187. [Google Scholar] [CrossRef]
- Fu, H.; Jiang, S.; Xiong, Y. Current status and prospects of farming the giant river prawn (Macrobrachium rosenbergii) and the oriental river prawn (Macrobrachium nipponense) in China. Aquac. Res. 2012, 43, 993–998. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, X.; Gu, Z.; Zhang, Y.; Wang, Z. Activity and transcriptional responses of hepatopancreatic biotransformation and antioxidant enzymes in the oriental river prawn Macrobrachium nipponense exposed to microcystin-LR. Toxins 2015, 7, 4006–4022. [Google Scholar] [CrossRef] [PubMed]
- Gui, J.; Tang, Q.; Li, Z.; Liu, J.; De Silva, S.S. Culture of the oriental river prawn (Macrobrachium nipponense). In Aquaculture in China: Success Stories and Modern Trends; Wiley: Hoboken, NJ, USA, 2018; pp. 218–225. [Google Scholar] [CrossRef]
- Pan, X.; Shen, J.; Li, J.; He, B.; Hao, G.; Xu, Y.; Yao, J. Identification and biological characteristics of the pathogen causing Macrobrachium nipponense soft-shell syndrome. Microbiology 2009, 36, 1571–1576. [Google Scholar] [CrossRef]
- Gao, X.; Tong, S.; Zhang, S.; Chen, Q.; Jiang, Z.; Jiang, Z.; Wei, W.; Zhu, J.; Zhang, X. Aeromonas veronii associated with red gill disease and its induced immune response in Macrobrachium nipponense. Rev. Aquac. 2020, 51, 5163–5174. [Google Scholar] [CrossRef]
- Lulijwa, R.; Rupia, E.J.; Alfaro, A.C. Antibiotic use in aquaculture, policies and regulation, health and environmental risks: A review of the top 15 major producers. Rev. Aquac. 2020, 12, 640–663. [Google Scholar] [CrossRef]
- Alderman, D.J.; Hastings, T.S. Antibiotic use in aquaculture: Development of antibiotic resistance–potential for consumer health risks. Int. J. Food Sci. Technol. 1998, 33, 139–155. [Google Scholar] [CrossRef]
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef]
- Limbu, S.M.; Zhou, L.; Sun, S.X.; Zhang, M.L.; Du, Z.Y. Chronic exposure to low environmental concentrations and legal aquaculture doses of antibiotics cause systemic adverse effects in Nile tilapia and provoke differential human health risk. Environ. Int. 2018, 115, 205–219. [Google Scholar] [CrossRef]
- Andersen, W.C.; Casey, C.R.; Nickel, T.J.; Young, S.L.; Turnipseed, S.B. Dye residue analysis in raw and processed aquaculture products: Matrix extension of AOAC international official method 2012.25. J. AOAC Int. 2018, 101, 1927–1939. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, C.; Zhou, L.I.; Dong, Y.; Su, Y.; Wang, X.; Qin, J.; Chen, L.; Li, E. Beneficial effects of dietary β-glucan on growth and health status of Pacific white shrimp Litopenaeus vannamei at low salinity. Fish Shellfish Immunol. 2019, 91, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Suphantharika, M.; Khunrae, P.; Thanardkit, P.; Verduyn, C. Preparation of spent brewer’s yeast β-glucans with a potential application as an immunostimulant for black tiger shrimp, Penaeus monodon. Bioresour. Technol. 2003, 88, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Saikia, S.K. Oxidative Stress in Fish: A Review. J. Sci. Res. 2020, 12, 145–160. [Google Scholar] [CrossRef]
- Das, U.N.; Das, U.N. Introduction to Free Radicals, Antioxidants, Lipid Peroxidation, and Their Effects on Cell Proliferation. In Molecular Biochemical Aspects of Cancer; Springer: Berlin/Heidelberg, Germany, 2020; pp. 41–65. [Google Scholar] [CrossRef]
- Staerck, C.; Gastebois, A.; Vandeputte, P.; Calenda, A.; Larcher, G.; Gillmann, L.; Papon, N.; Bouchara, J.; Fleury, M. Microbial antioxidant defense enzymes. Microb. Pathog. 2017, 110, 56–65. [Google Scholar] [CrossRef]
- El-Missiry, M.A. Antioxidant Enzyme; BoD–Books on Demand: Norderstedt, Germany, 2012. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2012, 101, 13–30. [Google Scholar] [CrossRef]
- Perveen, S.; Yang, L.; Zhou, S.; Feng, B.; Xie, X.; Zhou, Q.; Qian, D.; Wang, C.; Yin, F. β-1,3-Glucan from Euglena gracilis as an immunostimulant mediates the antiparasitic effect against Mesanophrys sp. on hemocytes in marine swimming crab (Portunus trituberculatus). Fish Shellfish Immunol. 2021, 114, 28–35. [Google Scholar] [CrossRef]
- Gu, M.; Ma, H.; Mai, K.; Zhang, W.; Bai, N.; Wang, X. Effects of dietary β-glucan, mannan oligosaccharide and their combinations on growth performance, immunity and resistance against Vibrio splendidus of sea cucumber, Apostichopus japonicus. Fish Shellfish Immunol. 2011, 31, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, K. Evolution of Innate Immunity: Clues from Invertebrates via Fish to Mammals. Front. Immunol. 2014, 5, 459. [Google Scholar] [CrossRef]
- Du, J.; Zhu, H.; Liu, P.; Chen, J.; Xiu, Y.; Yao, W.; Wu, T.; Ren, Q.; Meng, Q.; Gu, W.; et al. Immune responses and gene expression in hepatopancreas from Macrobrachium rosenbergii challenged by a novel pathogen spiroplasma MR-1008. Fish Shellfish Immunol. 2013, 34, 315–323. [Google Scholar] [CrossRef]
- Bu, X.; Lian, X.; Wang, Y.; Luo, C.; Tao, S.; Liao, Y.; Yang, J.; Chen, A.; Yang, Y. Dietary yeast culture modulates immune response related to TLR2-MyD88-NF-kβ signaling pathway, antioxidant capability and disease resistance against Aeromonas hydrophila for Ussuri catfish (Pseudobagrus ussuriensis). Fish Shellfish Immunol. 2019, 84, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, M.; Li, N.; Dong, Z.; Cai, L.; Wu, B.; Xie, J.; Liu, L.; Ren, L.; Shi, B. New insights into β-glucan-enhanced immunity in largemouth bass Micropterus salmoides by transcriptome and intestinal microbial composition. Front. Immunol. 2022, 13, 1086103. [Google Scholar] [CrossRef] [PubMed]
- Vu, N.T.; Nghia, N.T.; Thao, N.H.P. Synergic degradation of yeast β-glucan with a potential of immunostimulant and growth promotor for tiger shrimp. Aquacult. Rep. 2021, 21, 100858. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Panigrahi, A.; Sivakumar, M.R.; Sundaram, M.; Saravanan, A.; Das, R.R.; Katneni, V.K.; Ambasankar, K.; Dayal, J.; Gopikrishna, G. Comparative study on phenoloxidase activity of biofloc-reared pacific white shrimp Penaeus vannamei and Indian white shrimp Penaeus indicus on graded protein diet. Aquaculture 2020, 518, 734654. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, H.W. Innate immunity induced by fungal β-glucans via dectin-1 signaling pathway. Int. J. Med. Mushrooms 2014, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Man, X.; Huang, X.; Wang, Y.; Song, Q.S.; Hui, K.M.; Zhang, H.W. Identification of a C-type lectin possessing both antibacterial and antiviral activities from red swamp crayfish. Fish Shellfish Immunol. 2018, 77, 22–30. [Google Scholar] [CrossRef]
- Han, B.; Zhang, X.; Chang, E.; Wan, W.; Xu, J.; Zhao, C.; Miao, S. Effects of dietary Saccharomyces cerevisiae and β-glucan on the growth performance, antioxidant capacity and immunity response in Macrobrachium rosenbergii. Aquacult. Nutr. 2021, 27, 20–28. [Google Scholar] [CrossRef]
- Amparyup, P.; Sutthangkul, J.; Charoensapsri, W.; Tassanakajon, A. Pattern recognition protein binds to lipopolysaccharide and β-1,3-glucan and activates shrimp prophenoloxidase system. J. Biol. Chem. 2016, 291, 10949. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, C.H.; Tsai, C.H.; Chen, J.C. Molecular cloning and characterisation of a pattern recognition molecule, lipopolysaccharide-and β-1,3-glucan binding protein (LGBP) from the white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2005, 18, 297–310. [Google Scholar] [CrossRef]
- Liu, F.; Li, F.; Dong, B.; Wang, X.; Xiang, J. Molecular cloning and characterisation of a pattern recognition protein, lipopolysaccharide and β-1,3-glucan binding protein (LGBP) from Chinese shrimp Fenneropenaeus chinensis. Mol. Biol. Rep. 2009, 36, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Fernando, P.; Megeney, L.A. Is caspase-dependent apoptosis only cell differentiation taken to the extreme? FASEB J. 2007, 21, 8–17. [Google Scholar] [CrossRef]
- Yang, D.; Chai, L.; Wang, J.; Zhao, X. Molecular cloning and characterization of Hearm caspase-1 from Helicoverpa armigera. Mol. Biol. Rep. 2008, 35, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.; Huang, Y.; Hui, K.; Shi, Y.; Wang, W.; Ren, Q. Cloning and characterization of two different ficolins from the giant freshwater prawn Macrobrachium rosenbergii. Dev. Comp. Immunol. 2014, 44, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Xuan, F.; Fu, H.; Zhu, J.; Ge, X.; Wu, X. Molecular cloning, characterization and expression analysis of caspase-3 from the oriental river prawn, Macrobrachium nipponense when exposed to acute hypoxia and reoxygenation. Fish Shellfish Immunol. 2017, 62, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Dhuppar, S.; Mazumder, A. Measuring cell cycle-dependent DNA damage responses and p53 regulation on a cell-by-cell basis from image analysis. Cell Cycle 2018, 17, 1358–1371. [Google Scholar] [CrossRef] [PubMed]
- Mai, W.; Yan, J.; Wang, L.; Zheng, Y.; Xin, Y.; Wang, W. Acute acidic exposure induces p53-mediated oxidative stress and DNA damage in tilapia (Oreochromis niloticus) blood cells. Aquat. Toxicol. 2010, 100, 271–281. [Google Scholar] [CrossRef]
- Huang, P.; Du, J.; Cao, L.; Gao, J.; Li, Q.; Sun, Y.; Shao, N.; Zhang, Y.; Xu, G. Effects of prometryn on oxidative stress, immune response and apoptosis in the hepatopancreas of Eriocheir sinensis (Crustacea: Decapoda). Ecotoxicol. Environ. Saf. 2023, 262, 115159. [Google Scholar] [CrossRef]
- Wang, C.; Pan, J.; Wang, X.; Cai, X.; Lin, Z.; Shi, Q.; Li, E.; Qin, J.G.; Chen, L. N-acetylcysteine provides protection against the toxicity of dietary T-2 toxin in juvenile Chinese mitten crab (Eriocheir sinensis). Aquaculture 2021, 538, 736531. [Google Scholar] [CrossRef]
- Cheng, C.; Ma, H.; Ma, H.; Liu, G.; Deng, Y.; Feng, J.; Wang, L.; Cheng, Y.; Gou, Z. The role of tumor suppressor protein p53 in the mud crab (Scylla paramamosain) after Vibrio parahaemolyticus infection. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 246, 108976. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Li, X.; Wang, L.; Shao, Z. Changes in the intestinal bacterial community during the growth of white shrimp, Litopenaeus vannamei. Aquac. Res. 2016, 47, 1737–1746. [Google Scholar] [CrossRef]
- Hou, D.; Huang, Z.; Zeng, S.; Liu, J.; Weng, S.; He, J. Comparative analysis of the bacterial community compositions of the shrimp intestine, surrounding water and sediment. J. Appl. Microbiol. 2018, 125, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A. Nutritional immunity of fish intestines: Important insights for sustainable aquaculture. Rev. Aquac. 2021, 13, 642–663. [Google Scholar] [CrossRef]
- Pirarat, N.; Pinpimai, K.; Endo, M.; Katagiri, T.; Ponpornpisit, A.; Chansue, N.; Maita, M. Modulation of intestinal morphology and immunity in nile tilapia (Oreochromis niloticus) by Lactobacillus rhamnosus GG. Res. Vet. Sci. 2011, 91, e92–e97. [Google Scholar] [CrossRef] [PubMed]
- Hoseinifar, S.H.; Van Doan, H.; Dadar, M.; Ringø, E.; Harikrishnan, R. Feed additives, gut microbiota, and health in finfish aquaculture. In Microbial Communities in Aquaculture Ecosystems: Improving Productivity and Sustainability; Springer: Berlin/Heidelberg, Germany, 2019; pp. 121–142. [Google Scholar] [CrossRef]
- Eichmiller, J.; Hamilton, M.; Staley, C.; Sadowsky, M.; Sorensen, P.W. Environment shapes the fecal microbiome of invasive carp species. Microbiome 2016, 4, 44. [Google Scholar] [CrossRef]
- Yang, G.; Xu, Z.; Tian, X.; Dong, S.; Peng, M. Intestinal microbiota and immune related genes in sea cucumber (Apostichopus japonicus) response to dietary β-glucan supplementation. Biochem. Biophys. Res. Commun. 2015, 458, 98–103. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Meirelles, P.M.; Mino, S.; Suda, W.; Oshima, K.; Hattori, M.; Thompson, F.; Sakai, Y.; Sawabe, T.; Sawabe, T. Individual Apostichopus japonicus fecal microbiome reveals a link with polyhydroxybutyrate producers in host growth gaps. Sci. Rep. 2016, 6, 21631. [Google Scholar] [CrossRef]
- Dong, P.; Guo, H.; Huang, L.; Zhang, D.; Wang, K. Glucose addition improves the culture performance of Pacific white shrimp by regulating the assembly of Rhodobacteraceae taxa in gut bacterial community. Aquaculture 2023, 567, 739254. [Google Scholar] [CrossRef]

| Ingredients | B0 | B1 | B2 | B3 | B4 |
|---|---|---|---|---|---|
| Fish meal | 18 | 18 | 18 | 18 | 18 |
| Soybean meal | 40 | 40 | 40 | 40 | 40 |
| Rapeseed meal | 15 | 15 | 15 | 15 | 15 |
| Fish oil | 3 | 3 | 3 | 3 | 3 |
| Soybean oil | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Corn starch | 10 | 10 | 10 | 10 | 10 |
| Soy lecithin | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Cholesterol | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Vitamin premix a | 2 | 2 | 2 | 2 | 2 |
| Mineral premix b | 3 | 3 | 3 | 3 | 3 |
| Choline chloride | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Sodium carboxymethyl cellulose | 2 | 2 | 2 | 2 | 2 |
| Cellulose | 4.00 | 3.95 | 3.90 | 3.80 | 3.60 |
| β-1,3-glucan c | 0 | 0.05 | 0.10 | 0.20 | 0.40 |
| Total | 100 | 100 | 100 | 100 | 100 |
| Proximate composition | |||||
| Dry matter | 91.52 | 91.91 | 91.72 | 92.19 | 91.80 |
| Crude protein | 38.44 | 38.56 | 38.44 | 38.46 | 38.36 |
| Crude lipid | 9.73 | 9.77 | 9.78 | 9.70 | 9.78 |
| Ash content | 9.32 | 9.60 | 9.42 | 9.55 | 9.31 |
| Primer | Sequence (5′→3′) | GenBank | Product |
|---|---|---|---|
| (Mn) 18S rRNA-S | TACTGCTGAGCCGAAGAT | EU118285.1 | 159 |
| (Mn) 18S rRNA-A | CCACGGACTATTACTACCTAC | ||
| (Mn) lectin-S | AAGGGCAAGGTGTCTCTTCG | PP516428 | 160 |
| (Mn) lectin-A | CCTCCCATGGTGTCCATGTC | ||
| (Mn) LBP-S | GTCTGTCTAGCAAGGGCGTT | PP516429 | 159 |
| (Mn) LBP-A | AGTGTTGATGCGATGAGCGA | ||
| (Mn) LGBP-S | CTGCTGATATCGTCGACCCC | AGF86400.1 | 167 |
| (Mn) LGBP-A | GGCATAGCTGATGCTACGGT | ||
| (Mn) P53-S | TGCTTGCTCACAGCGATAAACTT | KT963043.1 | 112 |
| (Mn) P53-A | AGTCGCCGAGTGTCAAGTCAATAT | ||
| (Mn) caspase 3-S | TTGTCATGCAGTACTTGACTGAAGC | KX651496.1 | 171 |
| (Mn) caspase 3-A | CCTCATGGGTTGTGCATCATTATA |
| Diet | IBW (g) | FBW (g) | WG (%) | SGR (%/day) | SR (%) |
|---|---|---|---|---|---|
| B0 | 0.10 ± 0.01 | 0.51 ± 0.05 ab | 391.00 ± 47.33 ab | 2.91 ± 0.17 abc | 58.10 ± 0.83 b |
| B1 | 0.10 ± 0.01 | 0.57 ± 0.01 bc | 446.00 ± 5.20 bc | 3.10 ± 0.02 bc | 60.48 ± 2.18 b |
| B2 | 0.10 ± 0.01 | 0.60 ± 0.01 c | 480.67 ± 14.47 c | 3.20 ± 0.04 c | 69.52 ± 2.97 c |
| B3 | 0.10 ± 0.01 | 0.50 ± 0.07 ab | 375.52 ± 78.21 ab | 2.84 ± 0.28 ab | 69.05 ± 0.82 c |
| B4 | 0.10 ± 0.01 | 0.46 ± 0.04 a | 341.62 ± 42.74 a | 2.72 ± 0.17 a | 48.09 ± 6.75 a |
| Diet | Sequences | Chao | Shannon | Simpson | Coverage (%) |
|---|---|---|---|---|---|
| B0 | 89,538 ± 16,475 | 925.66 ± 97.81 | 4.53 ± 0.12 | 0.045 ± 0.02 | 99.87 ± 0.06 |
| B2 | 94,935 ± 27,033 | 763.73 ± 58.44 | 4.47 ± 0.21 | 0.033 ± 0.01 | 99.93 ± 0.02 |
| B4 | 89,658 ± 56,480 | 829.08 ± 77.77 | 4.40 ± 0.12 | 0.044 ± 0.01 | 99.90 ± 0.03 |
| Phylum | Experimental Diet | ||
|---|---|---|---|
| B0 | B2 | B4 | |
| Proteobacteria | 39.31 ± 14.65 | 56.99 ± 12.11 | 54.01 ± 13.98 |
| Cyanobacteria | 23.27 ± 7.37 | 15.55 ± 17.82 | 24.44 ± 13.09 |
| Actinobacteria | 10.24 ± 4.10 | 5.90 ± 2.56 | 5.98 ± 0.51 |
| Patescibacteria | 8.40 ± 4.05 | 5.67 ± 2.34 | 4.39 ± 1.06 |
| Genus | Experimental Diet | ||
|---|---|---|---|
| B0 | B2 | B4 | |
| Chloroplast_norank | 12.03 ± 8.31 | 10.48 ± 12.76 | 10.60 ± 2.89 |
| Candidatus_Hepatincola_norank | 10.37 ± 10.87 | 7.29 ± 3.28 | 16.91 ± 2.87 |
| Rhodobacteraceae_unclassified | 2.24 ± 0.90 a | 8.54 ± 3.78 b | 4.20 ± 3.21 ab |
| Saccharimonadales_norank | 7.44 ± 3.66 | 5.14 ± 2.07 | 4.00 ± 1.23 |
| Rhodobacter | 2.48 ± 0.67 a | 9.67 ± 3.18 b | 6.36 ± 4.47 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, T.; Wang, J.; Xu, H.; Wang, Z.; Liu, Y.; Bai, H.; Zhang, Y.; Kong, Y.; Liu, Y.; Ding, Z. Dietary β-1,3-Glucan Promotes Growth Performance and Enhances Non-Specific Immunity by Modulating Pattern Recognition Receptors in Juvenile Oriental River Prawn (Macrobrachium nipponense). Fishes 2024, 9, 379. https://doi.org/10.3390/fishes9100379
Xu T, Wang J, Xu H, Wang Z, Liu Y, Bai H, Zhang Y, Kong Y, Liu Y, Ding Z. Dietary β-1,3-Glucan Promotes Growth Performance and Enhances Non-Specific Immunity by Modulating Pattern Recognition Receptors in Juvenile Oriental River Prawn (Macrobrachium nipponense). Fishes. 2024; 9(10):379. https://doi.org/10.3390/fishes9100379
Chicago/Turabian StyleXu, Tailei, Junbao Wang, Hao Xu, Zifan Wang, Yujie Liu, Hongfeng Bai, Yixiang Zhang, Youqin Kong, Yan Liu, and Zhili Ding. 2024. "Dietary β-1,3-Glucan Promotes Growth Performance and Enhances Non-Specific Immunity by Modulating Pattern Recognition Receptors in Juvenile Oriental River Prawn (Macrobrachium nipponense)" Fishes 9, no. 10: 379. https://doi.org/10.3390/fishes9100379
APA StyleXu, T., Wang, J., Xu, H., Wang, Z., Liu, Y., Bai, H., Zhang, Y., Kong, Y., Liu, Y., & Ding, Z. (2024). Dietary β-1,3-Glucan Promotes Growth Performance and Enhances Non-Specific Immunity by Modulating Pattern Recognition Receptors in Juvenile Oriental River Prawn (Macrobrachium nipponense). Fishes, 9(10), 379. https://doi.org/10.3390/fishes9100379






